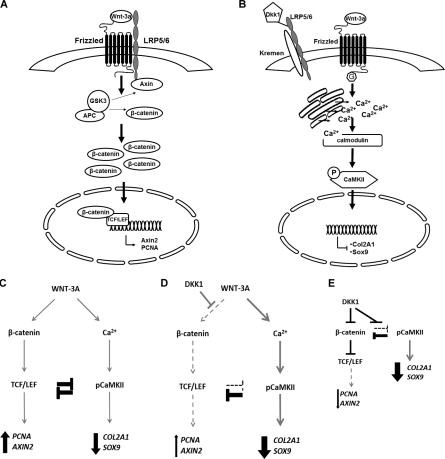
A single Wnt can simultaneously activate different pathways with distinct and independent outcomes and reciprocal regulation in human articular chondrocytes.
Abstract
Activation and disruption of Wnt/β-catenin signaling both result in cartilage breakdown via unknown mechanisms. Here we show that both WNT-3A and the Wnt inhibitor DKK1 induced de-differentiation of human articular chondrocytes through simultaneous activation of β-catenin–dependent and independent responses. WNT-3A activates both the β-catenin–dependent canonical pathway and the Ca2+/CaMKII noncanonical pathways, with distinct transcriptional targets. WNT-3A promotes cell proliferation and loss of expression of the chondrocyte markers COL2A1, Aggrecan, and SOX9; however, proliferation and AXIN2 up-regulation are downstream of the canonical pathway and are rescued by DKK1, whereas the loss of differentiation markers is CaMKII dependent. Finally, we showed that in chondrocytes, the Ca2+/CaMKII-dependent and β-catenin–dependent pathways are reciprocally inhibitory, thereby explaining why DKK1 can induce loss of differentiation through de-repression of the CaMKII pathway. We propose a novel model in which a single WNT can simultaneously activate different pathways with distinct and independent outcomes and with reciprocal regulation. This offers an opportunity for selective pharmacological targeting.
Introduction
The articular cartilage covers the articulating surface of bone elements, ensuring frictionless joint motion. Osteoarthritis (OA) is a leading cause of disability worldwide, affecting up to one third of the population over the age of 50 (Lawrence et al., 1998; Lawrence et al., 2008) and costing $185.5 million only in the US (Kotlarz et al., 2009). Cartilage breakdown is the hallmark of OA. Unfortunately, no treatment for OA is currently available, except for pain control and physiotherapy, until prosthetic joint replacement is required. Understanding the pathogenetic mechanisms leading to the disruption of homeostasis and cartilage breakdown is of paramount importance to the development of effective pharmacological treatments.
Degeneration of the articular cartilage is the final outcome of an imbalance between catabolic processes triggered by injurious factors (biomechanical or inflammatory) and compensatory anabolic processes. Genetic studies in humans (Loughlin et al., 2004) and experimentation in animal models (Lories et al., 2007; Wu et al., 2010) have convincingly demonstrated the involvement of Wnt signaling in OA pathogenesis.
Wnts are a family of 19 morphogens involved in several processes from embryonic morphogenesis to homeostasis of adult tissues (Logan and Nusse, 2004). Wnts signal through multiple pathways (Macdonald et al., 2007; Semenov et al., 2007), the best characterized of which is the Wnt/β-catenin signaling, also known as the “canonical” pathway. In this pathway, in the absence of ligands, the constitutively active kinase GSK-3β phosphorylates β-catenin within a destruction complex, addressing it for degradation through the proteasome pathway (Logan and Nusse, 2004). The engagement of some members of the Wnt family, such as WNT-1 and WNT-3A (Hinck et al., 1994;Shimizu et al., 1997) with Frizzled (FZD) receptors and the coreceptors low-density lipoprotein receptor-related receptor 5/6 (LRP5/6), results in the disruption of the destruction complex, accumulation of β-catenin in the cytosol, and its migration in the nucleus, where β-catenin interacts with T cell factor (TCF)/lymphoid enhancer–binding factor (LEF) transcription factors and supports the transcription of target genes (Molenaar et al., 1996). Other Wnts, such as WNT-5A and WNT-11 (Kühl et al., 2000), can instead activate other signaling cascades, generally referred as “noncanonical” pathways, such as the planar cell polarity pathway (PCP) and the Ca2+/CaMKII pathways (Sheldahl et al., 1999; Kühl et al., 2000; Semenov et al., 2007).
Activation of the canonical Wnt pathway is required and sufficient for embryonic joint specification (Hartmann and Tabin, 2001; Guo et al., 2004), and both canonical and noncanonical Wnt signaling regulate morphogenesis, proliferation, cell specification, and differentiation within the developing skeleton (Church et al., 2002; Enomoto-Iwamoto et al., 2002; Yang et al., 2003; Koyama et al., 2008).
In adult life, modulation of Wnt signaling has an important role in cartilage homeostasis. We demonstrated enhanced activation of the canonical Wnt pathway in osteoarthritic cartilage in humans (Dell’Accio et al., 2001) and after cartilage injury in mice (Dell’Accio et al., 2008; Eltawil et al., 2009). In addition, a loss of function polymorphism in the SFRP3 gene, encoding for a soluble Wnt inhibitor (Leyns et al., 1997; Wang et al., 1997), has been linked to an increased susceptibility to develop OA (Loughlin et al., 2004; Lories et al., 2007). These data, together with other in vivo and in vitro experimental evidence, suggest that an over-activation of the β-catenin–dependent/Wnt pathway might be involved in OA pathogenesis (Schett et al., 2008; Luyten et al., 2009).
However, the results of functional studies using mouse genetics contrast with each other and are difficult to interpret. For instance, both repression and forced activation of the canonical Wnt signaling resulted in chondrodysplasia in embryonic life (Akiyama et al., 2004) and in OA development postnatally (Lories et al., 2007; Chen et al., 2008; Koyama et al., 2008; Zhu et al., 2008, 2009).
We demonstrated that the prototypical canonical activator WNT-3A and the inhibitor of the canonical pathway DKK1 both induce down-regulation of cartilage phenotypic markers and reduction of glycosaminoglycan (GAG)-rich extracellular matrix in vitro. Although the fact that an activator and an inhibitor of the canonical Wnt pathway both lead to the same outcome is very surprising, nevertheless it is in keeping with the in vivo data mentioned previously and provided a convenient system to dissect the underpinning molecular mechanisms. We therefore used this system to propose a new model according to which WNT-3A can simultaneously activate the β-catenin–dependent and CaMKII-dependent signaling, each with specific outcomes; reciprocal inhibition of these two pathways explains how differentiation is induced by WNT-3A through activation of CaMKII, and by DKK1 through inhibition of the canonical pathway and consequent de-repression of the CaMKII pathway.
Results
Biological events associated with WNT-3A treatment of primary articular chondrocytes
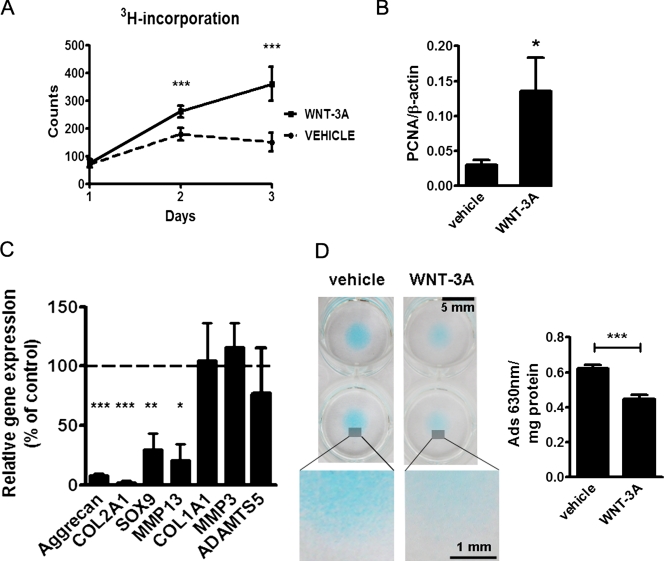
WNT-3A is a prototypical, well-characterized activator of the β-catenin–dependent canonical pathway (Shimizu et al., 1997), which has an established role in arthritis (Nakamura et al., 2005), and it is expressed in articular cartilage (Fig. S1). Taking advantage of the availability of WNT-3A as recombinant protein, we investigated the effects of this ligand on the proliferation, differentiation, and extracellular matrix production of articular chondrocytes. Treatment of primary porcine articular chondrocytes with 100 ng/ml of recombinant WNT-3A over a 3-d time course resulted in a statistically significant increase of cell proliferation compared with vehicle control (Fig. 1 A). The proliferative effect of WNT-3A was confirmed in adult human articular chondrocytes (AHACs), where the proliferation marker PCNA was up-regulated 24 h after stimulation with WNT-3A (Fig. 1 B).
Figure 1.
