Abstract
Iatrogenic spinal cord injury is the most feared complication of scoliosis surgery. The importance of combined somatosensory evoked potentials (SEP) and motor evoked potentials (MEP) monitoring during spine surgery is well known. The current authors retrospectively evaluated the results of neurophysiological intraoperative monitoring (IOM) in a large population of patients who underwent surgical treatment for spinal deformity. Intraoperative monitoring of SEPs and transcranial electrical stimulation MEPs (TES-MEP) was performed in 172 successive patients who underwent surgical treatment of idiopathic (128 pts), congenital (15 pts) or syndromic (29 pts) scoliosis. The first 106 patients (Group 1) underwent only SEP monitoring, while the other 66 patients (Group 2) underwent combined SEP and TES-MEP monitoring, when the technique was introduced in the current authors’ institution. Halogenate anaesthesia (Sevoflurane, MAC 0.6–1.2) was performed in Group 1 cases, total intravenous anaesthesia (Propofol infusion, 6–10 mg/kg/h) in Group 2 patients. A neurophysiological “alert” was defined as a reduction in amplitude (unilateral or bilateral) of at least 50% for SEPs and of 65% for TES-MEPs compared with baseline. In Group 1, two patients (1.9%) developed postoperative neurologic deficits following surgical correction of spinal deformity, consisting of permanent paraparesis in one case and transient paraparesis secondary to spinal cord ischaemia in the other. Twelve patients presented intraoperative significant changes of neurophysiological parameters that improved following corrective actions by surgeons and anaesthesiologists, and did not show any postoperative neurologic deficits. In ten cases the alert was apparently unrelated to surgical manoeuvres or to pharmacological interventions and no postoperative neurologic deficits were noted. Considering the patients of Group 2, two patients (3.0%) presented transient postoperative neurologic deficits preceded by significant intraoperative changes in SEPs and TES-MEPs. In five cases a transient reduction in the amplitudes of SEPs (1 patient) and/or TES-MEPs (5 patients) was recorded intraoperatively with no postoperative neurologic deficits. In conclusion, in the current series of 172 patients the overall prevalence of postoperative neurologic deficit was 2.3% (4 patients). When combined SEP and TES-MEP monitoring was performed, the sensitivity and specificity of IOM for sensory-motor impairment was 100 and 98%, respectively. Combined SEP and TES-MEP monitoring must be regarded as the neurophysiological standard for intraoperative detection of emerging spinal cord injury during corrective spinal deformity surgery. Early detection affords the surgical team an opportunity to perform rapid intervention to prevent injury progression or possibly to reverse impending neurologic sequelae.
Keywords: Neurophysiological intraoperative monitoring, Somatosensory evoked potentials, Motor evoked potentials, Scoliosis, Postoperative complications
Introduction
Iatrogenic spinal cord injury is the most feared complication of scoliosis surgery. The incidence of neurological complications for spinal deformity surgery has been estimated by the Scoliosis Research Society as 1%, except when a combined approach is used, where the rate increases to 1.87% [1]. Possible factors associated with higher risk of neurological complications of scoliosis surgery are represented by congenital scoliosis, scoliosis combined with hyperkyphosis, severity of the curve (Cobb angle over 90°), combined approach surgery, revision surgery and decreased spinal cord perfusion due to hypotension and/or significant haemorrhage. Procedures associated with higher risk include vertebral osteotomies and kyphosis correction [1, 2]. There exists an urgent need for a method to warn the spine surgeon of impending neurological deficits during surgery. Until the advent of neurophysiological IOM, the only other available method of observing spinal cord function was the Stagnara wake-up test [3]: this requires intraoperative reversal of general anaesthesia to permit the observation of voluntary lower-extremity movement. The technique permits assessment of the gross integrity of spinal cord motor tract function but it presents many limitations. The exact moment of neurologic injury remains obscure, and other possible complications exist including accidental extubation, air embolism or fractures of vertebral arches. Occasionally it may be dangerous to wake up patients with certain primary diseases intraoperatively [4]. Some patients may not be able to cooperate with the wake-up test because of age, language barriers or mental status.
Since 1970s the introduction of SEP for monitoring scoliosis surgery has significantly reduced the rate of intraoperative spinal cord injury [5–7]. In 1992 a Scoliosis Research Society study concluded that the use of intraoperative spinal cord neurophysiological monitoring during operative procedures including instrumentation should be considered “a viable alternative as well as an adjunct to the use of the wake-up test during spinal surgery” [8]. Nevertheless, it is well known that the use of SEPs alone may be ineffective in detecting a motor tract deficit [9, 10]. As a result, various methods for monitoring the motor tract of the spinal cord have been developed. The most commonly used stimulation technique is transcranial electric stimulation (TES) of the primary motor cortex by corkscrew electrodes placed in the scalp, to produce myogenic motor evoked potentials (TES-MEPs). The recordings are made from subcutaneous or intramuscular needle electrodes placed in multiple muscles in the arms and legs [11]. The combination of SEPs and TES-MEPs provides global monitoring coverage of spinal cord function.
