Abstract
Chronic lumbar pain due to degenerative disc disease affects a large number of people, including those of fully active age. The usual self-repair system observed in nature is a spontaneous attempt at arthrodesis, which in most cases leads to pseudoarthrosis. In recent years, many possible surgical fusion techniques have been introduced; PLIF is one of these. Because of the growing interest in minimally invasive surgery and the unsatisfactory results reported in the literature (mainly due to the high incidence of morbidity and complications), a new titanium lumbar interbody cage (I-FLY) has been developed to achieve solid bone fusion by means of a stand-alone posterior device. The head of the cage is blunt and tapered so that it can be used as a blunt spreader, and the core is small, which facilitates self-positioning. From 2003 to 2007, 119 patients were treated for chronic lumbar discopathy (Modic grade III and Pfirrmann grade V) with I-FLY cages used as stand-alone devices. All patients were clinically evaluated preoperatively and after 1 and 2 years by means of a neurological examination, visual analogue score (VAS) and Prolo Economic and Functional Scale. Radiological results were evaluated by polyaxial computed tomography (CT) scan and flexion–extension radiography. Fusion was defined as the absence of segmental instability on flexion–extension radiography and Bridwell grade I or II on CT scan. Patients were considered clinical “responders” if VAS evaluation showed any improvement over baseline values and a Prolo value >7 was recorded. At the last follow-up examination, clinical success was deemed to have been achieved in 90.5% of patients; the rate of bone fusion was 99.1%, as evaluated by flexion–extension radiography, and 92.2%, as evaluated by CT scan. Morbidity (nerve root injury, dural lesions) and complications (subsidence and pseudoarthrosis) were minimal. PLIF by means of the stand-alone I-FLY cage can be regarded as a possible surgical treatment for chronic low-back pain due to high-degree DDD. This technique is not demanding and can be considered safe and effective, as shown by the excellent clinical and radiological success rates.
Keywords: Discopathy, Posterior spinal fusion, Interbody arthrodesis, Interbody technique, Fusion
Introduction
Chronic lumbar discopathy is a very common disease. Generally affecting the ageing spine, it may also occur at a very early age. In spite of the anatomical and radiological classification of this disease, the relationships between its presence and a patient’s clinical complaints are somewhat unclear. Its anatomical classification mainly stems from the introduction of NMR studies, which have yielded two major classifications (Modic and Pfirrmann in 3° and 5°, respectively) [1–3], and is now clearly defined. A semi-quantitative definition of disc degeneration has also been provided by the NMR dGEMRIC study [4]. While the incidence and prevalence of discopathy in the various age groups and the mechanisms by which it becomes symptomatic remain fairly unclear, many different explanations have been put forward. L5–S1 disc degeneration takes place asymptomatically in early life in most human beings. It may be very well tolerated or it can give rise to excruciating pain. Why this is so remains obscure, even though many pathophysiological mechanisms have been advocated. As a rule, in subjects over 70 years of age it is, in most cases, completely asymptomatic; it therefore remains a considerable clinical disease only in the young population. Psychological conditions and personality patterns also seem to play a major role when considering the need to treat these symptomatic cases. Discopathy can be identified at single or multiple levels, with different degrees of severity. In recent years, conservative treatment has generally been undertaken. In the near future, new biological treatments may enable the chondrocyte–proteoglycan structure of the disc to be regenerated. However, restoration of the correct biomechanical function of the disc is likely to be very difficult. The usual self-repair mechanism observed in nature is a spontaneous attempt at arthrodesis. In most cases, this leads to pseudoarthrosis, which mainly occurs at an anterior site, as opposed to what takes place in the cervical spine. On account of the sometimes invalidating chronic lumbar pain, the better construction of the anatomical picture provided by NMR studies, and the introduction of new surgical devices, several surgical approaches have been widely adopted recently, such as interbody fusion. The pioneering surgical experience of Cloward [5] utilized posterior lumbar interbody fusion, which mimics the natural repair of discopathy. This gained a certain popularity and led to the introduction of the concept of stand-alone interbody fusion devices. A technical evolution of this procedure—percutaneous minimally invasive fusion [transforaminal lumbar interbody fusion (TLIF), and (extreme lateral interbody fusion) XLIF]—has recently been proposed to minimize the surgical risk of the PLIF. Actually, although a great variety of graft types and cages [such as titanium mesh, carbon fibre, and polyether ether ketone (PEEK)] [6–9] exist and promising results are described, these devices used as lumbar stand-alone cages are still considered with scepticism. The main criticisms levelled against the stand-alone devices concern the risk of nerve root injury during retraction, which may cause endoneural fibrosis and chronic radiculopathy, the risk of instability and subsequent pseudarthrosis and finally the risk of posterior extrusion of the graft [10–12]. Consequently, the use of interbody fusion in association with pedicle screw fixation became a popular surgical treatment.
Since the 1990s, lumbar prostheses for non-fusion treatment of single- or multiple-level chronic lumbar disc disease have been introduced. While initial follow-up studies were very promising, longer-term clinical assessments have painted a less encouraging picture. To date, no study has shown total disc replacement to be superior to spinal fusion in terms of clinical outcome [13]. Furthermore, the long-term benefits of total disc replacement in preventing adjacent-level disc degeneration and the efficacy of two- or multi-level disc replacement remain unproven. The main criticism levelled at this procedure is that it violates the abdominal cavity and retroperitoneal space, giving rise to morbidity and complications. In 2004, randomized controlled trials proposed by the FDA [14–16] were initiated; these compared the Charité technique of lumbar disc replacement with anterior lumbar interbody fusion, demonstrating equivalent clinical outcomes at 2 years. However, the results have been disappointing, in that only 57% of patients undergoing disc replacement and 46% of those undergoing anterior interbody fusion with a BAK cage were seen to meet the four criteria for success (>25% improvement in ODI at 24 months, no device failure, no major complications and no neurological deterioration) [14]. These results were also confirmed over 5 years of follow-up [16] (57.8 vs. 51.2%).