WNT-3A induces chondrocyte proliferation and de-differentiation in vitro. (A and B) 100 ng/ml WNT-3A stimulated proliferation of primary chondrocytes in vitro as evaluated by an [H3]thymidine incorporation assay in porcine articular chondrocytes (A; n = 6) and by up-regulation of the proliferation marker PCNA in primary AHACs by quantitative PCR (Q-PCR) (B; n = 4). (C and D) WNT-3A–induced down-regulation of the differentiation markers COL2A1, Aggrecan, SOX9, and MMP13, as evaluated by Q-PCR (C; n = 6), and reduced the accumulation of highly sulphated GAG in primary AHACs (D; by Alcian blue staining and spectrophotometric quantification; n = 4). Gene expression values are reported as a percentage of up- or down-regulation compared with control (100%, broken line in the graph). Statistical analysis was performed with an unpaired t test. Error bars indicate mean ± SEM. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
We then compared the expression of chondrocyte-lineage markers by confluent primary AHACs treated with either WNT-3A or vehicle control. COL2A1, Aggrecan, and SOX9 mRNA were statistically significantly down-regulated after WNT-3A treatment (Fig. 1 C). The chondrocyte hypertrophy marker MMP13 was also down-regulated. Other genes relevant to cartilage homeostasis/biology including COL1A1, MMP-3, and ADAMTS5 (Goldring, 2006) were unchanged. The main function of articular chondrocytes is the production of a specialized extracellular matrix rich in highly sulphated GAGs, which provide the elastic biomechanical properties required for motion and weight bearing. Therefore, to test whether WNT-3A affected this important function of chondrocytes, we exploited the capacity of chondrocytes to produce large amounts of such matrix when cultured in 3D micromasses. Treatment of AHAC micromasses with 100 ng/ml of WNT-3A for 4 d induced a statistically significant decrease in the accumulation of highly sulphated GAGs, as evaluated by Alcian blue staining and spectrophotometric quantitation (Fig. 1 D; De Bari et al., 2001). Collectively, these findings indicate that WNT-3A stimulation activates proliferation and de-differentiation of articular chondrocytes.
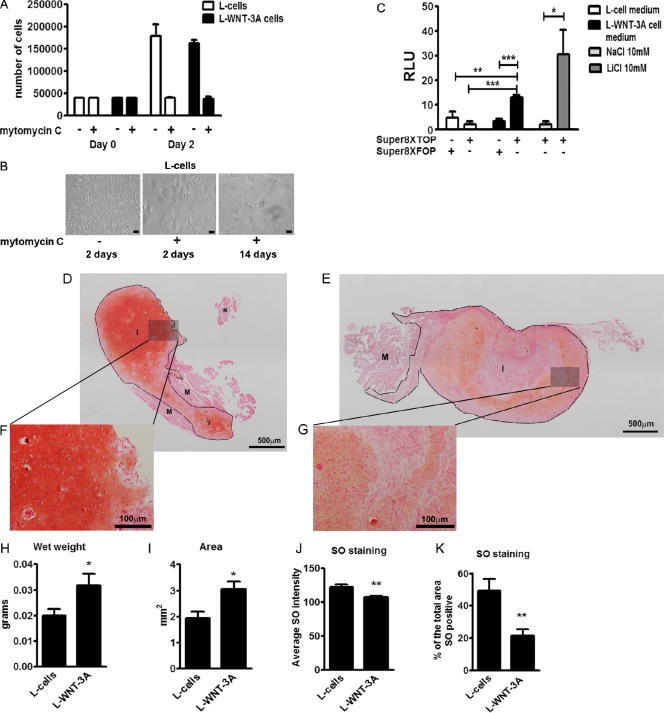
We confirmed the validity of these data in vivo using a well-established model that measures the capacity of chondrocytes to form hyaline-like cartilage when implanted ectopically in nude mice (Dell’Accio et al., 2001). To provide prolonged WNT-3A stimulation of the chondrocytes in vivo, we co-injected freshly isolated porcine articular chondrocytes with 10% of growth-arrested L cells stably overexpressing WNT-3A (L-WNT-3A), or L cells as control. Growth arrest of L cells was successfully achieved by mitomycin C treatment (Fig. 2, A and B), without compromising their capacity to secrete biologically active WNT-3A for at least 14 d (Fig. 2 C). Indeed, conditioned medium from L-WNT-3A cells that had been growth-arrested for 2 wk was able to activate the TCF/LEF1 reporter assay SUPER8XTOPFlash (Veeman et al., 2003) in porcine articular chondrocytes. 2 wk after the injection, the wet weight of the cartilage implants retrieved from mice injected with chondrocytes plus L-WNT-3A cells was statistically significantly higher compared with that of implants obtained from chondrocytes co-implanted with control L cells (Fig. 2, H and I) and statistically significantly less differentiated, as demonstrated by a weaker staining with safranin O (SO; Fig. 2, D–G, J, and K), which confirms that WNT-3A also mediated proliferation and loss of chondrocyte phenotype in an in vivo system. Similar results were obtained with primary AHACs (Fig. S2), thereby confirming that this biological outcome is conserved across mammals.
Figure 2.
WNT-3A induces proliferation and de-differentiation of articular chondrocytes in vivo. (A and B) WNT-3A induced chondrocyte de-differentiation in vivo in an ectopic cartilage formation assay. Isolated porcine articular chondrocytes were co-injected intramuscularly in nude mice together with either L cells stably expressing WNT-3A (L-WNT3A) or control L cells (L cells; 12 injections per condition). Before injection, L cells and L-WNT-3A cells were growth-arrested by mitomycin C treatment. To confirm growth arrest, 2 d after treatment, a sample of cells with or without mitomycin C treatment was detached and counted (A; n = 4). Mitomycin C–treated cells stopped proliferating. One additional well was kept in culture for 14 d. The cells persisted but never reached confluency (B). Bar, 10 µm. (C) To confirm that mitomycin C growth-arrested L-WNT-3A cells still produced biologically active WNT-3A, the conditioned medium (from the last change of medium) obtained 14 d after mitomycin C treatment was tested for the ability to activate the SUPER8XTOPFlash reporter assay in porcine articular chondrocytes. Indeed, conditioned medium from L-WNT-3A cells, but not from control cells, activated the SUPER8XTOPFlash reporter. The mutagenized SUPER8XFOPFlash vector was used to control for specificity (n = 4). (D–K) SO staining of cartilage implants obtained by the co-injection of porcine articular cartilage with L cells (D) or L-WNT-3A cells (E) in nude mice. Cartilage implants obtained from porcine articular chondrocytes co-injected with L-WNT-3A cells are on average larger (H and I) and less differentiated (J and K), as shown by a decreased SO staining in comparison with controls. Grayscale digital images from SO staining were used to calculate and compare the mean intensity of the staining and, after thresholding, the percentage of the total implant area that was positive for SO (n = 12 per condition). M, muscle; I, implant; RLU, relative luminescence unit. An unpaired t test was used for statistical evaluation. Error bars indicate mean ± SEM. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
WNT-3A mediates proliferation of AHACs via the β-catenin–dependent pathway and their de-differentiation via a β-catenin–independent mechanism
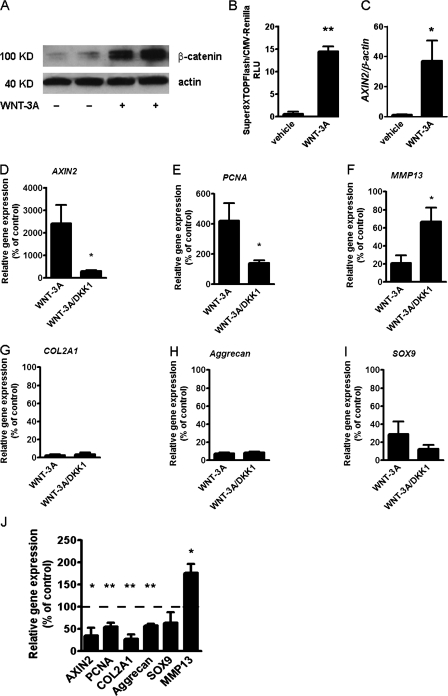
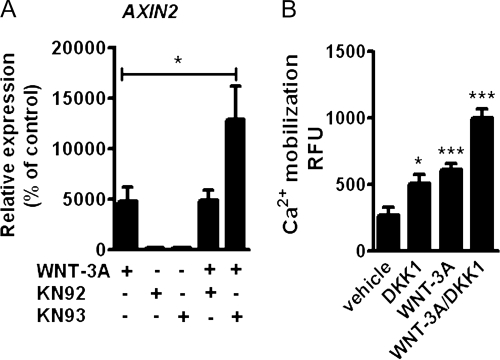
WNT-3A is known to activate the canonical Wnt pathway in a variety of cells. We therefore set out to confirm the activation of this pathway by WNT-3A in AHACs. Treatment with 100 ng/ml recombinant WNT-3A resulted in accumulation of β-catenin (Fig. 3 A), activation of the SUPER8XTOPFlash reporter assay (Fig. 3 B), and a 250-fold up-regulation of the endogenous target gene AXIN2 (Fig. 3 C), which is indicative of activation of the β-catenin–dependent canonical pathway (Yan et al., 2001).
Figure 3.
Inhibition of Wnt canonical pathway with DKK1 does not rescue the loss of the chondrocyte phenotype. (A–C) WNT-3A activates the Wnt canonical pathway in AHACs. (A) Western blotting for β-catenin in the cytoplasmic fraction of AHACs treated with 100 ng/ml WNT-3A or vehicle control for 24 h. (B) WNT-3A activated the SUPER8TOPFlash reporter assay in P0 AHACs after 24 h of stimulation (n = 3). (C) WNT-3A up-regulated the expression of the endogenous Wnt canonical target gene AXIN2 in AHACs as evaluated by Q-PCR. Values were normalized for the housekeeping gene β-actin. (D–I) Primary AHACs were treated for 24 h with 100 ng/ml of recombinant WNT-3A or DKK1, alone or in combination, and were subjected to gene expression analysis by PCR. Blockade of the Wnt canonical pathway by DKK1 rescued the modulation of AXIN2, PCNA, and MMP13 mRNA (D–F) but not the down-regulation of COL2A1, Aggrecan, and SOX9 (G–I). (J) In the absence of exogenous WNT-3A, treatment with 100 ng/ml DKK1 resulted, as expected, in down-regulation of the mRNA levels of AXIN2 and PCNA, but also in a paradoxical down-regulation of COL2A1 and Aggrecan similar to that induced by WNT-3A. Q-PCR data, n = 6. Gene expression values are reported as a percentage of up- or down-regulation compared with control (100% is indicated by the broken line in the graph). Data were analyzed with an unpaired t test. Error bars indicate mean ± SEM. *, P < 0.05; **, P < 0.005.