The current authors retrospectively evaluated the results of IOM performed with SEPs alone or combined SEPs and TES-MEPs in a large population of consecutive patients who underwent surgical treatment for spinal deformity. The aim of the study was to report the authors’ experience with the use of IOM in spinal deformity surgery at their institution.
Materials and methods
From 2005 to the end of 2007 the present authors performed the intraoperative monitoring of spinal function by SEP recording under halogenate anaesthesia for successive patients of their series. In 2008 the methodology of TES-MEP recording was introduced in the authors’ institution. It is well known that volatile anaesthetics reduce dramatically the amplitude and reproducibility of motor evoked responses: for this reason a shift to total intravenous anaesthesia (TIVA) with propofol and remifentanil was required. The current authors have reviewed the IOM records of 172 consecutive patients (131 females and 41 males, aged from 11 to 69 years) who underwent surgery for spinal deformity from October 2005 to May 2009 in the Spine Surgery Department of the Rizzoli Orthopaedic Institute in Bologna, Italy.
The clinical diagnosis of the patients is summarized in Table 1A and B. 163 patients underwent posterior fusion with instrumentation surgery, using pedicle screw only construct. Another seven patients underwent anterior spinal fusion by thoracotomy. A combined anterior and posterior approach was performed in two cases. The day before surgery all patients were informed about the IOM procedure, and a clinical and neurophysiological evaluation was performed.
Table 1.
Clinical diagnosis (A) and characteristics of spinal deformity (B) in Group 1 and Group 2 patients
| Overall population | Group 1 | Group 2 | |
|---|---|---|---|
| (A) Clinical diagnosis of patients in Group 1 and Group 2 | |||
| No of patients | 172 | 106 | 66 |
| No of adult patients (mean age, range) | 80 | 53 | 27 |
| (41, 18–70) | (41, 19–70) | (39, 18–65) | |
| M/F | 41/131 | 26/80 | 19/47 |
| Congenital scoliosis/kyphoscoliosis | 15 | 5 | 10 |
| Adolescent idiopathic scoliosis/kyphoscoliosis | 76 | 53 | 23 |
| Adult idiopathic scoliosis/kyphoscoliosis | 52 | 36 | 16 |
| Neurofibromatosis | 7 | 3 | 4 |
| Marfan syndrome | 4 | 2 | 2 |
| Miscellaneousa | 18 | 7 | 11 |
| Overall population (172 pts) | Group 1 (106 pts) | Group 2 (66 pts) | |
|---|---|---|---|
| (B) Characteristics of spinal deformity in Group 1 and Group 2 patients | |||
| Pre-operative Cobb angle (mean, range) | 71° (50°–25°) | 66° (55°–120°) | 80° (50°–125°) |
| Post-operative Cobb angle (mean, range) | 36° (10°–95°) | 30° (10°–84°) | 45° (20°–95°) |
| Percentage deformity correction (mean%, range%) | 50 (18–77) | 52 (22–77) | 45 (18–69) |
| Thoracic deformity | 83 | 51 | 32 |
| Lumbar deformity | 9 | 3 | 6 |
| Thoraco-lumbar deformity | 80 | 52 | 28 |
| Kyphotic component | 39 | 18 | 21 |
aNine syndromic encephalopathies; three kyphosis secondary to vertebral lesions; two arthrogryposis multiplex congenita; one Prader–Willi syndrome; one Duchenne’s syndrome, two kyphoscoliosis and syringomyelia
Two groups of patients were considered: Group 1 included the first 106 patients who underwent only SEP monitoring under halogenate anaesthesia (sevoflurane); in Group 2 there were 66 successive patients who underwent combined SEP and TES-MEP monitoring under TIVA.
Anaesthetics
In all the procedures, anaesthesia was induced with propofol (dose range 1.5–2 mg/kg) or thiopentone (4–5 mg/kg).
In patients of Group 1, anaesthesia was maintained with sevoflurane (MAC 0.6–1.2) supplemented with nitrous oxide (mean 50.0 ± 5.0%). During the 66 procedures of Group 2 cases, anaesthesia was maintained with a propofol infusion (mean 7 mg/kg/h, range 6–10). Analgesia was provided by intravenous fentanyl (range 1–2 γ/kg/h) or by remifentanil infusion (mean 0.25 γ/kg/min, range 0.2–0.35).