A less invasive procedure, which is currently proposed for the treatment of chronic lumbar pain due to discopathy, involves the implantation of dynamic interspinous fixation devices. However, no clear demonstration of its biomechanical and clinical efficacy has yet been provided [17].
In view of these considerations, about 6 years ago we developed a new self-positioning, self-threading, stand-alone device that could overcome the main disadvantages of previous stand-alone cages that could lead to clinical failure. This new device should yield a higher rate of solid interbody fusion. Since 2003, this device has been implanted in patients; the results of long-term follow-up on 119 patients are here presented and discussed in detail.
Materials and methods
The population comprised all patients admitted to the Neurosurgery Departments of the Istituto IRCCS Galeazzi and of the A. O. Fatebenefratelli e Oftalmico in Milan between September 2003 and December 2005 with a diagnosis of single-level lumbar degenerative discopathy who underwent stand-alone posterior lumbar interbody fusion with the I-FLY cage.
Inclusion criteria for this surgical treatment were as follows: (1) single-level lumbar degenerative changes classified as Modic type III [1, 3], and grade V in the Pfirrmann classification [2] documented by MRI study of the lumbar spine, (2) normal bone anatomy documented by lumbar CT scan, (3) chronic low-back pain with or without radiculopathy, (4) failure of conservative treatment with first-line non-steroidal anti-inflammatory drugs, sometimes corticosteroids (dexamethasone), and physiotherapy for a minimum of 3 months before admission, (5) preoperative flexion–extension radiography of the lumbar spine to exclude spinal instability. The presence of grade I spondylolisthesis without segmental instability (demonstrated by flexion–extension radiography) was not considered a contraindication for this surgical approach. Patients were usually admitted the day before surgery and discharged home within 72 h of the operation. They were instructed to wear a soft corset for the first 5 postoperative weeks, and to use a forward-sloping seat. They could perform ordinary daily activities (without carrying or lifting weights heavier than 5 kg) after 1 week.
On admission, all patients underwent detailed anamnesis and neurological evaluation; VAS [18] and the Prolo Economic and Functional Scale [19] were completed to determine the extent of back pain and quality of life, respectively. Prior to discharge, all patients underwent a postoperative CT scan to exclude major complications. Patients underwent clinical and radiological follow-up examinations 1 and 2 years after surgery.
Clinical evaluation included neurological examination; VAS and the Prolo Economic and Functional Scale were also completed. Radiological control was performed by means of a polyaxial CT scan centred on the treated segment, and flexion–extension radiography [20]: fusion rates were evaluated according to the Bridwell 4-point scale [21]. Patients were regarded as “responders” of the last VAS evaluation documented an improvement of at least 3 points, and they had a Prolo value >7.
Technical characteristics of the cage
A new cage (I-FLY) was designed in 2001 and produced in its final version for clinical use in 2002 (Medtronic, Minneapolis, State of Minnesota, USA). The device is made of titanium (Ti6Al4V named ASTM F 136-70) and is approved by the American Society for Testing and Material. Cages are 25 mm in length and vary from 7 to 10 mm in diameter, plus the 2 mm overall height of the threads.
Implantation of this new device should overcome the main disadvantages of previous stand-alone cages, i.e.:
the need to destroy facet joints
the need for a working tube to maintain the distance and alignment of vertebral endplates
the need to violate the endplates by disc trimming
the need to thread the endplates prior to implantation of the cages
the risk of positioning the cages in a wrong direction, thereby violating the vertebral body
the risk of the cage pulling out
the risk of cage subsidence.
This new device and the implantation instruments have been designed to enable the cage to be placed without interrupting the use of the microscope after bilateral discectomy.
To achieve these goals, and to obtain a higher rate of fusion, the main design modification concerns the shape of the head of the cage (Fig. 1), which is designed in such a way as to enable the two vertebral endplates to be impacted and distracted without any additional instrument. As soon as the first thread is inserted by impact, the cage is progressively screwed forward into place (about 5 mm from the posterior border of the endplates). The head of the cage is smooth and, as it advances, progressively distracts the two endplates, thereby ensuring a proper threading of the bone and alignment of the cage (self-positioning). The total length of the cage is 25 mm. A second major consideration regards the diameter of the cage. To avoid destroying the facet joints, the transverse distance between the superior (medial) facet joints and the spinous process has been considered. In the lumbar spine, this distance is maximal at L5–S1, raging from 15.3 to 18.7 mm, and decreases progressively in the cranial direction: L4–L5 (13.6–15.5 mm), L3–L4 (12.3–14.8 mm), L2–L3 (11.3–14.2 mm) and L1–L2 (11.2–13.9 mm) [7, 22–25]. Obviously, the external diameter of the implantable cage should not exceed this distance at each level (Fig. 2).
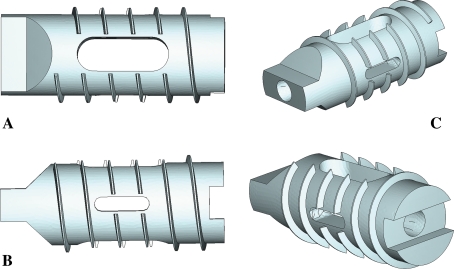
Fig. 1.