To confirm that the observed AHAC phenotypic changes were caused by the activation of the canonical pathway, primary AHACs were treated with either 100 ng/ml WNT-3A alone or in combination with 100 ng/ml of recombinant DKK1, which specifically inhibits the β-catenin–dependent pathway by binding to LRP coreceptors and preventing their association with FZDs (Mao et al., 2001). Co-treatment with DKK1 reverted the WNT-3A–induced up-regulation of AXIN2, PCNA, and MMP13 (Fig. 3, D–F), but, surprisingly failed to rescue the down-regulation of COL2A1, Aggrecan, and SOX9 mRNA (Fig. 3, G–I). To investigate whether this was caused by excessive, exogenous WNT-3A stimulation, we investigated whether treatment with DKK1 alone, in the absence of exogenous WNT-3A, modified the phenotype of AHACs. Strikingly, treatment of AHACs with DKK1, in the absence of WNT-3A, promoted by itself a down-regulation of COL2A1 and Aggrecan, similar to that induced by WNT-3A, and up-regulation of MMP13, although, as expected, it did reduce AXIN2 and PCNA mRNA expression (Fig. 3 J).
The finding that DKK1 not only did not rescue WNT-3A–induced loss of phenotypic markers but, on its own, promoted it, suggests that although proliferation and AXIN2 up-regulation are driven by activation of the canonical Wnt pathway, the loss of phenotypic markers and of extracellular matrix is regulated through a different pathway that is antagonized by the canonical one. To explain the apparent paradox that WNT-3A and DKK1 have the same effect, we hypothesized that, under our experimental conditions, the modulation of AXIN2, PCNA, and MMP13 and chondrocyte proliferation are dependent on the Wnt–β-catenin canonical pathway, whereas the WNT-3A–mediated down-regulation of COL2A1 and Aggrecan are β-catenin independent.
WNT-3A simultaneously activates the canonical and the Ca2+/CaMKII-dependent Wnt pathways in chondrocytes in a dose-dependent manner
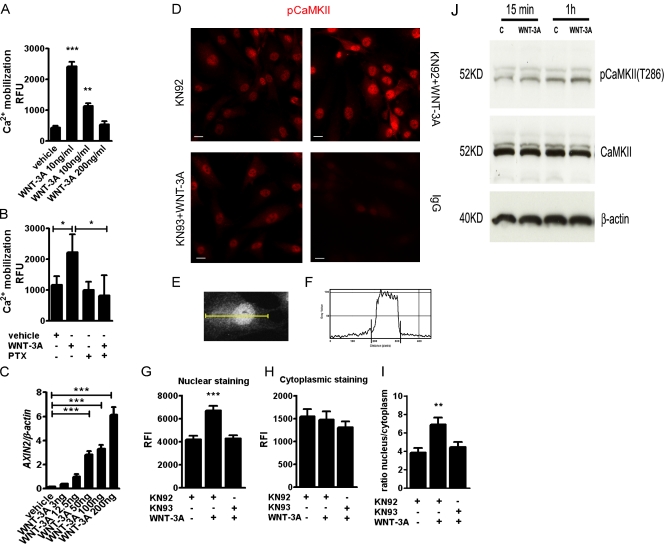
We therefore hypothesized that WNT-3A induces loss of chondrocyte differentiation through activation of a noncanonical Wnt pathway. We focused on the Wnt-Ca2+/CaMKII pathway because it has been shown to be active in chicken epiphyseal cartilage (Taschner et al., 2008) and to mediate Wnt signaling in zebrafish and Xenopus embryos (Slusarski et al., 1997a,b; Sheldahl et al., 1999; Kühl et al., 2000) antagonizing β-catenin. Therefore, to test whether WNT-3A activates the noncanonical Ca2+/CaMKII-dependent pathway, we treated primary AHAC cultures with increasing doses of WNT-3A while monitoring calcium mobilization (as a readout of the activation of the Ca2+-dependent pathway) and AXIN2 expression (as a readout for the activation of the canonical pathway). In keeping with our hypothesis, WNT-3A elicited activation of both pathways; notably, Ca2+ mobilization occurred preferably at low doses and AXIN2 up-regulation at higher doses (Fig. 4, A and C).
Figure 4.
WNT-3A promotes intracellular Ca2+-mediated phosphorylation of CaMKII and its nuclear translocation. (A) WNT-3A caused calcium mobilization in AHACs, particularly at low doses. Primary AHACs were treated with different amounts of recombinant WNT-3A or vehicle control for 5 min and then subjected to fluorometric determination of calcium mobilization (n = 9). (B) WNT3A-induced calcium mobilization assay in primary AHACs is G protein dependent. AHACs were preincubated overnight at 37°C with 1 µg/ml of the G protein inhibitor PTX and then treated for 5 min with 100 ng/ml of WNT-3A or vehicle control. PTX treatment blocked calcium mobilization induced by WNT-3A (n = 6). (C) Q-PCR for AXIN2 mRNA in primary chondrocytes exposed for 24 h to different doses of recombinant WNT-3A. WNT-3A induced dose-dependent up-regulation of AXIN2 in primary AHACs (n = 3). (D) WNT-3A induced nuclear accumulation of the phosphorylated form of CaMKII. Immunofluorescence staining for pCaMKII in P0 AHACs stimulated for 24 h with WNT-3A in combination with either the CaMKII inhibitor KN93 or its inactive analogue KN92 (both 10 µM). Bar, 20 µm. (E–I). Quantification of the pCaMKII fluorescence intensity in the nuclear and in the cytoplasmic fractions of stimulated AHACs. The pixel intensity profile was plotted over a linear section of a whole cell (E and F). The nuclear and cytoplasmic fluorescence intensity was then calculated as the area under the curve (G–I). (J) WNT3A promotes phosphorylation of CaMKII in T286 at 15 min and 1 h from WNT-3A stimulation. RFU, relative fluorescence unit; RFI, relative fluorescence intensity. Data were analyzed with an unpaired t test except in C, where a Kruskal Wallis with a Dunns post-test was used. Error bars indicate mean ± SEM. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
In zebrafish and Xenopus embryos, it was shown that the Ca2+/CaMKII pathway signals in a G protein–dependent manner (Slusarski et al., 1997a; Sheldahl et al., 1999; Kühl et al., 2000). In our experimental system, pertussis toxin (PTX), a Gαi, Gα0, and Gαt protein inhibitor (Slusarski et al., 1997a; Sheldahl et al., 1999), efficiently blocked WNT-3A–induced calcium mobilization (Fig. 4 B). Next, to evaluate the involvement of CaMKII, we treated AHACs with 100 ng/ml WNT-3A or vehicle control for 24 h and stained the monolayers with an antibody to detect the activated, phosphorylated form of CaMKII (Soderling, 1999). PhosphoCaMKII staining was increased in WNT-3A–treated samples with increased nuclear localization, confirming the activation of this pathway (Fig. 4, D–I). Such phosphorylation was abrogated in the presence of the selective CaMKII inhibitor KN93 (Fig. 4 D). CaMKII phosphorylation was also detected by Western blotting analysis, after 15 min and 1 h from the addition of WNT-3A to the cells (Fig. 4 J). Altogether, our data indicate that exogenous application of WNT-3A is sufficient to dose dependently activate both the canonical β-catenin–dependent and noncanonical Ca2+/CaMKII-dependent pathways (see Fig. 7, A and B).
Figure 7.
Simultaneous activation and reciprocal inhibition of β-catenin– and CaMKII–dependent pathways in AHACs. (A and B) In AHACs, WNT-3A up-regulates AXIN2 expression and proliferation through the Wnt canonical pathway (A), but induces loss of differentiation via a G protein–mediated Ca2+/CaMKII–dependent pathway (B). (C–E) These two pathways are reciprocally inhibitory and in equilibrium. Therefore, either exogenous WNT-3A (C) or blockade of the canonical pathway with DKK1 (D–E) will both result in loss of chondrocyte phenotype.
Wnt canonical and Wnt-CaMKII noncanonical pathways are reciprocally inhibitory in AHACs
In Xenopus, the Wnt Ca2+/CaMKII pathway has been shown to inhibit the Wnt–β-catenin–dependent pathway (Sheldahl et al., 1999; Kühl et al., 2000). To investigate the interaction between the CaMKII pathway with the Wnt canonical pathway after WNT-3A stimulation, we treated confluent passage 0 (P0) AHACs with 100 ng/ml WNT-3A and either the selective CaMKII inhibitor KN93 or its inactive analogue KN92 (both 10 µM) for 24 h. CaMKII inhibition by KN93 significantly increased the WNT-3A–induced up-regulation of AXIN2 mRNA (Fig. 5 A).In contrast, KN92, which is structurally similar to KN93 but does not inhibit CaMKII (Marley and Thomson, 1996), did not alter the expression of AXIN2 (Fig. 5 A). To study if the Wnt/β-catenin pathway modulates the Wnt-Ca2+/CaMKII pathway, we treated AHACs with WNT-3A and DKK1, alone or in combination. Both WNT-3A and DKK1 stimulation resulted in statistically significant activation of Ca2+ mobilization, which was further increased when DKK1 and WNT-3A were used together, thereby demonstrating that the selective inhibition of the canonical pathway enhances the activation of the Ca2+ mobilization (Fig. 5 B).
