Sahai uses 3D culture and intravital imaging to investigate how tumor cells invade surrounding tissues.
Sahai uses 3D culture and intravital imaging to investigate how tumor cells invade surrounding tissues.
During metastasis, tumor cells (as individuals or possibly in groups) depart the main body of a tumor and invade neighboring and remote tissues. Finding the right tools to investigate what drives cancerous cells to this invasive state and how they manage the invasion process has long been a knotty problem—one that Erik Sahai is determined to unpick.
Erik Sahai
PHOTO COURTESY OF DAVID BACON
Sahai is not the type to let a loose thread hang; instead, he's apt to grab it and follow it through the tangle to see where it leads him. A problem he first grasped as a graduate student—the ability of Rho small GTPases to regulate transformation via ROCK family kinases (1, 2)—eventually led Sahai to where he is today: tying his many observations together into a better understanding of invasive mechanisms (3–5). We called him at his lab at the Cancer Research UK London Research Institute to discuss how he's approached this gnarly problem.
FIRST THREAD
When did you decide you wanted a career in science?
I wasn't one of those kids who knew at age seven that they wanted to be a scientist. I think I didn't really start considering it as a career until, as an undergraduate, I got a summer job working in Jon Pines’ lab in Cambridge. I really enjoyed the atmosphere and the energy of being in a lab and doing experiments, but I also think science had the same appeal that just mucking around in the backyard had: playing with stuff, seeing what happens, and figuring out how things work.
What did you initially set out to work on in your graduate work?
When I did my PhD, I decided that I wanted to move to London because, as beautiful as Cambridge is, I was 20 years old and had become rather bored of the limited scope of activity outside the lab that Cambridge had to offer—I wanted to move to a big city. I joined Richard Treisman's lab, which was focused on the serum response factor (SRF) pathway.
“If you're a scientist you can't ignore experimental results.”
For my first project in his lab, I was actually trying to clone a gene that doesn't exist. I was looking for the mammalian homologue of a yeast protein, Ste12, which interacts with the yeast homologue of SRF. Today, if you want to know whether there's a mammalian Ste12, you can go on NCBI and work out that there isn't one in 10 seconds. But back then, genome sequencing hadn't yet taken hold, and the Internet barely existed, so you had to try all sorts of other approaches. I think I spent 18 months or so looking for this gene with no success before switching projects.
After struggling through that experience, what did you do?
Although it was unsuccessful in the end, I actually learned quite a lot from trying many different techniques and also from constantly having to think up new approaches to solve the problem. But after that I switched to trying to understand how the Rho small G protein signals to SRF. The approach I took toward that was trying to make some Rho effector mutants, with the idea being that these would give us some insight into the different things that Rho could regulate within the cell. Unfortunately, we weren't able to link a particular Rho effector to signaling to SRF. Instead, the one interesting thing to come out of the project was the fact that the Rho proteins regulate cell transformation through the ROCK family kinases.
TYING UP LOOSE ENDS
Sahai and postdoc Fernando Calvo discuss a recent result.
PHOTO COURTESY OF DAVID BACON
So what did you work on as a postdoc?
In my PhD I'd observed that growth control could be restored in some transformed cells by a small-molecule ROCK inhibitor, which suggested Rho-ROCK signaling was important for Ras-mediated transformation. But if you surveyed the literature as a whole, there were several papers claiming the opposite. So there was this apparent paradox in the literature, and I liked the intellectual challenge of working that out. I suppose one could simply shrug one's shoulders and say, “It's a different cell line,” but I've never found that response particularly satisfying. Therefore, assuming the experiments have been done properly, the data should tell you something. It might not fit nicely into your favorite hypothesis, but so long as it's not some aberration or artifact, then data are data and they have to be reconciled. I think if you're a scientist you can't ignore experimental results. They have to fit in somewhere, somehow, even if you don't understand how that is at the time that you get the results. It transpired that some aspects of Rho signaling promote the deregulated proliferation of transformed fibroblasts while other Rho-regulated processes hinder transformation.
You also investigated how Rho-ROCK signaling regulates tumor cell invasion…
About the time I joined Chris Marshall's lab for my postdoc, we were starting to play around with Matrigel as a 3D matrix, which others had used to set up what I thought were more realistic types of invasion assays—ones that weren't simply cells crawling around in 2D. So we started putting cancer cell lines into these 3D matrices and doing invasion assays with the ROCK inhibitor, trying to work out whether the sensitivity of the cell line correlated with whether or not it had a Ras mutation or whether or not it had high MAP kinase signaling.