A single bolus of non-depolarizing muscle relaxant (vecuronium or atracurium) was given at induction to facilitate tracheal intubation and ventilation; no muscle relaxants were administered after intubation.
Invasive blood pressure, ECG, end-tidal carbon dioxide concentration, pulse oximetry and body temperature were monitored. Mean blood pressures and blood loss were 69.7 ± 13.5 mmHg, 1,875 ml (range 1,000 ± 2,500) and 66 ± 8.6 mmHg, 1,762 ml (range 800 ± 2,750) in the propofol and sevoflurane groups, respectively. Patients were warmed actively throughout the procedure. The mean temperature was 35.4 ± 0.8°C in the propofol group and 35.7 ± 0.6°C in the sevoflurane group.
Somatosensory evoked potentials (SEP)
Both cortical and peripheral SEPs were elicited by a 200-μsec square-wave electrical pulse presented, in turn, to the posterior tibial and median nerves at a rate of 2.91 Hz. Stimulation intensity was adjusted individually and ranged from 20 to 40 mA. Cortical potentials were recorded from subdermal needle electrodes affixed to Cz′ referenced to Fpz and to C3′ referenced to C4′ (International 10-20 System) for the lower limb SEPs, and to C3′ and C4′ referenced to the contralateral mastoid for the upper limb SEPs. Peripheral responses were recorded similarly with electrodes placed at the popliteal fossa and at Erb’s point for the lower and the upper limb, respectively. Filtering was 30–1,500 Hz, with a 50 or 100 ms analysis time; averaging was stopped manually at such times as potentials were clearly reproducible and ranged from 100 to 200 repetitions.
Transcranial electrical stimulation motor evoked potentials
TES-MEPs were elicited by delivering a brief (50-μsec), high-intensity (up to 200 mA) anodal pulse train (5–7 pulses with a 4-msec interstimulus interval) between two pairs of corkscrew electrodes inserted subcutaneously over motor cortex regions C1–C2 and C3–C4 (International 10-20 System). The stimulation parameter values (i.e. the number of pulses and intensity) were optimized to elicit the maximum baseline amplitude possible for that particular patient. To avoid oropharyngeal airway bite or tongue bites, a bite block was used.
These myogenic responses were recorded bilaterally from the abductor pollicis brevis muscle in the upper extremities and bilaterally from, at a minimum, the tibialis anterior and abductor hallucis muscles in the lower extremities. Other sites included the iliopsoas, adductor magnus, quadriceps and gastrocnemius muscles, depending on the complexity of the curve and the incorporation and levels of pedicle screw fixation.
The filter bandpass was 40–5,000 Hz and the time base was 100 ms.
All stimulation and recording of SEPs and TES-MEPs were performed using a commercially available neurophysiological monitoring workstation (Protektor, Xltek®).
Definition of significant change and intervention
A neurophysiological change was defined significant (i.e. an “alert”) when it consisted of a persistent unilateral or bilateral reduction in amplitude ≥50% for SEPs and ≥65% for TES-MEPs compared with baseline. Response latency shift was not considered an alert suggestive of emerging spinal cord injury, unless it was associated with a notable reduction in amplitudes.
A critical neurophysiologic alert triggered a sequence of interventional steps based on a predetermined algorithm [12]. If the neurophysiological change was time-related to a specific surgical manoeuvre, the precipitating manoeuvre was promptly reversed. Regardless of whether the change was related to a particular surgical action, the anaesthesiologist was always directed to raise the mean arterial blood pressure to at least 90 mm Hg to promote better spinal cord perfusion. If, after temporary cessation of the surgery and institution of hemodynamic management, the response amplitude failed to show signs of recovery over the course of 10 min, corrective forces were reversed. If the amplitude still did not improve, even after reversal of correction and implant removal, cessation of the procedure was considered. A Stagnara wake-up test was performed when required by the surgeon.
The reliability of IOM in the two groups included sensitivity (i.e. whether early cord changes can be detected by waveform changes) and specificity (i.e. whether the detected changes are associated with cord compromise and can be distinguished from other less important changes).
Results
None of the patients had epileptic seizures during or after the operation. No burn marks occurred at stimulation sites.
Group 1—SEP monitoring under inhalational anaesthesia
Two patients (1.9%) developed postoperative neurologic deficits following surgical correction manoeuvres of spinal deformity (Table 2A).
Table 2.
