Abstract
Wnt proteins can activate different branches of the Wnt signaling pathway, raising the question of specificity. In this issue, Nalesso et al. (2011. J. Cell Biol. doi:10.1083/jcb.201011051) provide an answer to this conundrum by showing that different concentrations of Wnt ligands can elicit different intracellular responses. These findings not only provide new insights into the molecular mechanisms underlying Wnt signaling, but also indicate how Wnt gradients might contribute to tissue patterning during embryogenesis.
Wnt proteins are secreted glycoproteins that regulate cellular processes such as proliferation, differentiation, migration, and cell polarity. To achieve these diverse effects, Wnt proteins can activate different intracellular signaling branches upon binding to Frizzled receptors. These include the canonical Wnt pathway involving the multifunctional protein β-catenin, which interacts with transcription factors to activate target gene transcription. In contrast, noncanonical, β-catenin–independent pathways among others include the release of intracellular calcium and subsequent activation of calcium-calmodulin dependent kinase (CamKII; Fig. 1). What is not yet fully understood is how signaling specificity is achieved. Frizzled coreceptors such as LRP5/6 or Ror2 are thought to determine how a Wnt signal is interpreted. Whereas LRP5/6 are specific for the Wnt/β-catenin pathway, Ror2 couples to noncanonical Wnt/JNK signaling. In both cases, Wnt proteins induce a heterodimerization of Frizzled and its respective co-receptor (Grumolato et al., 2010). At least to our current knowledge, the noncanonical Wnt/Ca2+ pathway does not involve any co-receptor but is G protein coupled (Slusarski et al., 1997a; Kühl et al., 2000; Koval and Katanaev, 2011).
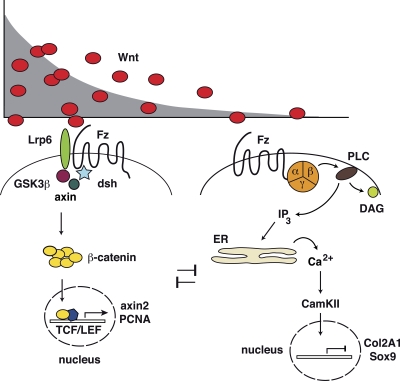
Figure 1.
Concentration-dependent activation of Wnt pathways. Wnt/β-catenin signaling is activated by high concentrations of Wnt ligands, resulting in the stabilization of β-catenin (left). Binding of Wnt protein results in LRP6/Fz heterodimer formation. Intracellular components of canonical Wnt signaling thereby are recruited to the receptor complex including disheveled (dsh), axin, and GSK3β. As a consequence, β-catenin accumulates in the cytoplasm, enters the nucleus, and interacts with transcription factors such as TCF/LEF, resulting in target gene activation. In contrast, Wnt/Ca2+ signaling is favored by lower concentrations of Wnt ligands (right). Wnt/Frizzled interaction results in a G protein (orange circle)-mediated activation of phospholipase Cβ (PLC) that generates diacylglycerol (DAG) and inositol-3,4,5-trisphosphate (IP3). IP3 production results in release of calcium ions from the ER that in turn activate CamKII. Target genes are indicated as in Nalesso et al. (2011). Both pathways reciprocally inhibit each other.
In this issue, Nalesso et al. now provide us with novel insights into the specificity of Wnt signal transduction by studying cartilage development in primary human articular chondrocytes. Curiously, either activation or blockade of the Wnt/β-catenin pathway resulted in loss of cartilage in these cells. Using biochemical assays to monitor different branches of the Wnt signaling pathway, the authors showed that Wnt3a can activate canonical and noncanonical Wnt signaling in the same cell type, thereby regulating different target genes (Fig. 1).
It has been observed previously that a single Wnt ligand can activate different signaling branches in the same cell; in mouse ST2 cells, Wnt3a activates canonical and noncanonical pathways (Tu et al., 2007). Nalesso et al. (2011) now show that the type of Wnt signaling activated depends on the concentration of the Wnt ligand: low concentrations of Wnt3a trigger Wnt/Ca2+ signaling as measured by calcium release as well as activation of CamKII. However, high concentrations of Wnt3a activate Wnt/β-catenin signaling (Fig. 1). Certainly, one would like to see these findings confirmed in other cell types, and microfluidic devices that generate stable and reproducible Wnt concentration gradients might be a valuable tool to do so (Cimetta et al., 2010). Nevertheless, these findings have immediate implications on our current understanding of Wnt signaling.