Perspective images of the I-FLY: a lateral surface, b superior surface, c oblique views
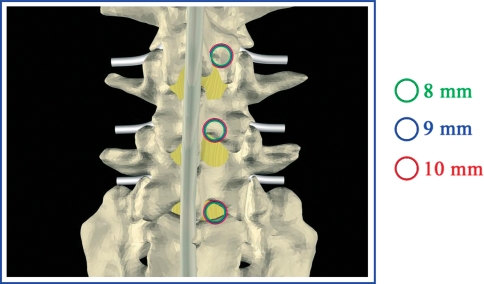
Fig. 2.
a Posterior diameter need for the surgical approach. b View of the bilateral surgical exposition of the disc and nerve root necessary to implant the I-FLY
The cage has an internal cavity, which can be packed with autologous bone removed by means of partial laminectomy, and wide apertures in the superior and inferior surfaces to facilitate bone fusion. Smaller apertures in the lateral surfaces enable the correct, symmetrical positioning of the cage to be verified. Because of these simple modifications, implantation requires a cylindrical working space which corresponds exactly to the outer diameter of the cage: 7–8–9–10 mm plus the thread diameter (2 mm). If the disc exceeds 10 mm in height, this procedure is poorly indicated, owing to the need for larger cages, leading to excessive destruction of the facet joints. The ideal indication for these cages is a very severe chronic discopathy with a very narrow disc space (Pfirrmann grade V) and without instability.
Statistical analysis
Statistical analysis was performed by means of SPSS Version 13. Continuous data are shown as average + standard deviation (SD), and categorical data as absolute and relative frequencies. Chi-square test was used to compare categorical variables, while continuous data were analysed by means of t test or Mann–Whitney non-parametric test, according to the normality of distribution.
Comparisons between the preoperative condition and that observed at the first and last follow-up examinations were made by means of a generalized linear model for repeated measurements. A logistic regression was applied to ascertain whether baseline characteristics could predict the outcome. The multivariable model adopted a block-entry procedure and included all variables with a p value <0.02 on univariate analysis. Calibration of the model was calculated by means of the Hosmer–Lemeshow test, and discrimination by means of C-statistics. Agreement between clinical and radiological improvement was evaluated by means of the Kappa test.
Technical note
The patient is positioned prone on gel rolls to promote physiological lordosis. Median skin incision is carried out (from 4 to 6 cm). Standard bilateral interlaminar exposure is achieved. Fluoroscopy can be made available for intra-operative control. Under the microscope, bilateral standard interlaminotomy and flavectomy (with rongeurs and a high-speed pneumatic drill) are performed to expose the dural sac. Conventional discectomy is performed by incising the annulus lateral to the dural sac with a 15-gauge scalpel blade. After this has been done bilaterally, soft fragments from the intradiscal space or extruded fragments are then removed with the disc rongeurs in a conventional fashion. Blunt bipolar cautery is used during dissection if scarring from a previous surgical procedure is present. The main goal of this step is to remove any extruded fragments, to decompress neural elements and to provide entry to the disc space for distraction with no or minimal nerve root retraction. If the disc space collapses significantly, complete discectomy may not be possible until disc distraction is accomplished. The spinous processes, the interspinous ligaments and the articular facets are left intact. Distraction of the disc should be initiated on one side to minimize trauma to the articular facets when ligamentum flavum and laminar bone removal have been performed on both sides. The endplates are progressively distracted until appropriate disc space height is obtained and the foraminal opening is restored. With the T-handle attached, the smallest disc distractor is inserted with the flat surfaces parallel to the endplates. It is then rotated 90° to distract and the distractor is left in the disc space and the T-handle is removed (Fig. 3a). At this point, preparation of the disc space is completed on the opposite side: remaining soft tissue or cartilaginous endplate is removed with the scraper or with curettes to allow optimal graft incorporation. On completion of the discectomy, the width and depth of the disc removal are carefully checked on both sides by using the disc distractor as a probe.
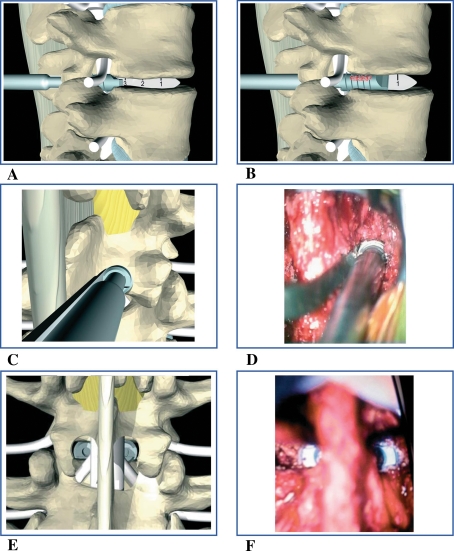
Fig. 3.
Operative steps: a insertion of the disc distractor and its rotation of 90° achieving distraction of the endplates. On the opposite side the head of the cage is impacted until the second threads. b The cage is threaded. c, d Posterior view of cage insertion and threading. e, f Final position of the cages in a posterior view
The depth of the disc removal should be at least 30 mm (the implant length plus approximately 5 mm); the width varies according to the implant size, and can also be verified by positioning the implant within the exposed disc space. The cage size should exceed the initial height of the intervertebral space by more than 3 mm to achieve stable construction. The implant is packed with autograft acquired from local bony elements. While the dural sac is protected and retracted with the root retractor, the cage, which is attached to the holder (Fig. 3c, d) is gently impacted until the first two threads are inserted within the intervertebral disc space (Fig. 3a). The cage is then threaded into the disc until it is at least 5 mm below the posterior wall of the vertebral body (Fig. 3b). At this point, the implant holder, root retractor and disc retractor on the opposite side are removed and the position of the cage can be checked fluoroscopically. The same steps are repeated on the opposite side until the cage is correctly placed (Fig. 3e, f).