Figure 5.
Wnt canonical and Wnt-CaMKII noncanonical pathways are reciprocally inhibitory in AHACs. (A) Q-PCR for AXIN2 of primary AHACs treated with 100 ng/ml WNT-3A in the presence of the CaMKII inhibitor KN93 or its inactive analogue KN92 (10 µM). (B) DKK1 promoted intracellular calcium accumulation in P0 AHACs. The inhibition of the canonical pathway with DKK1 enhanced intracellular calcium accumulation induced by WNT-3A. In all the experiments, n = 4. Data in A were analyzed with ANOVA; data in B were analyzed with an unpaired t test. Error bars indicate mean ± SEM. *, P < 0.05; ***, P < 0.0005.
Collectively, our data demonstrate reciprocal inhibition of the Wnt–β-catenin and Wnt-Ca2+/CaMKII pathways after WNT-3A stimulation in articular chondrocytes, and that the inhibition of one causes de-repression of the other (see Fig. 7, C–E)
To gain a better insight into this mechanism and specifically examine whether the selectivity of the blockade for the canonical pathway was essential for Ca2+ mobilization or if general out-titration of Wnts also induced Ca2+ mobilization, we repeated the same experiment using sFRP1 as a Wnt inhibitor. We chose sFRP1 because it is known to bind and inhibit several Wnts, including WNT-3A (Galli et al., 2006; Wawrzak et al., 2007), preventing their interaction with FZD receptors. sFRP1 treatment prevented WNT-3A–induced intracellular calcium accumulation, but, in contrast to DKK1, did not induce Ca2+ mobilization on its own, thereby confirming that Ca2+ mobilization induced by DKK1 is caused by selective inhibition of the canonical pathway and consequent de-repression of the Ca2+/CaMKII pathway (Fig. S3).
Distinct effects in chondrocytes of the WNT-3A–mediated activation of β-catenin and CaMKII pathways
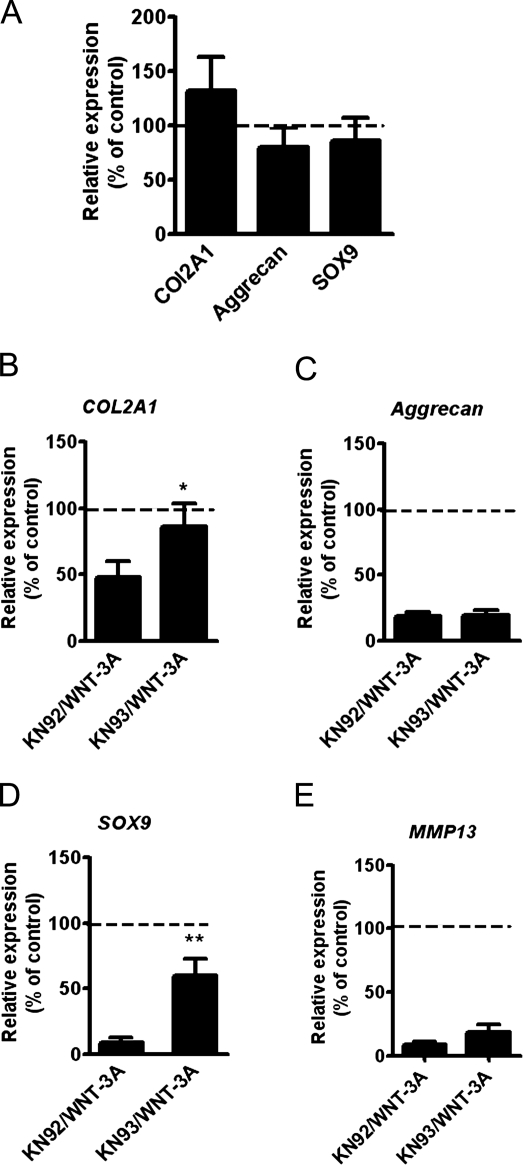
Having demonstrated that WNT-3A can signal simultaneously through two intracellular pathways, we set out to discriminate CaMKII-dependent from β-catenin–dependent transcriptional targets. To this end, we treated chondrocytes with WNT-3A in the presence of either the CaMKII inhibitor KN93 or the inactive compound KN92. As expected, WNT-3A induced a significant down-regulation of COL2A1, Aggrecan, and SOX9 mRNA compared with control, but CaMKII blockade with KN93 resulted in a statistically significant rescue of COL2A1 and SOX9 expression (Fig. 6. B and D), which demonstrated that WNT-3A–induced chondrocyte de-differentiation is at least in part CaMKII dependent. In contrast, KN93 did not rescue the down-regulation of MMP13 mRNA (Fig. 6 E). Therefore WNT-3A up-regulates AXIN2 and PCNA through the canonical pathway and down-regulates SOX9 and COL2A1 in a CaMKII-dependent manner (Fig. 7, A and B).
Figure 6.
Inhibition of the Ca2+/CaMKII pathway rescued the loss of COL2A1 and SOX9 mRNA expression in AHACs. (A) Blockade of CaMKII with KN93 did not alter the basal levels of Aggrecan, COL2A1, and SOX9. Primary AHACs were treated for 24 h with 10 µM KN93 or its inactive analogue KN92. (B–E) CaMKII blockade rescues WNT-3A–induced down-regulation of COL2A1 and SOX9 mRNA. Primary AHACs were cultured for 24 h in the presence of WNT-3A or vehicle control, and either the CaMKII inhibitor KN93 or the inactive control KN92. Gene expression was evaluated by Q-PCR. The values were normalized for β-actin and expressed as a percentage of the KN92-treated group (100% is indicated by the broken line in the graphs; n = 6). Statistical analysis was performed with an unpaired t test. Error bars indicate mean ± SEM. *, P < 0.05; **, P < 0.005.
In contrast, nonspecific forced Ca2+ mobilization using phorbol 12-myristate 13-acetate (PMA) resulted in down-regulation of AGGRECAN but not SOX9 or COL2A1 (Fig. S4). This, and the fact that instead CaMKII blockade rescued the expression of COL2A1 as well as SOX9, suggests that the WNT-3A–induced effects on these three genes rely specifically on the CaMKII-dependent Ca2+-pathway.
Discussion
We have demonstrated that chondrocyte exposure to exogenous WNT-3A can simultaneously activate both the β-catenin–dependent and the G protein–mediated Ca2+/CaMKII/pathways, which are in equilibrium because of reciprocal inhibition. These two pathways modulate the transcription of distinct target genes involved in different biological processes: WNT-3A promotes chondrocyte proliferation through β-catenin and differentiation through CaMKII. According to our model, blockade of the canonical/β-catenin pathway will also cause articular-chondrocyte de-differentiation through de-repression of the CaMKII pathway (Fig. 7, A and B).
This model may help explaining contradictions in the current literature. In particular, both activation and disruption of the canonical Wnt signaling result in chondrodysplasia with delayed maturation of chondrocytes (Enomoto-Iwamoto et al., 2002; Akiyama et al., 2004) and in cartilage breakdown in adulthood (Chen et al., 2008; Koyama et al., 2008; Zhu et al., 2008, 2009). Similarly, in the mouse embryo (Yamaguchi et al., 1999; Yang et al., 2003), both the overexpression and the knockout of WNT-5A, which in zebrafish and Xenopus has been shown to activate the Ca2+/CaMKII pathway (Slusarski et al., 1997b; Kühl et al., 2000), result in a similar phenotype of chondrodysplasia, in both cases with reduced chondrocyte proliferation and hypertrophic maturation (Yamaguchi et al., 1999; Yang et al., 2003). Although this phenotype, in vivo, may be partially determined by compensatory mechanisms involving modulation of the Ihh–PTHrP pathway, COL2A1 expression was up-regulated in the growth plate of WNT-5A knockout mice and down-regulated in mice overexpressing WNT-5A under the transcriptional control of the collagen type 2 promoter (Yang et al., 2003). In addition, in vitro, WNT-5A dose-dependently down-regulated the activity of a COL2A1 reporter in mouse chondrocytes. Importantly, this study showed that WNT-1, a well validated canonical Wnt, could also decrease COL2A1 reporter activity, though in a TCF-independent manner, because overexpression of TCF1 failed to achieve the same result (Yang et al., 2003). This may suggest that WNT-3A may not be the only canonical Wnt to signal through multiple pathways.
In keeping with our data, Taschner et al. (2008) found that forced activation of CaMKII in the chick epiphyseal cartilage resulted in down-regulation of cell-cycle regulators and acceleration of hypertrophic differentiation, which is notoriously associated with down-regulation of COL2A1 and SOX9 (Lefebvre et al., 1998; Taschner et al., 2008). However, care must be applied in comparing our results with Taschner et al. (2008) because of the intrinsically different biology of epiphyseal chondrocytes, which are destined to undergo hypertrophic differentiation and eventually be replaced by bone, and the articular chondrocytes, which are resistant to hypertrophy and to endochondral bone formation. In addition, in Taschner et al. (2008), proliferation is driven by noncell autonomous mechanisms including secretion of Ihh and consequent PTHrP signaling. With all these limitations in comparing the experimental setups, this paper and a recent similar paper from Li et al. (2011), in which overexpression of constitutively active CaMKII was complemented by loss of function experiments, show the role of this kinase in the initiation of hypertrophy, a process that is associated with the loss of stable chondrocyte markers including SOX9 and COL2A1 (Lefebvre et al., 1998).