Intra-operative changes and clinical outcomes in 14 patients of Group 1 (A) and in 7 patients of Group 2 (B)
| No. | Sex | Age | Diagnosis | Cobb angle | Procedure | Intra-operative alert | Stage of surgery | Presumed cause | Wake-up test | Post-operative outcome | IOM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) Group 1 | |||||||||||
| 1 | M | 19 | Neuromuscular kyphoscoliosis Sensory-motor axonal neuropathy |
120 | P | P40a bilateral loss | Deformity correction | Excessive corrective forces | P | Permanent paraparesis | TP |
| 2 | M | 19 | Kyphosis in tetraparesis and mental retardation | 116 | P | Bilateral P40a amplitude reduction | Deformity correction | Excessive corrective forces | P | Unchanged | TP |
| 3 | F | 14 | Congenital kyphoscoliosis | 88 | P | Bilateral P40a amplitude reduction | Deformity correction | Excessive corrective forces | P | Unchanged | TP |
| 4 | F | 13 | Adolescent idiopathic scoliosis | 82 | P | Transient, bilateral N20b and P40a amplitude reduction | Implant of instrumentation | Systemic hypotension, spinal cord ischaemia | NP | Transient paraparesis | FN |
| 5 | F | 46 | Adult idiopathic scoliosis | 40 | P | Transient bilateral P40a amplitude reduction | Spine exposure | ? | NP | Unchanged | FP |
| 6 | M | 23 | Adolescent idiopathic scoliosis | 115 | A | Transient bilateral P40a amplitude reduction | Implant of instrumentation | ? | NP | Unchanged | FP |
| 7 | F | 12 | Adolescent idiopathic scoliosis | 80 | P | Bilateral P40a amplitude reduction | Implant of instrumentation | ? | N | Unchanged | FP |
| 8 | F | 15 | Adolescent idiopathic scoliosis | 48 | P | Bilateral P40a amplitude reduction | Implant of instrumentation | ? | N | Unchanged | FP |
| 9 | F | 15 | Adolescent idiopathic scoliosis | 55 | P | Bilateral P40a amplitude reduction | Implant of instrumentation | ? | N | Unchanged | FP |
| 10 | F | 54 | Adult idiopathic scoliosis | 58 | P | Bilateral P40a amplitude reduction | Implant of instrumentation | ? | N | Unchanged | FP |
| 11 | F | 15 | Adolescent idiopathic scoliosis | 74 | P | Bilateral P40a amplitude reduction | Deformity correction | Excessive corrective forces?c | N | Unchanged | FP |
| 12 | M | 16 | Adolescent idiopathic scoliosis | 40 | P | Bilateral P40a amplitude reduction | Implant of instrumentation | ? | N | Unchanged | FP |
| 13 | F | 13 | Adolescent idiopathic scoliosis | 70 | P | Transient bilateral P40a amplitude reduction | Spine exposure | ? | NP | Unchanged | FP |
| 14 | F | 39 | Adult idiopathic scoliosis | 65 | P | Bilateral P40a amplitude reduction | Deformity correction | Excessive corrective forces?c | N | Unchanged | FP |
| (B) Group 2 | |||||||||||
| 1 | F | 17 | Adolescent idiopathic scoliosis | 90 | P | Bilateral loss of SEP (P40) and MEP responses at lower limbs | Implant of instrumentation | Pedicle screw malpositioning | N | Transient paraparesis (recovered in 3 months) | TP |
| 2 | F | 13 | Acquired kyphosis (vertebral rhabdomyosarcoma) | 125 | P | Transient bilateral reduction in SEP (P40) and MEP responses at lower limbs | Implant of instrumentation | Systemic hypotension | N | Unchanged | TP |
| 3 | F | 20 | Congenital kyphoscoliosis | 95 | P | Transient bilateral reduction in SEP (P40) and MEP responses at lower limbs | Implant of instrumentation | Systemic hypotension | NP | Unchanged | TP |
| 4 | F | 17 | Kyphoscoliosis in neurofibromatosis | 58 | A | Unilateral loss of peripheral and cortical SEPs and MEPs (ABP musclea) at the right upper limb | Spine exposure | Arm malpositioning | NP | Transient right arm monoparesis secondary to brachial plexopathy | TP |
| 5 | F | 13 | Adolescent idiopathic scoliosis | 110 | P | Loss of MEP responses recorded at the left lower limb (TA and AH musclesb) | Deformity correction | Excessive corrective forces | N | Unchanged | TP |
| 6 | F | 20 | Kyphoscoliosis in neurofibromatosis; pre-operative paraparesis | 82 | P | Transient bilateral reduction in MEP responses recorded at lower limbs | Deformity correction | Excessive corrective forces | NP | Unchanged | TP |
| 7 | F | 13 | Acquired kyphoscoliosis pre-operative paraparesis (C4-D1 ependymoma) | 115 | P | Bilateral loss of MEP responses recorded at lower limbs | Implant of instrumentation | Pharmacological depression of cortical activity | N | Unchanged | FP |
aLower limb cortical response P40
bUpper limb cortical response N20
cThe reversal of corrective forces was not followed by cortical SSEPs improvement but the patient did not show any motor deficit during the wake-up test
ABP abductor pollicis brevis muscle, TA tibialis anterior muscle, AH abductor hallucis muscle
Procedure: P posterior approach, A anterior approach; Wake-up test: P positive, N negative, NP not performed; IOM: TP true positive, FN false negative, FP false positive
One patient (No. 1) with severe scoliosis (Cobb angle 120°) combined with kyphosis and pre-existing mild sensory-motor axonal neuropathy and lower limb hypopallesthesia developed a persistent postoperative paraparesis. The complete loss of lower limb cortical SEPs was recorded during the instrumentation positioning and deformity correction (true positive).