Another critical thing about those studies is that we were actually looking down the microscope at the cells to evaluate whether or not they were invading, as opposed to using assays that score invasion by swabbing one side of a filter and measuring the amount of cellular material present. And while I don't think we resolved the paradox for why 3D invasion by some cells is sensitive to ROCK inhibition while in other cells it's not, we did learn some of the rules about how you could tell whether or not a cell line would require ROCK function to invade into a three-dimensional matrix. More generally, we learned that cancer cells can have different modes of invasion and that this has implications for therapeutic strategies to stop invasion.
How does ROCK drive invasion by cancer cells?
ROCK drives actomyosin contractility, which is responsible for the rounded morphology we observe in some invading cells. We think the cell also utilizes actomyosin contractility to produce force with which to deform the extracellular matrix. So, if you think about the extracellular matrix, it forms a barrier in front of tumor cells that keeps them from invading. One solution is to elbow it out of the way and squeeze through, and that's what we think is going on to a significant degree in these contractile, ROCK-dependent cells. The other solution to this problem is to chop the barrier up, and that's what ROCK-independent cells (which typically have a very different, elongated morphology) do. Both forms of invasion can be observed in vivo; cancers work pretty much every way that's conceivable.
UNRAVELING COMPLEXITY
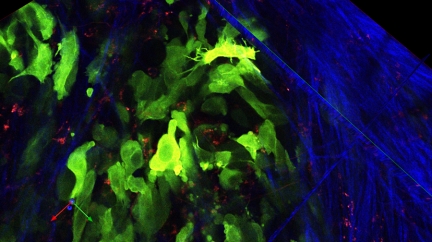
Melanoma cells (green) invading into a collagen-rich matrix (blue) containing phagocytic cells (red).
IMAGE COURTESY OF ERIK SAHAI
How did this lead you into intravital imaging?
There was a lingering question about what matrix material is closest to the physiological environment and whether any of them actually represent a good model of a real tumor's setting. At that time, John Condeelis’ lab had just published some of their early intravital imaging of cancer cells moving around in their mammary tumor models. That technology was amazing and would enable me to answer my questions about whether reconstituted matrices mimic what's going on in tumors. So I got a one-year fellowship to go work in the Condeelis lab and learn this technology before setting up my own lab.
What have you chosen to pursue in your own lab?
Our intravital imaging has revealed that there is great heterogeneity in behavior within tumors: some cells invade, many don't. The causes and consequences of this heterogeneity represent an interesting biological problem and probably relate to the uneven response of tumors to therapy. One thing we wanted to probe was whether particular transcriptional programs might be heterogeneously active within the tumor—we've obtained some interesting insights by simultaneously imaging cell behavior and transcriptional readouts.
Another area we've been working on is the heterogeneous interactions between the tumor and its environment, focusing on cancer-associated fibroblasts and how they might promote invasion. For example, we observed that the cancer-associated fibroblast acts as the leading cell of a cohort of tumor cells. It clears a path through the surrounding tissue, like a snowplow clearing a track for other cars to use. This process therefore starts to resemble aspects of developmental biology and morphogenesis and poses interesting questions concerning how cells move when there's a whole group of them moving together. For example, how is movement coordinated and organized? And how do cancer cells trick fibroblasts into doing their dirty work for them?
“Cancers work pretty much every way that's conceivable.”
We're watching tumors in all their complexity, then trying to unpick what's going on. Hopefully we can learn a lot more and make a small contribution to the benefit of cancer patients.
References
- 1.Sahai E., Alberts A.S., Treisman R.. 1998. EMBO J. 17:1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahai E., et al. 1999. Curr. Biol. 9:136–145. [DOI] [PubMed] [Google Scholar]
- 3.Sahai E., Marshall C.J.. 2003. Nat. Cell Biol. 5:711–719. [DOI] [PubMed] [Google Scholar]
- 4.Gaggioli C., et al. 2007. Nat. Cell Biol. 9:1392–1400. [DOI] [PubMed] [Google Scholar]
- 5.Giampieri S., et al. 2009. Nat. Cell Biol. 11:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]