The reciprocal inhibition of the canonical and CaMKII-dependent pathways activated by WNT-3A may represent a hub through which different stimuli active in cartilage and known to influence CaMKII can dramatically influence the outcome of Wnt signaling by switching between β-catenin and CaMKII-dependent target genes. Such stimuli include inflammatory molecules (Pritchard and Guilak, 2006; Racioppi and Means, 2008), biomechanics (Valhmu and Raia, 2002; Shimazaki et al., 2006), and PTHrP activity (Taschner et al., 2008).
Although we and others (Slusarski et al., 1997a; Sheldahl et al., 1999; Kühl et al., 2000) have shown that Ca2+/CaMKII activation is G protein–coupled receptor (GPCR) dependent, it is still unclear how Wnts activate G proteins. Based on the similarity between FZD receptors and other GPCR (Wang et al., 2006), it is tempting to postulate that, in the absence of DKK-engaged LRP, FZD receptors may function as GPCRs (Wang et al., 2006; Koval and Katanaev, 2011). However, we cannot exclude that in our system, G protein activation may be indirect, e.g., through the activation of a different GPCR.
A separate issue is as to whether, in our system, individual FZD receptors can activate both pathways, or if the simultaneous activation of canonical and CaMKII pathways is caused by the coexpression, on chondrocytes, of FZD receptors with different signaling specificity. Slusarski et al. (1997a) demonstrated that in zebrafish, expression of rat frizzled 2 receptor (Rfz-2), but not Rfz-1, increased intracellular calcium accumulation in a way that was enhanced by the coexpression of Xenopus Wnt-5a (Xwnt-5a). In addition, in a mammalian cell line, Xwnt-5a but not Xwnt-8 stimulated calcium accumulation via Rfz-2 in a PTX-sensitive way (Liu et al., 1999). Subsequently, Kühl et al. (2000) demonstrated that in Xenopus, WNT-5A–induced Ca2+ mobilization resulted in CaMKII activation in a FZD-specific manner. Only some FZD receptors were able to signal through CaMKII, and such signal was also strictly ligand-specific.
We have demonstrated that AHACs express different FZD receptors (Fig. S5) known to mediate both the canonical (Gazit et al., 1999; Terasaki et al., 2002; Karasawa et al., 2002; Kemp et al., 2007) and the Ca2+-dependent pathways (Kühl et al., 2000; Robitaille et al., 2002; Nishita et al., 2010). Therefore, it is possible that the receptor repertoire of chondrocytes may determine the balance of the WNT-3A signaling.
Alternatively we cannot exclude the possibility that mechanisms downstream of FZD receptors may activate CaMKII: GSK3 inhibitor LiCl also down-regulated COL2A1 expression (Ryu et al., 2002; Ryu and Chun, 2006) in different systems, with the caveat that lithium is also an inhibitor of the inositol pathway (Berridge and Irvine, 1989). Hence, these data are difficult to interpret.
Whichever the mechanism, it is unlikely that, in our system, the activation of the Ca2+/CaMKII pathway is indirect, as Ca2+ mobilization occurred as early as 5 min after the addition of WNT-3A.
Equally, although it cannot be completely excluded, it is unlikely that exogenous WNT-3A may have caused promiscuous activation of receptors, first because Ca2+ release took place particularly at low doses of WNT-3A, whereas β-catenin activation was observed only at the higher doses; and second, because DKK1 also caused Ca2+ release and chondrocyte de-differentiation in the absence of exogenous WNT-3A (Figs. 3 J, 5 B, and 7, D and E), which cannot be explained by promiscuous receptor activation by supraphysiological levels of exogenous WNT-3A. Intriguingly, in addition, direct activation of the PKC pathway by WNT-3A was found in the ST2 cell line mediating osteogenesis (Tu et al., 2007).
In our system, activation of CaMKII was associated with its nuclear localization, which could be blocked with the CaMKII inhibitor KN93. A similar activation-dependent nuclear localization of phosphoCaMKII has been described in smooth muscle cells (Cipolletta et al., 2010) and very recently in epiphyseal chondrocytes (Li et al., 2011) in vivo. Nuclear localization of CaMKII is regulated by several factors including splicing of an NLS, phosphorylation of serines downstream of the NLS, and binding to anchoring and interacting molecules (Griffith et al., 2003). The specific function of the nuclear localization in this context is not known; however, subcellular localization of CaMKII has been shown to restrict/regulate substrate specificity in different cell types or in response to other signals (Griffith et al., 2003; Tsui et al., 2005). Therefore, it is tempting to speculate that CaMKII may have a nuclear function, either in mediating chondrocyte de-differentiation or in suppression of the canonical pathway. In this regard, it has been shown that CaMKII activation in colon cancer cells mediates nuclear export of TCF isoforms (Najdi et al., 2009).
One novel and important aspect of our study is that the β-catenin–dependent pathway and the Ca2+/CaMKII noncanonical pathway have different transcriptional targets: AXIN2 and cell proliferation are specifically regulated by the canonical pathway, whereas the loss of chondrocyte phenotype (COL2A1 and SOX9) are mediated by the Ca2+/CaMKII arm of WNT-3A stimulation. This has important implications from the pharmacological point of view. In fact, for its role on cartilage and joint homeostasis, the Wnt signaling is an attractive therapeutic target. Unfortunately, the multitude of processes regulated by Wnts and the complexity of their regulation has long been a hurdle. Understanding how downstream biological events are individually regulated by Wnts in different cells represents an opportunity to achieve a higher degree of target specificity. Indeed, the availability of molecules and drugs that can selectively interfere with either CaMKII or the canonical Wnt pathway offers the possibility of targeting individual outcomes of Wnt stimulation and avoiding concerns related, for instance, to the role of β-catenin in cancer development (Morin et al., 1997; Sunaga et al., 2001).
Materials and methods
Cartilage harvest and chondrocyte isolation
Adult human articular cartilage was obtained from patients who underwent joint replacement for knee OA after obtaining informed consent. The cartilage samples were provided by P. Achan (Barts and the London National Health Service Trust, London, England, UK). All procedures were approved by the East London and The City Research Ethics Committee 3.
Cartilage tissue was dissected from preserved areas of the femoral condyles and the patellar groove. The cartilage was sliced full thickness, excluding the mineralized cartilage and the subchondral bone. From each sample, one full thickness specimen was processed for histological grading, and the rest was used for chondrocyte isolation. The aliquot destined to chondrocyte isolation was washed twice in high-glucose DME (DME/F-12 1:1 plus GlutaMax; Invitrogen) containing 10% FBS, 1 mM sodium pyruvate (NaPyr), and 2% antibiotic antimycotic solution (Invitrogen). Cartilage was then digested for 30 min at 37°C in 1 mg/ml pronase (Roche) and then overnight at 37°C in 1 mg/ml collagenase P (Roche) prepared in complete medium (same composition as above, with 1% antibiotic/antimycotic solution) under agitation. The AHACs recovered from the digestion were then resuspended in complete medium and seeded at a density of 10,000 cells/cm2. The cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. The aliquot destined for histological scoring was fixed in 4% paraformaldehyde in PBS at pH 7.4, paraffin embedded, sectioned, stained, and scored for features of OA as described previously (Mankin et al., 1971, Dell’Accio et al., 2008). Only cells obtained from samples with a Mankin score <4 were used for subsequent experiments. All experiments were performed using freshly isolated or confluent P0 cells.
Porcine chondrocytes were isolated from the metatarsal joints of adult pigs obtained within 24 h of death from a local abattoir. The articular cartilage was dissected in sterile conditions and processed for cell isolation as described for human chondrocytes.
Titrated thymidine incorporation
Freshly isolated porcine articular chondrocytes were seeded at a density of 6,000 cells/cm2 and left to attach overnight in standard culture conditions. The day after seeding and at all the time points analyzed, the cells were treated for 6 h with 100 ng/ml of recombinant WNT-3A (R&D Systems) or vehicle (0.1% BSA in PBS) in complete medium at 37°C. 2 μCu/µl/cm2 of [3H]thymidine was then added, and after 12 h of incubation, the cells were lysed in trichloroacetic acid. The radioactivity (recorded as counts per minute) was measured using a 1900 Liquid Scintillation analyzer (Packard).
Gene expression analysis
Total RNA was extracted from AHACs using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. 500 ng of total RNA from each sample was reverse transcribed using Thermoscript Reverse transcription kit (Invitrogen) with oligo dT primer. Quantitative and semiquantitative PCR was performed with hot-start DNA polymerase (QIAGEN) in the presence of 0.1× SYBR green (Sigma-Aldrich) and 0.2× ROX dye (Invitrogen) using, respectively, a T7900 HD or a 96 well GeneAmp PCR system 9700 machine (Applied Biosystems). Primer sequences are listed in Table I.
Table I.