One patient (No. 4) with idiopathic scoliosis (Cobb angle 82°) presented transient reduction of upper and lower limb cortical SEPs related to hypotension during the pedicle screws positioning. Cortical SEPs recovered partially following haemodynamic management of blood pressure parameters and the procedure was concluded. The patient awoke with paraparesis due to anterior spinal cord ischaemia. The motor deficits recovered completely in 3 months after implant removal. The transient change in SEPs did not match with the extent of postoperative damage, so it was defined a false negative result.
In 92 patients the intraoperative evoked responses did not show any significant changes and there were no postoperative neurologic sequelae (true negative).
The other 12 patients presented intraoperative significant changes of neurophysiological parameters that justify corrective actions by surgeons and anaesthesiologists, but did not show any postoperative neurologic deficits. Among these, two patients (No. 2, 3) presented a marked reduction in the amplitude of the lower limb cortical SEPs attributed to surgical manoeuvres during the correction of severe kyphosis. The reduction in corrective forces was followed by the complete recovery of the evoked responses and both patients did not show any postoperative deficit (true positive).
In ten cases (No. 5–14) the significant intraoperative reduction in the amplitudes of lower limb cortical SEPs was apparently unrelated to surgical manoeuvres or to pharmacological interventions and no postoperative neurologic deficits were noted (false positive). The neuromonitoring alerts occurred during the implant of the instrumentation in five patients and during correction of spinal deformity in two, but temporary cessation of the surgery, hemodynamic and pharmacological management, implant removal or reversal of correction were not followed by a significant improvement in the amplitude of cortical responses. The wake-up test never showed any motor deficits but it was followed by the recovery of cortical SEPs in all patients.
Group 2—SEP and TES-MEP monitoring under TIVA anaesthesia
Two patients (3.0%) presented postoperative neurologic deficits preceded by significant intraoperative changes in SEPs and TES-MEPs (true positive). In one case (No. 1) the surgical manoeuvres had been stopped on account of the sudden drop in the amplitudes of somatosensory and motor evoked responses at the lower limbs following the correction of spinal deformity. The reversal of correction and implant removal was followed by a significant improvement of SEP and TES-MEP evoked potentials. A wake-up test failed to detect any motor deficit. The patient developed transient postoperative myelopathy, with thermodolorific hypoesthesia (upper level T12) and marked proximal hyposthenia to lower limbs with preservation of flexion and extension movements of the foot and achieved normal walking 5 months later.
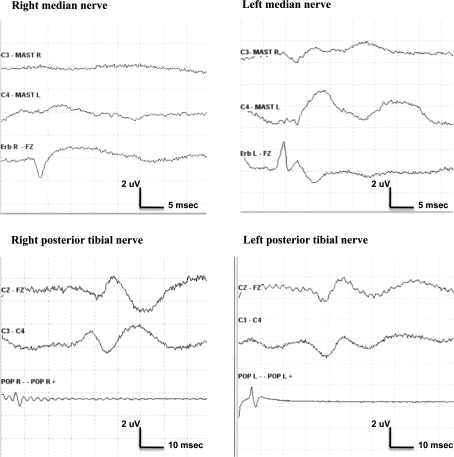
An 18-year-old female with a severe thoracic scoliosis and Neurofibromatosis type I (No. 6) underwent an anterior spinal release procedure. The preoperative neurological examination was normal. SEP and TES-MEP were performed preoperatively after positioning the patient in a left lateral decubitus, the right arm fixed to an arm support above the head, and repeated regularly throughout the operation. Soon after the patient positioning there was an acute complete loss of MEPs from the right ABP muscle with preservation of responses from the left ABP and from lower-extremity recording sites. At the same time, the peripheral and cortical SEP responses following stimulation of right median nerve were abolished, whereas potentials obtained by stimulation of the left median nerve and of posterior tibial nerves were unchanged (Fig. 1). The repositioning of the right arm was followed by the recovery of motor response from the right ABP muscle. Also peripheral and cortical right median nerve SEPs re-emerged, but at reduced amplitudes relative to pre-positioning baselines. No more neurophysiological changes were recorded up to the end of the operation.