How can these different responses be explained? One could assume that individual members of the Frizzled family specifically activate different Wnt signaling branches. In light of these novel findings, this would indicate that those Frizzled receptors that activate the Wnt/Ca2+ pathway have a higher affinity for certain Wnt ligands than those activating the Wnt/β-catenin pathway. However, some Frizzled receptors have been shown to activate Wnt/β-catenin as well as Wnt/Ca2+ signaling, shifting the focus to the co-receptors. Recently, dimerization of Wnts has been shown to favor canonical signaling (Cha et al., 2009). Dimer formation in turn depends on Wnt concentrations and might stipulate heterodimerization of different Wnt receptors. More detailed biochemical studies will indicate which of these two possibilities (or both) are correct and should also take into account the fact that Frizzled receptors can also form oligomers (Kaykas et al., 2004).
Canonical and noncanonical Wnt signaling branches are highly interconnected, and cross-regulate each other. The Wnt/Ca2+ pathway inhibits the Wnt/β-catenin pathway (Slusarski et al., 1997a,b; Kühl et al., 2000; Ishitani et al., 2003), and Nalesso et al. (2011) now indicate that the opposite also occurs. Assuming a Wnt morphogen gradient in a tissue either generated by diffusion or by graded transcript distribution, the concentration-dependent activation of two Wnt pathways results in two domains characterized by distinct pathway activities. The inhibitory cross-regulation of both pathways resembles a two-repressor mechanism (Cherry and Adler, 2000), generating a sharp boundary and a switch-like behavior (Fig. 2).
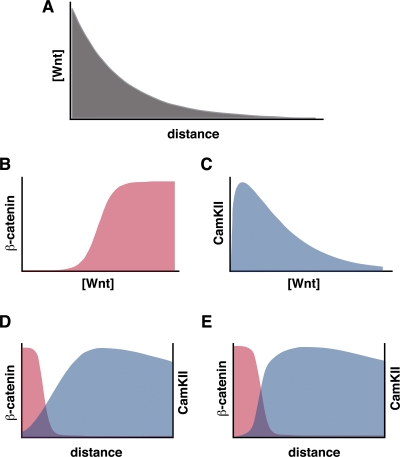
Figure 2.
Generating a Wnt switch. Schematic drawing of a Wnt switch. A Wnt gradient in a tissue (A) activates different Wnt pathways in a concentration-dependent manner (B and C), which results in spatially organized Wnt signaling activities (D). Inhibitory cross-regulation sharpens the boundary, setting up a Wnt switch (E).
Is there an example for such a Wnt switch? The generation of the dorso-ventral body axis of a Xenopus laevis embryo is set up within the first hours of development. The maternally stored Wnt11 ligand is enriched on the dorsal side of the embryo and present in lower concentrations on the ventral side (Schroeder et al., 1999), representing such a Wnt gradient. It has been shown that maternally stored Wnt11 activates the Wnt/β-catenin pathway and is required for dorsal axis formation (Tao et al., 2005). Wnt11 has also been shown to activate CamKII on the ventral side of the embryo, where both Wnt11 and CamKII are required for ventral marker gene expression (Kühl et al., 2000). Now, the paper of Nalesso et al. (2011) provides a unifying explanation for these puzzling facts. A higher concentration of Wnt11 on the dorsal side favors β-catenin and the lower Wnt11 activity on the ventral side supports Wnt/Ca2+ signaling. Modulating one component of the switch would shift the spatial position of the switch within the tissue. This has indeed been done by introducing constitutive active or kinase-dead versions of CamKII into the X. laevis embryo, resulting in an altered expression of the ventral marker Vent1. Such a Wnt switch could also act in a time-dependent manner, first allowing Wnt/β-catenin signaling, which is then turned off upon degradation of the extracellular ligand through activating a negative CamKII feedback.