Results
From September 2003 to December 2005, 120 patients underwent surgery with stand-alone I-FLY cages for degenerative discopathy. Outcomes were assessed and statistical analyses were completed in 119 cases (99.2% of the cohort) after 1 year and in 116 cases (96.6% of the cohort) after 2 years: 4 patients (3.4%) were lost to follow-up. Fifty-three patients (44.5%) were male and 66 (55.5%) were female. Their mean age was 49 years (range 21–74 years, SD ± 9). On admission, 8 patients (6%) were unemployed or retired from work; of the 111 patients who worked, 46 (39%) did heavy work (labourers, artisans). Forty-eight (40%) were smokers. Two patients (2%) suffered from coronary artery disease. The mean duration of symptoms was 34 months ± 44.
Preoperative symptoms included back pain in all cases; a sensory deficit was detected in 58 (49%) patients, and a strength deficit in 17 (14%). Fifty-two patients (44%) had previously undergone lumbar surgery (microdiscectomy) at the degenerated level. Surgery was performed at the L3–L4 level in 9 patients (7.5%), at L4–5 in 36 patients (30%), and at L5–S1 in 74 cases (62%). Thirteen (11%) patients were treated in 2003, 46 (39%) in 2004, and 60 (50%) in 2005. In 69 cases (58%) 8 mm diameter cages were used, in 43 cases (36.1%) 9 mm cages, and in 7 (5.9%) 10 mm cages. No 7 mm cages were used.
The mean time required for surgery was 75 min (SD 0.7); estimated blood loss was 75 ± 30 ml and no blood transfusions were required. No intra-operative dural lesions were found. No mortality was recorded in our series. All patients were discharged on the third postoperative day. Of the 17 patients (14%) with a radicular motor deficit on preoperative evaluation, only one (0.8%) showed persistence of the deficit at the last follow-up examination. At the same follow-up time, 18 (15%) of the 58 patients in whom a sensory deficit had been detected still presented some sensory deficit. Average preoperative and postoperative (at 1 and 2 years) Prolo and VAS values are presented in detail in Table 1.
Table 1.
Average preoperative and postoperative values of the evaluation scales for the surgical series
| Variable | Pre-operative | First follow-up examination | Second follow-up examination |
|---|---|---|---|
| Prolo E, average (SD) | 2.5 (0.9) | 4.1 (1.1)* | 4.4 (0.8)* |
| Prolo F, average (SD) | 2.0 (0.4) | 3.9 (1.3)* | 4.3 (0.9)* |
| Prolo, average (SD) | 4.5 (1.1) | 8.0 (2.3)* | 8.7 (1.5)* |
| VAS, average (SD) | 9.2 (1.2) | 3.5 (3.0)* | 2.6 (2.4)* |
* P < 0.05 in comparison with the previous value observed
Prolo outcome
Economic and physical Prolo values recorded at the last follow-up examination were summed to define the Prolo scale response to therapy. Categories were defined as follows: Prolo ≤6, not satisfactory (non-responder); Prolo 7–8, fair (responder); Prolo 9–10, good (responder). At the 1-year follow-up examination, the Prolo outcome was: good = 70 patients (58.8%); fair = 33 patients (27.7%) and not satisfactory = 16 (13.5%). According to the Prolo criterion, 103 (86.5%) patients were responders. At the 2-year follow-up examination, Prolo results showed a higher number of responders: good = 92 patients (79.3%); fair = 17 patients (14.6%) and not satisfactory = 7 (6.1%). According to the Prolo criterion, 109 (93.9%) patients were responders.
A logistic regression was applied to ascertain whether baseline characteristics were able to predict the outcome. The multivariable model is a forward stepwise model based on a likelihood-ratio (LR) test and includes variables with p < 0.2 on univariate analysis. The multivariable analysis revealed that age was the only independent predictor of Prolo outcome. Specifically, the probability of being a responder according to the Prolo criterion decreased among older people. Model calibration and discrimination were good (Table 2).
Table 2.
Statistical analysis of baseline characteristics of the population and Prolo results. Multivariate model
| Variable | Univariate | Multivariable* | ||||
|---|---|---|---|---|---|---|
| p value | OR | 95% CI | p value | OR | 95% CI | |
| Gender (male) | 0.505 | 1.45 | 0.49–4.29 | |||
| Age | 0.020 | 0.94 | 0.89–0.99 | 0.032 | 0.94 | 0.89–1.00 |
| Year of procedure | 0.441 | 0.74 | 0.34–1.60 | |||
| Unemployed (yes) | 0.626 | 0.65 | 0.11–3.77 | |||
| Diameter of the cage | 0.467 | 1.42 | 0.55–3.62 | |||
| Months of symptoms | 0.947 | 1 | 0.99–1.01 | |||
| Previous surgery (yes) | 0.118 | 2.60 | 0.79–8.63 | 0.180 | 2.31 | 0.68–7.87 |
| Smoker (yes) | 0.801 | 0.87 | 0.30–2.53 | |||
| Cardiopathy (yes) | 1.000 | – | – | |||
| Hours of surgery | 0.595 | 0.82 | 0.40–1.69 | |||
* 116 cases included in the analysis, Hosmer–Lemeshow test: p = 0.986, c-statistic = 0.705
VAS outcome
The difference between postoperative and preoperative VAS scale values was used to define the response to therapy. Categories were defined as follows: ΔVAS >3, improved (responder); ΔVAS ≤0, not improved (non-responder). A degree of improvement in the VAS score was recorded after 1 and 2 years in 108 (90.7%) and 110 patients (94.9%), respectively, while unsatisfactory results were found in 11 (9.3%) and 6 patients (5.1%). No variables proved significant in the multivariable model. Model calibration and discrimination were fairly good (Table 3).
Table 3.