Fig. 1.
Sudden loss of right median nerve peripheral and cortical SEP responses following positioning of the patient in a lateral decubitus
The patient presented transient postoperative weakness of the right upper limb due to brachial plexopathy and recovered completely in 4 weeks.
In 59 patients the intraoperative evoked responses did not show any significant changes and there were no postoperative neurologic sequelae (true negative).
In five cases a transient reduction in the amplitudes of somatosensory (2 patients) and/or motor (5 patients) evoked potentials was recorded intraoperatively with no postoperative neurologic deficits. The alert was related to hypotension in two cases and to surgical manoeuvres in two cases (true positive). One patient (No. 7) with preoperative pathologic motor responses due to myelopathy showed a complete loss of MEPs during intervention with no neurologic sequelae (false positive).
In conclusion, in the current series the overall prevalence of postoperative neurologic deficits was 2.9% (4/172). Motor impairment resolved completely within 5 months after surgery in three cases. One patient developed persistent paraparesis due to excessive corrective forces.
When only SEPs were recorded (Group 1) the sensitivity and specificity of IOM for spinal cord function were 75 and 90%, respectively, due to the high rate of false positives and the presence of one false negative result. On the contrary, when combined SEP and TES-MEP monitoring was performed (Group 2), the sensitivity and specificity of IOM for sensory-motor impairment rose to 100 and 98%, respectively. In Group 2 the sensitivity and specificity of each IOM technique were 67 and 100% for SEP and 100 and 98% for TES-MEP.
Discussion
Neurologic deficit is one of the most severe risks of scoliosis surgical treatment and its incidence varies among different studies. Timely detection of impending spinal cord damage is paramount. The first consensus meeting on intraoperative monitoring during spine surgery in Verona in 2006 [10] recommended the use of IOM in all spinal surgical procedures bearing a potential risk of damaging neural structures. In particular, the consensus group agreed that IOM can be recommended for corrections of spinal deformities with scoliosis greater than 45° and corrections of congenital spinal anomalies.
The current authors report the outcome of a large and heterogeneous population of adolescent and adult patients who underwent surgery for spinal deformity: the study presents some limitations, such as being retrospective and non-randomized. On the other hand it presented data of a large, consecutive population of patients operated on by four surgeons in a single institution, under IOM performed by the same neurophysiologist.
Three out of 172 patients showed postoperative neurologic complications involving the spinal cord, consisting of persistent paraparesis in one patient and transient paraparesis in two. All three patients had a severe scoliosis (Cobb angle 82–120°) combined with kyphosis and one patient had pre-existing neurological deficits consisting of mild sensory-motor axonal neuropathy and lower limb hypopallesthesia. Moreover, another four patients showed a significant drop in the amplitude of lower limb cortical evoked responses during the correction of severe kyphosis. The reduction in corrective forces avoided postoperative neurologic sequelae. The results of the present study are coherent with those of the literature [13], which indicates a higher risk of a neurological complication for patients with congenital scoliosis, scoliosis combined with hyperkyphosis, severe scoliosis with Cobb angle over 90° and with a pre-existing neurological deficit.
During spine surgery, neurologic complications may arise as the result of mechanical and/or vascular etiologies.
Neurophysiological monitoring of the dorsal column function using SEPs has been used for over 20 years in spine surgery. Intraoperative decreases in amplitude over 50% from baseline are uniformly considered signs of dysfunction of the sensory pathways and potentially complete spinal cord compromise. Nevertheless, false negative intraoperative SEP changes with regard to postoperative motor impairment have been reported in several studies [14, 15]. In fact, mechanical injury, vascular injury or hypotensive anaesthesia can result in motor changes without concomitant sensory changes in neuromonitoring. The occurrence of transient SEP changes reverting to baseline before the end of surgery is also possible, correlating to postoperative neurologic deficits [16]. Many factors can affect the amplitude and latency of SEP waveforms during surgery [17]. Somatosensory evoked potential amplitude decreases with ischaemia and anoxia because of temporal dispersion of the afferent volley and conduction block in damaged axons. In conjunction with surgical manipulations, minor drops in blood pressure may result in substantial SEP changes that reverse when perfusion pressure is increased. Marked temperature-related drops in SEP amplitude may occur after exposure of the spine but before instrumentation and deformity correction, with an increase in false negative outcomes. Moreover, intraoperative neurophysiologic monitoring is affected by the choice and management of the anaesthetic regimen. Inhalational anaesthetic agents have been widely used in spine surgery during SEP intraoperative monitoring [18–21] but they are known to have effects on neural synaptic and axonal function and cause a dramatic dose-dependent depression of SEP and cMEP responses [22].