Collectively, these novel findings by Nalesso et al. (2011) have a broad impact on the Wnt field, as they provide a unifying mechanism explaining how Wnt signaling specificity is achieved, how this might contribute to the diversity of Wnt mediated cellular effects, and how this can contribute to pattern formation during embryogenesis. The reaction–diffusion model of interacting morphogens can explain biological pattern formation in many contexts (Kondo and Miura, 2010). It will be of high interest to investigate how the novel findings by Nalesso et al. (2011) will extend these models.seca
Acknowledgments
We thank Susanne Gessert for help with preparing the figures.
Work of the authors is funded by the Deutsche Forschungsgemeinschaft (SFB497/A6 to M. Kühl and GSC270 to M. Kühl and Hans A. Kestler).
References
- Cha S.W., Tadjuidje E., White J., Wells J., Mayhew C., Wylie C., Heasman J. 2009. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr. Biol. 19:1573–1580 10.1016/j.cub.2009.07.062 [DOI] [PubMed] [Google Scholar]
- Cherry J.L., Adler F.R. 2000. How to make a biological switch. J. Theor. Biol. 203:117–133 10.1006/jtbi.2000.1068 [DOI] [PubMed] [Google Scholar]
- Cimetta E., Cannizzaro C., James R., Biechele T., Moon R.T., Elvassore N., Vunjak-Novakovic G. 2010. Microfluidic device generating stable concentration gradients for long term cell culture: application to Wnt3a regulation of β-catenin signaling. Lab Chip. 10:3277–3283 10.1039/c0lc00033g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L., Liu G., Mong P., Mudbhary R., Biswas R., Arroyave R., Vijayakumar S., Economides A.N., Aaronson S.A. 2010. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 24:2517–2530 10.1101/gad.1957710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T., Kishida S., Hyodo-Miura J., Ueno N., Yasuda J., Waterman M., Shibuya H., Moon R.T., Ninomiya-Tsuji J., Matsumoto K. 2003. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 23:131–139 10.1128/MCB.23.1.131-139.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaykas A., Yang-Snyder J., Héroux M., Shah K.V., Bouvier M., Moon R.T. 2004. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat. Cell Biol. 6:52–58 10.1038/ncb1081 [DOI] [PubMed] [Google Scholar]
- Kondo S., Miura T. 2010. Reaction-diffusion model as a framework for understanding biological pattern formation. Science. 329:1616–1620 10.1126/science.1179047 [DOI] [PubMed] [Google Scholar]
- Koval A., Katanaev V.L. 2011. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem. J. 433:435–440 10.1042/BJ20101878 [DOI] [PubMed] [Google Scholar]
- Kühl M., Sheldahl L.C., Malbon C.C., Moon R.T. 2000. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275:12701–12711 10.1074/jbc.275.17.12701 [DOI] [PubMed] [Google Scholar]
- Nalesso G., Sherwood J., Bertrand J., Pap T., Ramachandran M., De Bari C., Pitzalis C., Dell’Accio F. 2011. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J. Cell Biol. 193:551–564 10.1083/jcb.201011051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder K.E., Condic M.L., Eisenberg L.M., Yost H.J. 1999. Spatially regulated translation in embryos: asymmetric expression of maternal Wnt-11 along the dorsal-ventral axis in Xenopus. Dev. Biol. 214:288–297 10.1006/dbio.1999.9426 [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Corces V.G., Moon R.T. 1997a. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 390:410–413 10.1038/37138 [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Yang-Snyder J., Busa W.B., Moon R.T. 1997b. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 182:114–120 10.1006/dbio.1996.8463 [DOI] [PubMed] [Google Scholar]
- Tao Q., Yokota C., Puck H., Kofron M., Birsoy B., Yan D., Asashima M., Wylie C.C., Lin X., Heasman J. 2005. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 120:857–871 10.1016/j.cell.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Tu X., Joeng K.S., Nakayama K.I., Nakayama K., Rajagopal J., Carroll T.J., McMahon A.P., Long F. 2007. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell. 12:113–127 10.1016/j.devcel.2006.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]