Statistical analysis of baseline characteristics of the population and VAS results. Multivariate model
| Variable | Univariate | Multivariable* | ||||
|---|---|---|---|---|---|---|
| p value | OR | 95% CI | p value | OR | 95% CI | |
| Gender (male) | 0.252 | 2.62 | 0.51–13.59 | |||
| Age | 0.091 | 0.94 | 0.88–1.01 | 0.117 | 0.95 | 0.88–1.01 |
| Year of procedure | 0.215 | 0.48 | 0.15–1.53 | |||
| Unemployed (yes) | 0.097 | 0.17 | 0.02–1.38 | 0.149 | 0.20 | 0.02–1.79 |
| Diameter of the cage | 0.625 | 1.38 | 0.38–4.94 | |||
| Months of symptoms | 0.326 | 0.99 | 0.98–1.01 | |||
| Previous surgery (yes) | 0.774 | 1.24 | 0.28–5.48 | |||
| Smoker (yes) | 0.362 | 2.15 | 0.41–11.17 | |||
| Cardiopathy (yes) | 1.000 | – | – | |||
| Hours of surgery | 0.830 | 0.90 | 0.33–2.41 | |||
* 116 cases included in the analysis, Hosmer–Lemeshow test: p = 0.514, c-statistic = 0.728
Combined outcome
Patients were evaluated by combining the Prolo and VAS criteria; the results obtained at the two follow-up times are described in Table 4. Age proved to be the only independent predictor of combined outcome. Specifically, the probability of being a responder according to the combined criteria decreased among older people. Model calibration and discrimination were fairly good. On multivariable analysis, patient age was found to correlate with “non-responder” status (OR = 0.94, 95% CI = 0.89–0.99, p = 0.024). Calibration of the model was 0.668 on the Hosmer–Lemeshow test, and discrimination was 0.696 (C-statistic) (Table 5).
Table 4.
Clinical results combining VAS and Prolo evaluation at follow-up time
| Combined results | First follow-up examination | Second follow-up examination |
|---|---|---|
| NR Prolo + NR VAS | 6 (5.0%) | 3 (2.6%) |
| NR Prolo + R VAS | 10 (8.5%) | 4 (3.5%) |
| R Prolo + NR VAS | 2 (1.7%) | 0 (0%) |
| R Prolo + R VAS | 101 (84.8%) | 109 (93.9%) |
R responder, NR non-responder
Table 5.
Statistical analysis of baseline characteristics of the population and combined results. Multivariate model
| Variable | Univariate | Multivariable* | ||||
|---|---|---|---|---|---|---|
| p value | OR | 95% CI | p value | OR | 95% CI | |
| Gender (male) | 0.567 | 1.35 | 0.48–3.79 | |||
| Age | 0.017 | 0.94 | 0.89–0.99 | 0.024 | 0.94 | 0.89–0.99 |
| Year of procedure | 0.277 | 0.66 | 0.31–1.40 | |||
| Unemployed (yes) | 0.778 | 0.78 | 0.14–4.47 | |||
| Diameter of the cage | 0.281 | 1.67 | 0.66–4.23 | |||
| Months of symptoms | 0.960 | 1.00 | 0.99–1.01 | |||
| Previous surgery (yes) | 0.179 | 2.14 | 0.71–6.49 | 0.255 | 1.93 | 0.62–6.03 |
| Smoker (yes) | 0.713 | 0.83 | 0.30–2.29 | |||
| Cardiopathy (yes) | 1.000 | – | – | |||
| Hours of surgery | 0.879 | 0.946 | 0.46–1.94 | |||
* 116 cases included in the analysis, Hosmer–Lemeshow test: p = 0.668, c-statistic = 0.696
A polyaxial CT scan of the segment involved and a flexion–extension radiography were performed during follow-up, the former to establish the fusion rate according to the Bridwell scale [9] and the latter to exclude segmental instability. A Bridwell grade 1 (defined as bony bridge formation) was observed in 52.1% of patients after 1 year; after 2 years, this percentage had risen to 92.2% (Fig. 4). All the patients in whom a Bridwell grade 2 (stable construct but without clear bony bridge formation) had been recorded after 1 year (36.1%) presented a Bridwell grade 1 at the second follow-up CT scan. Of the 14 patients with grade 3 at the first follow-up CT scan (11.8%), 7 (6.9%) were still classified as grade 3 and one (0.9%) as grade 4 at the second examination. Of the 8 patients who presented a Bridwell grade 3 or 4 at the second follow-up CT scan, only the grade-4 patient failed to meet the clinical criteria for “responders” (Prolo 6 despite an improvement in the VAS value), while the other 7 were responders. In this last patient, flexion–extension radiography revealed an initial extrusion of the I-FLY cage and a grade I listhesis at the level of the operation (L5–S1). This patient underwent further surgery involving posterior instrumentation with screws and rods, without removal of the cages; a subsequent examination revealed a great improvement in both VAS and Prolo scores.
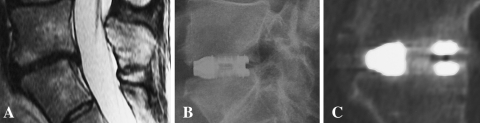
Fig. 4.
a Preoperative T-2 weighted sagittal MRI: discopathy of L5–S1 level. b Postoperative (2-year follow-up) radiography: correct position of I-FLY. c Polyaxial sagittal CT scan (2-year follow-up): successful bony bridge formation classified as Bridwell grade 1
Radiolucency at the endplate (named “halo sign”), with no collapse of the cage, was documented in 4 patients who showed solid bony bridge formation. The “halo sign” documented at both radiological examinations was found to be of no prognostic value (Kappa test p = 0.278) with respect to “responder” status. A good, statistically significant (Chi-square p = 0.001) correlation between an improvement in the radiological picture according to the Bridwell scale and “responder” status was found. No statistical correlation was found between the level of the operation and clinical or radiological outcome (Chi-square 0.466).