From 2005 to 2007 SEP intraoperative monitoring was performed during spinal deformity surgery under halogenate anaesthesia (Group 1). The sensitivity and specificity of IOM for spinal cord function were 75 and 90%, respectively, due to the high rate of false positives (10/106 patients) and the presence of one false negative result. Two patients presented significant changes of lower limb SEPs during spine exposure, typically related to temperature drop and increased anaesthetic depth.
The other eight false positive results are related to amplitude reduction of cortical responses occurring during the implant of instrumentation and the correction of deformity. Spinal cord perfusion may be compromised even at normal systemic blood pressure when intraoperative mechanical stress is applied to neural tissue [23]. In the cases reviewed by the authors, the change in SEP amplitude may be the multifactorial result of either mechanical stress, hypotension or pharmacological overdose leading to subclinical spinal cord damage. All patients showed a marked improvement in cortical SEPs following a negative wake-up test. Awakening a patient requires prolonged cessation of the surgery and progressive reduction of the pharmacologic load with a consequent rise in blood pressure. The manoeuvre per se might improve spinal cord function. A false positive result is defined by a significant change in signal not related to any surgical event and resulting in no new postoperative neurologic change. However, in practice it may be difficult to verify whether an abnormal signal obtained intraoperatively is true positive or negative. It may be possible to record neurophysiological signals in the presence of nitrous oxide or inhalational agents, however, at the expense of smaller and more variable responses, thus adding interpretive ambiguity.
In 2007, the monitoring of the corticospinal tract by TES-MEP was introduced in the present authors’ institution and a total intravenous anaesthesiologic regimen, with controlled rates of propofol and remifentanil was adopted. When combined SEP and TES-MEP monitoring was performed (Group 2), the sensitivity and specificity of IOM for sensory-motor impairment were 100 and 98%, respectively.
The use of TIVA raised the specificity of SEP monitoring to 100% due to the absence of false positive results. The sensitivity of the technique to detect impending spinal cord damage was still as low as 67% due to the occurrence of two false negative results. In group 2, 6/66 patients presented an intraoperative alarm related to spinal cord damage. The signal change consisted of bilateral loss of lower limb TES-MEPs in six patients and bilateral loss of lower limb cortical SEPs in three. In patients who presented a change in both somatosensory and motor responses, the reduction of motor amplitudes always preceded the SEP change. The only patient who developed postoperative motor deficit presented a loss of both somatosensory and motor responses during instrumentation positioning. Neither of the three patients who presented isolated change of TES-MEPs with no SEP changes developed postoperative deficits.
The differential sensitivities of TES-MEP potentials and SEP to evolving spinal cord injury may be related to differences in the neural pathways that mediate these responses and to the mechanism of spinal cord injury.
Spinal cord contusion causes a transient spinal cord conduction block, resulting in marked amplitude suppression (50–75%) of SEPs and/or TES-MEPs that typically resolves within 15–20 min. Reversal of these changes may be aided by increasing mean arterial blood pressure to improve spinal cord perfusion and by temporarily stopping further surgical manoeuvres [24]. Spinal cord sensory and motor pathways are physically separated from each other and have separate vascular supplies (i.e. anterior and posterior spinal arteries). It is possible that selective ischaemia of the anterior spinal cord region may manifest as a loss of motor-evoked potential amplitude in the absence of concurrent change in SEPs. Moreover, the vascular supply to the motor pathways is also less redundant than is the supply to the posterior sensory columns, adding to this vulnerability. Since most neurologic injuries during scoliosis surgery appear to be related to ischaemia, TES-MEPs are more likely to change under these conditions than SEPs. Prolonged hypotension can result in spinal cord vascular injury. TES-MEPs are particularly sensitive to blood pressure changes and can be used quite effectively to titrate the degree of hypotensive state that the spinal cord will withstand [25].
These data should be interpreted as suggesting that SEP monitoring complements TES-MEP monitoring by being sensitive to injury limited to the posterior sensory columns. Consensual change of SEP and MEP responses may have a worsened prognostic significance in terms of possible postoperative clinical impairment.
The Stagnara wake-up test was the first widely used method for intraoperative spinal monitoring of spinal cord functionality during scoliosis surgery. The current study indicates the possibility of false negative results of wake-up test, when the consequence of spinal cord lesion is proximal lower limb hyposthenia with sparing of distal leg movements. On the other hand, the wake-up test remains mandatory when only SEPs are used for IOM purpose due to the possibility of isolated lesion of corticospinal tract in the presence of unmodified SEP responses.