Primer sequences used for quantitative and semiquantitative gene expression analysis
| Gene | Sense | Antisense | Length |
| β-actin | 5′-CACGGCTGCTTCCAGCTC-3′ | 5′-CACAGGACTCCATGCCCAG-3′ | 134 bp |
| ADAMTS-5 | 5′-GACCGATGGCACTGAATGTA-3′ | 5′-TGTACAGCTGGAGTTGTCTCCT-3′ | 145 bp |
| Aggrecan | 5′-GTTGTCATCAGCACCAGCATC-3′ | 5′-ACCACACAGTCCTCTCCAGC-3′ | 509 bp |
| Axin-2 | 5′-TACCGGAGGATGCTGAAGGC-3′ | 5′-CCACTGGCCGATTCTTCCTT-3′ | 345 bp |
| Col1A1 | 5′-GCCCTGTCTGCTTCCTGTAA-3′ | 5′-GGTTCAGTTTGGGTTGCTTG-3′ | 104 bp |
| Col2A1 | 5′-CTGCTCGTCGCCGCTGTCCTT-3′ | 5′-AAGGGTCCCAGGTTCTCCATC-3′ | 432 bp |
| MMP-3 | 5′-CAACCGTGAGGAAAATCGATGCAG-3′ | 5′-CGGCAAGATACAGATTCACGCTCAA-3′ | 440 bp |
| MMP-13 | 5′-ACGGACCCATACAGTTTGAATACAGC-3′ | 5′-CCATTTGTGGTGTGGGAAGTATCATC-3′ | 360 bp |
| PCNA | 5′-GGAGAACTTGGAAATGGAAAC-3′ | 5′-CTGCATTTAGAGTCAAGACCC-3′ | 548 bp |
| Sox9 | 5′-GAACGCACATCAAGACGGAG-3′ | 5′-TCTCGTTGATTTCGCTGCTC-3′ | 631 bp |
| WNT-3A | 5′-CCATCCTCTGCCTCAAATTC-3′ | 5′-TGGACAGTGGATATAGCAGCA-3′ | 70 bp |
| FZD1 | 5′-TTCAGCAGCACATTCTGAGG-3′ | 5′-CCTGCACACATTTTCCCTTT-3′ | 154 bp |
| FZD2 | 5′-TCACGGTCTACATGATCAAA-3′ | 5′-GCAACCTAAAAGTGAAATGG-3′ | 266 bp |
| FZD3 | 5′-GGATGATCAAAGAAGCAAAG-3′ | 5′-TTGAGCCGATGAGAACTACT-3′ | 186 bp |
| FZD4 | 5′-GACTTTGGAAGGAACCTTTT-3′ | 5′-TGAAACCCCGTCTCTACTAA-3′ | 238 bp |
| FZD5 | 5′-GGTTTGGTGCAGGTGAATTT-3′ | 5′-CTACAGCATGGGATAGGCACT-3′ | 125 bp |
| FZD6 | 5′-TTCGGCAGCTCACTAGGATT-3′ | 5′-CATCAGAAAATCTTGCCCAA-3′ | 190 bp |
| FZD7 | 5′-CTGGAGTTCTTTGAAATGTGCT-3′ | 5′-AAGGTTAGCTCCCATGATTCTC-3′ | 133 bp |
| FZD8 | 5′-CGGTTTGGGTATTCTTAATG-3′ | 5′-ACAGGGGTAAGCCTCTAAAC-3′ | 215 bp |
| FZD9 | 5′-AACACAGAGAAGCTGGAGAA-3′ | 5′-ACCACCAGTGACATGAAAAT-3′ | 251 bp |
| FZD10 | 5′-AGAAACCCTTCAGTGCTACA-3′ | 5′-AAAGTGTCTCTGCCAACCTA-3′ | 205 bp |
SDS-PAGE and Western blotting
After treatment, cells were washed once in ice-cold PBS containing phosphatase and protease inhibitors (Roche). Total cell extracts were obtained by scraping the cells in extraction buffer (10 mM Hepes, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.05% NP-40, pH 7.9) and leaving the lysates on ice for 30 min. Protein concentration of the samples was determined by a bicinchoninic acid assay (Thermo Fisher Scientific).
The extracts were resolved by SDS-PAGE, and transferred to a nitrocellulose membrane (GE Healthcare). Human β-catenin was detected with anti–β-catenin rabbit monoclonal (diluted 1:1000; Cell Signaling Technology), whereas β-actin was detected with anti–β-actin mouse polyclonal antibody (diluted 1:10,000; Sigma-Aldrich), followed by incubation with HRP-conjugated secondary antibodies (diluted 1:2,000; Santa Cruz Biotechnology, Inc.). Antibodies were diluted in 5% nonfat milk/0.01% Tween 20 in PBS. In the case of CaMKII and phosphoCaMKII analysis, the membranes were blocked in 5% BSA/0.01% Tween 20 in PBS, and the antibodies (1:2,000 anti-CaMKII [Abcam]; 1:1000 anti-pT286CaMKII [Cell Signaling Technology]) were diluted in the same buffer.
Transfections and reporter assay
Subconfluent AHACs and porcine articular chondrocytes were cotransfected with SUPER8XTOP/FOPFlash TCF/LEF–firefly luciferase reporter vector (gift of R. Moon, University of Washington, Seattle, WA) and CMV-Renilla luciferase vector (in a ratio 1:10) by using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. 24 h after transfection, the medium was replaced and the cells were stimulated for 24 h with recombinant WNT-3A 100 ng/ml (R&D Systems) or WNT-3A conditioned medium at 37°C, 5% CO2. Luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega) and a TD-20/20 Luminometer (Turner Designs). Firefly luciferase activity was normalized by Renilla luciferase activity and expressed as relative luciferase units.
Ectopic cartilage formation assay in vivo
The ectopic cartilage formation assay was performed as described previously (Dell’Accio et al., 2001). In brief, with a few modifications, for each injection 5 million freshly isolated porcine chondrocytes were mixed with either 500,000 growth-arrested (treated with 7.5 µg/ml of mitomycin C [Sigma-Aldrich] diluted in complete medium for 2 h at 37°C) L cells overexpressing WNT-3A (L-WNT-3A cells, American Type Culture Collection) or with the same amount of mitomycin C–treated control cells (L cells, American Type Culture Collection).
Each sample was resuspended in 50 µl of sterile PBS and injected intramuscularly in the posterior compartment of the thigh of 4-wk-old female CD1nu/nu mice (Dell’Accio et al., 2001). We performed 12 injections per condition (24 in total). The same experiment was replicated with human articular chondrocytes (n = 3 implants for each condition). Animals were maintained in isolator cages under an unrestricted diet. The animals were then killed after 2 wk and the cartilage implants were retrieved. All procedures were approved by the Local Ethics committee and the UK Home Office.
The implants were paraffin embedded for histological analysis.
Histology
Images of L cells treated with mitomycin C were captured with an inverted microscope (Eclipse TS100; Nikon) using a Plan-Fluor 10× 0.30 NA objective lens at room temperature. Images were acquired with a camera (Coolpix 4500; Nikon) and analyzed with Photoshop 7.0 (Adobe). 5-µm-thick sections were obtained from the center of the cartilage implants isolated from the nude mice, thereby representing the maximum sectional area, and stained with SO according to standard protocols. All the sections were mounted in DPX (BDH chemicals). Images of the sections were taken with a microscope (BX61; Olympus) by using UPlan-Apochromat 4× NA 0.16 and 20× NA 0.70 objective lenses. Images were acquired at room temperature, with an F-View II (SIS; Olympus) camera and Cell P software. Three different parameters for each section were assessed by using ImageJ software: the area covered by the cartilage tissue, the mean of the intensity of the staining, and the percentage of the total area of the section with an intensity of staining equal to a fixed, thresholded value (130). The values were then averaged. In the case of implants obtained from porcine articular chondrocytes, one section per implant was analyzed. For the implants obtained by human chondrocytes, five sections for each implant were analyzed, covering a total thickness of 350 µm.
The contrast of the images was enhanced using Photoshop 7.0 for better rendering without altering the relationship of the target to the control images.
Calcium mobilization assay
Calcium mobilization was evaluated using Fluo-4-Direct kit (Invitrogen) in accordance to manufacturer’s specifications. In brief, P0 chondrocytes were labeled for 1 h (30 min at room temperature and 30 min at 37°C) with the Fluo-4-Direct calcium assay buffer containing Probenecid (Invitrogen), and were subjected to treatments with growth factors or inhibitors as indicated for individual experiments. After treatments, the fluorescence emitted by the cells (excitation wavelength 494 nm, emission wavelength 516 nm) was recorded using a fluorometer (Galaxy; Fluo-Star). The fluorescence was expressed as relative fluorescence units.
Micromass culture and Alcian blue staining
Freshly isolated human articular chondrocytes were resuspended at a density of 2.0 × 107 cells/ml in complete medium, and micromass cultures were obtained by pipetting 20-µl drops of cell suspension into each well of a 24-well plate. The cells were left to attach for 3 h and then 1 ml of compete medium was added. 24 h later, micromasses were exposed to 100 ng/ml of WNT-3A or vehicle for 4 d, changing medium every 48 h. Finally, micromasses were fixed and whole-mount stained with Alcian blue as described previously (De Bari et al., 2001). After proteoglycan extraction with 6 M guanidine HCl, the absorbance was read at 630 nm with a GENios spectrophotometer (Tecan). Images of the micromasses were acquired at room temperature with a camera (Coolpix 4500; Nikon), with the macro setting and daylight.
The contrast of the pictures was then modified with Photoshop 7.0 for best graphic rendering, equally for the vehicle-treated and for the WNT-3A treated micromasses.