Discussion
The purpose of this study was to analyse the results achieved in a series of 119 consecutive patients with symptomatic high-grade degenerative disc disease (DDD) who underwent a surgical procedure of lumbar interbody fusion (PLIF) by means of a newly designed stand-alone cage (I-FLY). Pioneered by Cloward [5], PLIF has never been widely adopted for the treatment of DDD, mainly on account of its technical difficulty and because the cause of spinal pain is still not completely understood and remains controversial [26]. In the last year, however, interest in PLIF has grown, principally because transverse process fusion, although biomechanically effective in stabilizing the spine, often does not eliminate micromotion at the disc [27]; this observation has led to the concept of the “360-degree approach”.
The increased popularity of PLIF, whether associated with the use of pedicle screws or not, is also attributable to the introduction of new interbody devices and application sets which provide an easier surgical approach. Indeed, compared with the other surgical procedures of anterior–posterior fusion, PLIF offers equal patient satisfaction, is more economical, and enables patients to resume work and other activities sooner [28].
Nevertheless, in spite of these results, posterior lumbar interbody fusion has gained popularity as a fusion procedure associated with pedicle screw fixation, while it has mostly been abandoned as a stand-alone procedure (which mainly involves the use of threaded cages) owing to the unfavourable clinical and anatomical reports on large clinical series. The main criticisms levelled against cylindrical stand-alone cages (BAK, Ray Threaded Fusion, TFC, and Interfix) concern the subsidence of the cages, mostly described in the upper vertebral body [29, 30], the high rate of pseudoarthrosis (4–24%) [28, 31, 32], cage migration [33] and dural tearing (3–10%) [34, 35] and chronic radiculopathy, both arising from excessive spinal root distraction during the cage-positioning procedure [36–38]. However, the reasons for these anatomical and clinical failures have not been fully analysed.
First of all, these stand-alone devices are threaded cages designed for anterior lumbar interbody fusion (ALIF). All the instrument sets are therefore designed for a wide approach to the disc. Moreover, additional space is required by the working tube, which is needed to keep a regular distance between the vertebral endplates throughout the entire procedure. Given that the outer diameter of the smallest cage is 12 mm, the additional 2–3 mm of the working tube would require about 15 mm of minimal working space on either side. In the case of larger cages, the transverse space required would increase to 17 mm or more on either side. Clearly, this working space requirement will make it impossible to spare the facet joints at any level above L5–S1 (mean interpeduncular distance is 17 mm at L5–S1, 14.5 mm at L4–L5, 13.5 mm at L3–L4, 12.7 mm at L2–L3 and 12.5 mm at L1–L2) [7, 22–25]. This means that the first step of this stabilizing procedure involves a highly destabilizing removal of the facet joints. Moreover, the working tube is applied in such a way that it ensures a stable interbody distance only in the posterior portion of the vertebral bodies and it may be angled superiorly or inferiorly. Consequently, the further surgical steps (shaving, trimming and threading) could result in inappropriate cranial or caudal angles, leading to violation of the endplates, asymmetrical positioning of the cages and subsequent subsidence. It should also be mentioned that the working tube has to remain in place for a fairly long time during the procedure, causing severe root and dural sac distraction, completely obscuring the view of the operating field and preventing use of the surgical microscope. These considerations have led to the introduction of minimally invasive procedure as TLIF and XLIF.
After implanting different types of lumbar cages (BAK and Interfix) from 1998 to 2002, we determined to exploit this initial experience to develop a different stand-alone device for posterior lumbar fusion to overcome these critical points. It was clear that the working tube should be replaced by a “self-positioning” cage which would invariably reach a perfectly symmetrical position within the disc space and at the same time provide very good primary bone grip and a high long-term rate of bone fusion. This was achieved simply by modifying the head of the cage, which is blunt and tapered so that it can be used as a blunt spreader which will align and distract the endplates as the cages are advanced; this invariably yields perfectly symmetrical threading of the endplates and final positioning of the cages. A biomechanical study [38] demonstrated optimal mechanical behaviour in terms of good stiffness and uniform distribution of stress over the cancellous surface of the I-FLY cage. Owing to its special design, the I-FLY cage allows extremely straight, minimally invasive surgical exposure and implantation. The facet joints are therefore preserved and destruction of the posterior and facet-joint ligaments and of the bony endplates is minimal, conditions which are crucial to successful bone fusion [39, 40]. In addition to the design of the superior and inferior surfaces of the cages, the fact that no preliminary trimming, shaving and threading of the endplates is required makes cage subsidence fairly unlikely. Clearly, the advantages of avoiding facet joint destruction and of achieving firm adherence of the two vertebral bodies are again limited by the size of the cages. Even though the working space is minimized and the risk of undue endplate violation is avoided, these cages are not suitable, as stand-alone cages, for the treatment of high discs (exceeding 10 mm in height); their use is strongly indicated only in cases of chronic discopathy classified as grade V according to the Pfirrmann classification [41]. This procedure, in our experience, has become suitable in treating failed back syndrome and recurrent herniated discs of reduced height (44% of our population), making this approach preferable to other interbody fusion techniques such as TLIF and XLIF.
Literature reports indicate that satisfactory clinical outcome ranges from 70 to 86% [8, 23, 24, 34, 42–44], without significant differences between the various interbody techniques [45]. Our clinical results after 2 years were slightly better (90.5%) than those reported in the literature. This was probably due mainly to the minimal incidence of morbidity (no nerve root injury, no dural lesions, minimal blood loss) and complications (subsidence and pseudoarthrosis) leading to re-operation.
Concerning radiological results, there is a lack of consensus regarding the exact criteria that should be adopted in defining solid interbody fusion. While some authors define fusion as absolute or relative lack of mobility at the operated segment [46–48], others include additional criteria, such as the absence of lucency or halo around the implant on CT scan [21, 46, 49, 50]. In our series, flexion–extension radiography of the lumbar spine did not document abnormal mobility at the operated level (99.1%), except in one case, and bony bridge formation was documented inside the cages in 92.2% of patients. Nevertheless, no statistically significant correlation was found between the clinical outcome and the radiographic results.
Conclusions
In our opinion, PLIF by means of the stand-alone I-FLY cage can be regarded as a possible surgical treatment of chronic low-back pain due to high-degree DDD. This technique is not demanding (requiring the technical skills needed for bilateral discectomy, without preliminary trimming, shaving and threading of the endplates) and can be considered safe and effective, as shown by the excellent clinical and radiological success rates. It must, however, be emphasized that these stand-alone cages can be used only for discs which do not exceed 10 mm in height. Furthermore, these cages are suitable for any interbody fusion associated with pedicle screw fixation.
Conflict of interest
None.
References
- 1.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disc disease: assessment of changes in vertebral body marrow with MRI Imaging. Radiology. 1988;166:194–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 2.Pfirrmann CWA, Matzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;17:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Modic MT. Current assessment of spinal degenerative disease with magnetic resonance imaging. Clin Orthop Relat Res. 1992;279:68–81. [PubMed] [Google Scholar]
- 4.Vaga S, Raimondi MT, Caiani EG, Costa F, Giordano C, Perona F, et al. Quantitative assessment of intervertebral disc glycosaminoglycan distribution by gadolinium-enhanced MRI in orthopedic patients. Magn Reson Med. 2008;59(1):85–95. doi: 10.1002/mrm.21433. [DOI] [PubMed] [Google Scholar]
- 5.Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion I. Indications, operative technique, after care. J Neurosurg. 1953;10(2):154–168. doi: 10.3171/jns.1953.10.2.0154. [DOI] [PubMed] [Google Scholar]
- 6.Branch CL, Branch CL., Jr Posterior lumbar interbody fusion with the keystone graft: technique and results. Surg Neurol. 1987;27:449–454. doi: 10.1016/0090-3019(87)90252-7. [DOI] [PubMed] [Google Scholar]
- 7.Brantigan JW, Steffee AD, Geiger JM. A carbon fiber implant to aid interbody lumbar fusion. Mechanical testing. Spine. 1991;16:S277–S282. doi: 10.1097/00007632-199106001-00020. [DOI] [PubMed] [Google Scholar]
- 8.Ray CD (1997) Threaded titanium cages for lumbar interbody fusions. Spine. 22:667–679 (discussion 79–80) [DOI] [PubMed]
- 9.Simmons JW. Posterior lumbar interbody fusion with posterior elements as chip grafts. Clin Orthop Relat Res. 1985;193:85–89. [PubMed] [Google Scholar]
- 10.Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2(2):118–126. doi: 10.1007/s12178-009-9053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishna M, Pollock RD, Bhatia C. Incidence, etiology, classification, and management of neuralgia after posterior lumbar interbody fusion surgery in 226 patients. Spine J. 2008;8:374–379. doi: 10.1016/j.spinee.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Sears W. Posterior lumbar interbody fusion for degenerative spondylolisthesis: restoration of sagittal balance using insert-and rotate interbody spacers. Spine J. 2005;5:170–179. doi: 10.1016/j.spinee.2004.05.257. [DOI] [PubMed] [Google Scholar]
- 13.Freeman BJ, Davenport J (2006). Total disc replacement in the lumbar spine: a systematic review of the literature. Eur Spine J 15(Suppl 3):S439–S447 [DOI] [PMC free article] [PubMed]
- 14.Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT et al (2005) A prospective, randomised multi-centre food and drug administration investigational device exemption study of lumbar total disc replacement with the Charite′ artificial disc versus lumbar fusion. Part I: evaluation of clinical outcomes. Spine 30:1565–1575 (discussion E387–E391) (erratum in Spine 2005 Oct 15;30(20):2356) [DOI] [PubMed]
- 15.Geisler FH, Blumenthal SL, Guyer RD, McAfee PC, Regan JJ, Johnson JP, et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicentre, prospective, randomized investigational device exemption study of Charite intervertebral disc. J Neurosurg (Spine) 2004;1:143–154. doi: 10.3171/spi.2004.1.2.0143. [DOI] [PubMed] [Google Scholar]
- 16.Guyer RD, McAfee PC, Banco RJ, Bitan FD, Cappuccino A, Geisler FH, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five-year follow-up. Spine J. 2009;9(5):374–386. doi: 10.1016/j.spinee.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Bono CM, Vaccaro AR. Interspinous process devices in the lumbar spine. J Spinal Disord Tech. 2007;20(3):255–261. doi: 10.1097/BSD.0b013e3180331352. [DOI] [PubMed] [Google Scholar]
- 18.AN MOR. The meaning and measurement of pain. Practitioner. 1948;160(956):136–144. [PubMed] [Google Scholar]
- 19.Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine. 1986;11(6):601–606. doi: 10.1097/00007632-198607000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion. J Neurosurg Spine. 2005;2(6):653–657. doi: 10.3171/spi.2005.2.6.0653. [DOI] [PubMed] [Google Scholar]
- 21.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior structural allografts in the thoracic and lumbar spine. Spine. 1995;20:1410–1418. [PubMed] [Google Scholar]