Phospho CaMKII immunofluorescence
AHACs used for immunofluorescence were seeded on chamber slides (Lab-Tek) and treated for 24 h with 100 ng/ml of recombinant WNT-3A in combination with 10 µM KN92 or KN93 (EMD). The cells were then washed in PBS and fixed in 4% buffered PFA, pH 7.4, for 5 min at room temperature. Autofluorescence was quenched in 50 mM NH4Cl for 10 min. After 1 h of blocking in FBS diluted 1:5 in PBS/0.2% Triton X-100 (blocking buffer), sections were incubated overnight at 4°C with anti–phospho-CaMKII rabbit polyclonal antibody (Cell Signaling Technology) or rabbit IgG (Dako) as a negative control, diluted 1:200 in blocking buffer. After washing, cells were incubated with cy3-conjugated goat anti–rabbit antibody 1:300 (Jackson ImmunoResearch Laboratories, Inc.) for 1 h. Slides were mounted in Mowiol (EMD), and images were acquired with a fluorescence microscope (BX61; Olympus) using a Uplan-Fluor 40× NA 0.85 objective lens. Images were acquired at room temperature, by using an F-View II (SIS) camera and Cell P software. After acquisition and densitometric analysis (see the next paragraph), the contrast of the images was enhanced for best graphic rendering using Photoshop 7.0, all with the same parameters, without altering the relationship of the target to control the images. The fluorescence intensity was measured by using ImageJ software on the original photographs. The average pixel intensity profile (y axis: 0 = black, 255 = white) was plotted over a linear section of a whole cell. The mean nuclear and cytoplasmic fluorescence intensity, obtained by the plot profile as shown in Fig. 4 E, were generated by analyzing 40 cells per field in duplicate for each condition.
Statistical analysis
Parametric data were compared with a t test. For multiple comparisons, we used analysis of variance (ANOVA) analysis, with a Dunnet post-test. Nonparametric values were analyzed by a Kruskal-Wallis test, with a Dunns post-test. P-values <0.05 were considered significant. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. All the data in the graphs are expressed as mean ± SEM.
Online supplemental material
Fig. S1 shows the expression of WNT-3A in human articular cartilage. Fig. S2 shows that cartilage implants obtained by the co-injection of human articular chondrocytes with L cells overexpressing WNT-3A are bigger and less differentiated than controls. Fig. S3 shows that sFRP1 does not promote intracellular calcium mobilization and partially blocks the ability of WNT-3A to increase intracellular calcium levels. Fig. S4 shows that PMA promotes intracellular calcium accumulation in articular chondrocytes and induces down-regulation of Aggrecan. Fig. S5 shows the gene expression levels of FZD receptors in human articular chondrocytes cultured in monolayer (P0) and in human cartilage explants. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201011051/DC1.
Acknowledgments
We would like to thank Mr. Pramod Achan (Barts and the London National Health Service trust) for providing human articular cartilage samples, Prof. R. Moon for providing the SUPER8XTOPFlash reporter vector, and Dr. F. D’Aquisto for providing assistance in the [3H]thymidine incorporation assay and in the reporter assay. We also thank Dr. T. Kamalati and Dr. A.Marrelli for critically reviewing the manuscript.
We wish to thank the Arthritis Research UK for funding this work (grants 17971, 19344, and 17859), the Medical Research Council for funding G. Nalesso’s studentship. C. De Bari is a fellow of the Medical Research Council UK (G108/620).
Footnotes
Abbreviations used in this paper:
- AHAC
- adult human articular chondrocyte
- FZD
- frizzled
- GAG
- glycosaminoglycan
- GPCR
- G protein–coupled receptor
- LEF
- lymphoid enhancer–binding factor
- LRP
- low-density lipoprotein receptor-related receptor
- OA
- osteoarthritis
- PMA
- phorbol 12-myristate 13-acetate
- PTX
- pertussis toxin
- Q-PCR
- quantitative PCR
- SO
- safranin O
- TCF
- T cell factor
References
- Akiyama H., Lyons J.P., Mori-Akiyama Y., Yang X., Zhang R., Zhang Z., Deng J.M., Taketo M.M., Nakamura T., Behringer R.R., et al. 2004. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 18:1072–1087 10.1101/gad.1171104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Irvine R.F. 1989. Inositol phosphates and cell signalling. Nature. 341:197–205 10.1038/341197a0 [DOI] [PubMed] [Google Scholar]
- Chen M., Zhu M., Awad H., Li T.F., Sheu T.J., Boyce B.F., Chen D., O’Keefe R.J. 2008. Inhibition of beta-catenin signaling causes defects in postnatal cartilage development. J. Cell Sci. 121:1455–1465 10.1242/jcs.020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church V., Nohno T., Linker C., Marcelle C., Francis-West P. 2002. Wnt regulation of chondrocyte differentiation. J. Cell Sci. 115:4809–4818 10.1242/jcs.00152 [DOI] [PubMed] [Google Scholar]
- Cipolletta E., Monaco S., Maione A.S., Vitiello L., Campiglia P., Pastore L., Franchini C., Novellino E., Limongelli V., Bayer K.U., et al. 2010. Calmodulin-dependent kinase II mediates vascular smooth muscle cell proliferation and is potentiated by extracellular signal regulated kinase. Endocrinology. 151:2747–2759 10.1210/en.2009-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari C., Dell’Accio F., Luyten F.P. 2001. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 44:85–95 [DOI] [PubMed] [Google Scholar]
- Dell’Accio F., De Bari C., Luyten F.P. 2001. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 44:1608–1619 [DOI] [PubMed] [Google Scholar]
- Dell’accio F., De Bari C., Eltawil N.M., Vanhummelen P., Pitzalis C. 2008. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 58:1410–1421 10.1002/art.23444 [DOI] [PubMed] [Google Scholar]
- Eltawil N.M., De Bari C., Achan P., Pitzalis C., Dell’accio F. 2009. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage. 17:695–704 10.1016/j.joca.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M., Kitagaki J., Koyama E., Tamamura Y., Wu C., Kanatani N., Koike T., Okada H., Komori T., Yoneda T., et al. 2002. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev. Biol. 251:142–156 10.1006/dbio.2002.0802 [DOI] [PubMed] [Google Scholar]
- Galli L.M., Barnes T., Cheng T., Acosta L., Anglade A., Willert K., Nusse R., Burrus L.W. 2006. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev. Dyn. 235:681–690 10.1002/dvdy.20681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Yaniv A., Bafico A., Pramila T., Igarashi M., Kitajewski J., Aaronson S.A. 1999. Human frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene. 18:5959–5966 10.1038/sj.onc.1202985 [DOI] [PubMed] [Google Scholar]
- Goldring M.B. 2006. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract. Res. Clin. Rheumatol. 20:1003–1025 10.1016/j.berh.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Griffith L.C., Lu C.S., Sun X.X. 2003. CaMKII, an enzyme on the move: regulation of temporospatial localization. Mol. Interv. 3:386–403 10.1124/mi.3.7.386 [DOI] [PubMed] [Google Scholar]
- Guo X., Day T.F., Jiang X., Garrett-Beal L., Topol L., Yang Y. 2004. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 18:2404–2417 10.1101/gad.1230704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C., Tabin C.J. 2001. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 104:341–351 10.1016/S0092-8674(01)00222-7 [DOI] [PubMed] [Google Scholar]
- Hinck L., Nelson W.J., Papkoff J. 1994. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J. Cell Biol. 124:729–741 10.1083/jcb.124.5.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T., Yokokura H., Kitajewski J., Lombroso P.J. 2002. Frizzled-9 is activated by Wnt-2 and functions in Wnt/beta -catenin signaling. J. Biol. Chem. 277:37479–37486 10.1074/jbc.M205658200 [DOI] [PubMed] [Google Scholar]
- Kemp C.R., Willems E., Wawrzak D., Hendrickx M., Agbor Agbor T., Leyns L. 2007. Expression of Frizzled5, Frizzled7, and Frizzled10 during early mouse development and interactions with canonical Wnt signaling. Dev. Dyn. 236:2011–2019 10.1002/dvdy.21198 [DOI] [PubMed] [Google Scholar]
- Kotlarz H., Gunnarsson C.L., Fang H., Rizzo J.A. 2009. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 60:3546–3553 10.1002/art.24984 [DOI] [PubMed] [Google Scholar]
- Koval A., Katanaev V.L. 2011. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem. J. 433:435–440 10.1042/BJ20101878 [DOI] [PubMed] [Google Scholar]
- Koyama E., Shibukawa Y., Nagayama M., Sugito H., Young B., Yuasa T., Okabe T., Ochiai T., Kamiya N., Rountree R.B., et al. 2008. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 316:62–73 10.1016/j.ydbio.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl M., Sheldahl L.C., Malbon C.C., Moon R.T. 2000. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275:12701–12711 10.1074/jbc.275.17.12701 [DOI] [PubMed] [Google Scholar]
- Lawrence R.C., Helmick C.G., Arnett F.C., Deyo R.A., Felson D.T., Giannini E.H., Heyse S.P., Hirsch R., Hochberg M.C., Hunder G.G., et al. 1998. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 41:778–799 [DOI] [PubMed] [Google Scholar]
- Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A., Gabriel S., Hirsch R., Hochberg M.C., Hunder G.G., et al. ; National Arthritis Data Workgroup 2008. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 58:26–35 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., Li P., de Crombrugghe B. 1998. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17:5718–5733 10.1093/emboj/17.19.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns L., Bouwmeester T., Kim S.H., Piccolo S., De Robertis E.M. 1997. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 88:747–756 10.1016/S0092-8674(00)81921-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Ahrens M.J., Wu A., Liu J., Dudley A.T. 2011. Calcium/calmodulin-dependent protein kinase II activity regulates the proliferative potential of growth plate chondrocytes. Development. 138:359–370 10.1242/dev.052324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu T., Slusarski D.C., Yang-Snyder J., Malbon C.C., Moon R.T., Wang H. 1999. Activation of a frizzled-2/beta-adrenergic receptor chimera promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via Galphao and Galphat. Proc. Natl. Acad. Sci. USA. 96:14383–14388 10.1073/pnas.96.25.14383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C.Y., Nusse R. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20:781–810 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- Lories R.J., Peeters J., Bakker A., Tylzanowski P., Derese I., Schrooten J., Thomas J.T., Luyten F.P. 2007. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 56:4095–4103 10.1002/art.23137 [DOI] [PubMed] [Google Scholar]
- Loughlin J., Dowling B., Chapman K., Marcelline L., Mustafa Z., Southam L., Ferreira A., Ciesielski C., Carson D.A., Corr M. 2004. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc. Natl. Acad. Sci. USA. 101:9757–9762 10.1073/pnas.0403456101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten F.P., Tylzanowski P., Lories R.J. 2009. Wnt signaling and osteoarthritis. Bone. 44:522–527 10.1016/j.bone.2008.12.006 [DOI] [PubMed] [Google Scholar]
- Macdonald B.T., Semenov M.V., He X. 2007. SnapShot: Wnt/beta-catenin signaling. Cell. 131:1204.e1–1204.e2 10.1016/j.cell.2007.11.036 [DOI] [PubMed] [Google Scholar]
- Mankin H.J., Dorfman H., Lippiello L., Zarins A. 1971. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 53:523–537 [PubMed] [Google Scholar]
- Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. 2001. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 411:321–325 10.1038/35077108 [DOI] [PubMed] [Google Scholar]
- Marley P.D., Thomson K.A. 1996. The Ca++/calmodulin-dependent protein kinase II inhibitors KN62 and KN93, and their inactive analogues KN04 and KN92, inhibit nicotinic activation of tyrosine hydroxylase in bovine chromaffin cells. Biochem. Biophys. Res. Commun. 221:15–18 10.1006/bbrc.1996.0536 [DOI] [PubMed] [Google Scholar]
- Molenaar M., van de Wetering M., Oosterwegel M., Peterson-Maduro J., Godsave S., Korinek V., Roose J., Destrée O., Clevers H. 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 86:391–399 10.1016/S0092-8674(00)80112-9 [DOI] [PubMed] [Google Scholar]
- Morin P.J., Sparks A.B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K.W. 1997. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 275:1787–1790 10.1126/science.275.5307.1787 [DOI] [PubMed] [Google Scholar]
- Najdi R., Syed A., Arce L., Theisen H., Ting J.H., Atcha F., Nguyen A.V., Martinez M., Holcombe R.F., Edwards R.A., et al. 2009. A Wnt kinase network alters nuclear localization of TCF-1 in colon cancer. Oncogene. 28:4133–4146 10.1038/onc.2009.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nawata M., Wakitani S. 2005. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am. J. Pathol. 167:97–105 10.1016/S0002-9440(10)62957-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M., Itsukushima S., Nomachi A., Endo M., Wang Z., Inaba D., Qiao S., Takada S., Kikuchi A., Minami Y. 2010. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol. Cell. Biol. 30:3610–3619 10.1128/MCB.00177-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard S., Guilak F. 2006. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 54:2164–2174 10.1002/art.21941 [DOI] [PubMed] [Google Scholar]
- Racioppi L., Means A.R. 2008. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveller. Trends Immunol. 29:600–607 10.1016/j.it.2008.08.005 [DOI] [PubMed] [Google Scholar]
- Robitaille J., MacDonald M.L., Kaykas A., Sheldahl L.C., Zeisler J., Dubé M.P., Zhang L.H., Singaraja R.R., Guernsey D.L., Zheng B., et al. 2002. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet. 32:326–330 10.1038/ng957 [DOI] [PubMed] [Google Scholar]
- Ryu J.H., Chun J.S. 2006. Opposing roles of WNT-5A and WNT-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J. Biol. Chem. 281:22039–22047 10.1074/jbc.M601804200 [DOI] [PubMed] [Google Scholar]
- Ryu J.H., Kim S.J., Kim S.H., Oh C.D., Hwang S.G., Chun C.H., Oh S.H., Seong J.K., Huh T.L., Chun J.S. 2002. Regulation of the chondrocyte phenotype by beta-catenin. Development. 129:5541–5550 10.1242/dev.00110 [DOI] [PubMed] [Google Scholar]
- Schett G., Zwerina J., David J.P. 2008. The role of Wnt proteins in arthritis. Nat. Clin. Pract. Rheumatol. 4:473–480 10.1038/ncprheum0881 [DOI] [PubMed] [Google Scholar]
- Semenov M.V., Habas R., Macdonald B.T., He X. 2007. SnapShot: noncanonical Wnt signaling pathways. Cell. 131:1378.e1–1378.e2 10.1016/j.cell.2007.12.011 [DOI] [PubMed] [Google Scholar]
- Sheldahl L.C., Park M., Malbon C.C., Moon R.T. 1999. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol. 9:695–698 10.1016/S0960-9822(99)80310-8 [DOI] [PubMed] [Google Scholar]
- Shimazaki A., Wright M.O., Elliot K., Salter D.M., Millward-Sadler S.J. 2006. Calcium/calmodulin-dependent protein kinase II in human articular chondrocytes. Biorheology. 43:223–233 [PubMed] [Google Scholar]
- Shimizu H., Julius M.A., Giarré M., Zheng Z., Brown A.M., Kitajewski J. 1997. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 8:1349–1358 [PubMed] [Google Scholar]
- Slusarski D.C., Corces V.G., Moon R.T. 1997a. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 390:410–413 10.1038/37138 [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Yang-Snyder J., Busa W.B., Moon R.T. 1997b. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 182:114–120 10.1006/dbio.1996.8463 [DOI] [PubMed] [Google Scholar]
- Soderling T.R. 1999. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 24:232–236 10.1016/S0968-0004(99)01383-3 [DOI] [PubMed] [Google Scholar]
- Sunaga N., Kohno T., Kolligs F.T., Fearon E.R., Saito R., Yokota J. 2001. Constitutive activation of the Wnt signaling pathway by CTNNB1 (beta-catenin) mutations in a subset of human lung adenocarcinoma. Genes Chromosomes Cancer. 30:316–321 [DOI] [PubMed] [Google Scholar]
- Taschner M.J., Rafigh M., Lampert F., Schnaiter S., Hartmann C. 2008. Ca2+/Calmodulin-dependent kinase II signaling causes skeletal overgrowth and premature chondrocyte maturation. Dev. Biol. 317:132–146 10.1016/j.ydbio.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Terasaki H., Saitoh T., Shiokawa K., Katoh M. 2002. Frizzled-10, up-regulated in primary colorectal cancer, is a positive regulator of the WNT - beta-catenin - TCF signaling pathway. Int. J. Mol. Med. 9:107–112 [PubMed] [Google Scholar]
- Tsui J., Inagaki M., Schulman H. 2005. Calcium/calmodulin-dependent protein kinase II (CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation. J. Biol. Chem. 280:9210–9216 10.1074/jbc.M407653200 [DOI] [PubMed] [Google Scholar]
- Tu X., Joeng K.S., Nakayama K.I., Nakayama K., Rajagopal J., Carroll T.J., McMahon A.P., Long F. 2007. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell. 12:113–127 10.1016/j.devcel.2006.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valhmu W.B., Raia F.J. 2002. myo-Inositol 1,4,5-trisphosphate and Ca(2+)/calmodulin-dependent factors mediate transduction of compression-induced signals in bovine articular chondrocytes. Biochem. J. 361:689–696 10.1042/0264-6021:3610689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman M.T., Slusarski D.C., Kaykas A., Louie S.H., Moon R.T. 2003. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13:680–685 10.1016/S0960-9822(03)00240-9 [DOI] [PubMed] [Google Scholar]
- Wang S., Krinks M., Lin K., Luyten F.P., Moos M., Jr 1997. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 88:757–766 10.1016/S0092-8674(00)81922-4 [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Liu T., Malbon C.C. 2006. Structure-function analysis of Frizzleds. Cell. Signal. 18:934–941 10.1016/j.cellsig.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Wawrzak D., Métioui M., Willems E., Hendrickx M., de Genst E., Leyns L. 2007. Wnt3a binds to several sFRPs in the nanomolar range. Biochem. Biophys. Res. Commun. 357:1119–1123 10.1016/j.bbrc.2007.04.069 [DOI] [PubMed] [Google Scholar]
- Wu Q., Zhu M., Rosier R.N., Zuscik M.J., O’Keefe R.J., Chen D. 2010. Beta-catenin, cartilage, and osteoarthritis. Ann. N. Y. Acad. Sci. 1192:344–350 10.1111/j.1749-6632.2009.05212.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T.P., Bradley A., McMahon A.P., Jones S. 1999. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 126:1211–1223 [DOI] [PubMed] [Google Scholar]
- Yan D., Wiesmann M., Rohan M., Chan V., Jefferson A.B., Guo L., Sakamoto D., Caothien R.H., Fuller J.H., Reinhard C., et al. 2001. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc. Natl. Acad. Sci. USA. 98:14973–14978 10.1073/pnas.261574498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Topol L., Lee H., Wu J. 2003. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 130:1003–1015 10.1242/dev.00324 [DOI] [PubMed] [Google Scholar]
- Zhu M., Chen M., Zuscik M., Wu Q., Wang Y.J., Rosier R.N., O’Keefe R.J., Chen D. 2008. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 58:2053–2064 10.1002/art.23614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Tang D., Wu Q., Hao S., Chen M., Xie C., Rosier R.N., O’Keefe R.J., Zuscik M., Chen D. 2009. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J. Bone Miner. Res. 24:12–21 10.1359/jbmr.080901 [DOI] [PMC free article] [PubMed] [Google Scholar]