- 22.Amonoo Kuofi HS. Maximum and minimum interpedicular distances in normal adult Nigerians. Am J Anat. 1982;135:225–233. [PMC free article] [PubMed] [Google Scholar]
- 23.Amonoo Kuofi HS, Patel PJ, Fatani JA. Transverse diameter of the lumbar spinal canal in normal adult Saudis. Acta Anat. 1990;137:124–128. doi: 10.1159/000146870. [DOI] [PubMed] [Google Scholar]
- 24.Chhabra S, Gopinathan K, Chhibber SR. Transverse diameter of the lumbar vertebral canal in North Indians. J. Anat. Soc India Vol. 1991;41(1):25–32. [Google Scholar]
- 25.Hinck VC, Clark WM, Hopkins CE. Normal interpediculate distances (minimum and maximum) in children and adults. Am. J. Roentgnol. 1966;97:141–153. doi: 10.2214/ajr.97.1.141. [DOI] [PubMed] [Google Scholar]
- 26.Zdeblick TA. The treatment of degenerative lumbar disorders. A critical review of the literature. Spine. 1995;20:126S–137S. doi: 10.1097/00007632-199512151-00009. [DOI] [PubMed] [Google Scholar]
- 27.Cristoferson LA, Selland B. Intervertebral implants following excision of protruded lumbar discs. J Neurosurg. 1975;42:401–405. doi: 10.3171/jns.1975.42.4.0401. [DOI] [PubMed] [Google Scholar]
- 28.Hacker RJ. Comparison of interbody fusion approaches for disabling low back pain. Spine. 1997;22:660–666. doi: 10.1097/00007632-199703150-00017. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y. Prediction of mechanical behaviors at interfaces between bone and two interbody cages of lumbar spine segments. Spine. 2001;26:1437–1442. doi: 10.1097/00007632-200107010-00010. [DOI] [PubMed] [Google Scholar]
- 30.Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine. 2000;25:1077–1084. doi: 10.1097/00007632-200005010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Barnes B, Rodts GE, McLaughlin MR, Haid RW., Jr Threaded cortical bone dowels for lumbar interbody fusion: over 1-year follow-up in 28 patients. J Neurosurg. 2001;95:1–4. doi: 10.3171/jns.2001.95.1.0001. [DOI] [PubMed] [Google Scholar]
- 32.Hee HT, Castro FP, Jr, Majd ME, Holt RT, Myers L. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord. 2001;14:533–540. doi: 10.1097/00002517-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Yang H, Tang T. Cage migration in spondylolisthesis treated with posterior lumbar interbody fusion using BAK cages. Spine. 2005;30(19):2171–2175. doi: 10.1097/01.brs.0000180402.50500.5b. [DOI] [PubMed] [Google Scholar]
- 34.Brantigan JW, Steffee DA, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Bratigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system. Spine. 2000;25:1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 35.Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD (1998) The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine Jun 1; 23 (11):1267–1278 (discussion 1279) [comment in Spine 1999 Sep 1; 24(17):1857] [DOI] [PubMed]
- 36.Brislin B, Vaccaro AR. Advances in posterior lumbar interbody fusion. Orthop Clin N Am. 2002;33(2):367–374. doi: 10.1016/S0030-5898(01)00013-X. [DOI] [PubMed] [Google Scholar]
- 37.DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16(3):130–139. doi: 10.5435/00124635-200803000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Fantigrossi A, Galbusera F, Raimondi MT, Sassi M, Fornari M. Biomechanical analysis of cages for posterior lumbar interbody fusion. Med Eng Phys. 2007;29(1):101–109. doi: 10.1016/j.medengphy.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Vishteh AG, Crawford NR, Chamberlain RH, Thramann JJ, Park SC, Craigo JB et al (2005) Biomechanical comparison of anterior versus posterior lumbar threaded interbody fusion cages. Spine. Feb 1; 30 (3):302–310 [DOI] [PubMed]
- 40.Weiner BK, Fraser RD (1998) Spine update lumbar interbody cages. Spine 23(5):634–640 [DOI] [PubMed]
- 41.Piera V, Rodroguez A, Cobos A, Hernandez R, Cobos P. Morphology of lumbar vertebral canal. Acta Anat. 1988;131:25–40. doi: 10.1159/000146482. [DOI] [PubMed] [Google Scholar]
- 42.Elias WJ, Simmons NE, Kaptain GJ, Chadduck JB, Whitehill R. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg. 2000;93:45–52. doi: 10.3171/spi.2000.93.1.0045. [DOI] [PubMed] [Google Scholar]
- 43.Freeman BJC, Licina P, Mehdian SH. Posterior lumbar interbody fusion combined with instrumented postero-lateral fusion: 5-year results in 60 patients. Eur Spine J. 2000;9:42–46. doi: 10.1007/s005860050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vamvanij V, Fredrickson BE, Thorpe JM, Stadnick ME, Yuan HA (1998) Surgical treatment of internal disc disruption: an outcome study of four fusion techniques. J Spinal Disord 11(5):375–382 [PubMed]
- 45.Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG et al (2005) Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine 2 (6):692–699 [DOI] [PubMed]
- 46.Brodsky AE, Kovalsky ES, Khalil MA (1991) Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine Jun; 16(6 Suppl):S261–S265 [DOI] [PubMed]
- 47.Burkus JK, Dorchak JD, Sanders DL (2003). Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. Feb 15; 28(4):372–377 [DOI] [PubMed]
- 48.Cleveland M, Bosworth DM, Thompson FR (1948) Pseudarthrosis in the lumbosacral spine. J Bone Jt Surg Am 30A(2):302–312 [PubMed]
- 49.Lang P, Genant HK, Chafetz N, Steiger P, Morris JM. Three-dimensional computed tomography and multiplanar reformations in the assessment of pseudarthrosis in posterior lumbar fusion patients. Spine. 1998;13(1):69–75. doi: 10.1097/00007632-198801000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Shah RR, Mohammed S, Saifuddin A, Taylor BA (2003) Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J Aug; 12(4):378–385 [DOI] [PMC free article] [PubMed]