Finally, complications related to patient positioning on the operating table may consist of severe motor deficit secondary to brachial plexopathy. Patients with skeletal dysplasia syndromes, neurofibromatosis, polyneuropathy should be regarded as particularly vulnerable to spinal cord and brachial plexus lesions due to head, neck or upper limb malpositioning [26]. The patient of the current series, affected by Neurofibromatosis type I, presented neurophysiological signs of brachial plexus damage immediately after positioning in a lateral decubitus. Sudden repositioning of the right arm prevented persistent lesion of the brachial plexus. A tangential benefit of IOM is the ability of SEPs and/or TES-MEPs to identify impending brachial plexopathy [27].
Conclusion
Intraoperative monitoring of SEPs and MEPs is a reliable method to provide information regarding spinal cord and peripheral nerves integrity during spinal deformity surgery. Correction of spinal deformity is in fact considered the most dangerous stage of scoliosis surgery. However, it is possible that minor drops in blood pressure in conjunction with surgical manipulation may lead to spinal cord damage at any stage of the operation. Lesions of motor pathways with sparing of dorsal column function due to selective ischaemia in the anterior spinal artery territory is possible. For this reason, a multimodal approach with SEPs and TES-MEPs under TIVA is mandatory to assess the maximum amount of information about spinal cord integrity, including both the descending motor and ascending sensory pathways and to minimize the rate of false positive and false negative results.
Conflict of interest
None.
References
- 1.Diab M, Smith AR, Kuklo TR, The Spinal Deformity Study Group et al. Neural complications in the surgical treatment of adolescent idiopathic scoliosis. Spine. 2007;32:2759–2763. doi: 10.1097/BRS.0b013e31815a5970. [DOI] [PubMed] [Google Scholar]
- 2.Qiu Y, Wang S, Wang B, et al. Incidence, risk factors of neurological deficits of surgical correction for scoliosis. analysis of 1373 cases at one Chinese institution. Spine. 2008;33:519–526. doi: 10.1097/BRS.0b013e3181657d93. [DOI] [PubMed] [Google Scholar]
- 3.Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop. 1973;93:173–178. doi: 10.1097/00003086-197306000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Mostegl A, Bauer R, Eichenbauer M. Intraoperative somatosensory potential monitoring: a clinical analysis of 127 surgical procedures. J Spine. 1988;13(4):396–400. doi: 10.1097/00007632-198804000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Tamaki T, Noguchi T, Takano H, et al. Spinal cord monitoring as a clinical utilization of the spinal evoked potential. Clin Orthop Relat Res. 1984;184:58–64. [PubMed] [Google Scholar]
- 6.Padberg AM, Wilson-Holden TJ, Lenke LG, Bridwell KH. Somatosensory and motor evoked potential monitoring without a wake-up test during idiopathic scoliosi surgery. Spine. 1992;23:1392–1400. doi: 10.1097/00007632-199806150-00018. [DOI] [PubMed] [Google Scholar]
- 7.Nuwer MR, Dawson EG, Carlson LG, et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. doi: 10.1016/0013-4694(94)00235-D. [DOI] [PubMed] [Google Scholar]
- 8.Scoliosis Research Society (1992) Position Statement on Somatosensory Evoked Potential Monitoring of Neurological Spinal Cord Function. Scoliosis Research Society 1992
- 9.Luk KDK, Hu Y, Wong YW, Cheung KMC. Evaluation of various evoked potenial techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26(16):1772–1777. doi: 10.1097/00007632-200108150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Sutter M, Deletis V, Dvorak J, et al. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J. 2007;16(2):S232–S237. doi: 10.1007/s00586-007-0421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajewski TN, Arlet V, Phillips LH. Current approach on spinal cord monitoring: the point of view of the neurologist, the anesthesiologist and the spine surgeon. Eur Spine J. 2007;16(2):S115–S129. doi: 10.1007/s00586-007-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz DM, Sestokas AK. A systems-based algorithmic approach to intraoperative neurophysiological monitoring during spinal surgery. Semin Spine Surg. 2002;14:136–145. [Google Scholar]
- 13.Mac Ewen GD, Bunnel WP, Sriram K. Acute neurological complications in the treatment of scoliosis. A report of the Scoliosis Research Society. J Bone Joint surg. 1975;57-A(3):404–408. [PubMed] [Google Scholar]
- 14.Ginsburg HH, Shetter AG, Raudzens PA. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials. J Neurosurg. 1985;63:296–300. doi: 10.3171/jns.1985.63.2.0296. [DOI] [PubMed] [Google Scholar]
- 15.Pelosi L, Lamb J, Grevitt M, et al. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113:1082–1091. doi: 10.1016/S1388-2457(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 16.Noonan KJ, Walker T, Feinberg JR, et al. Factors related to false- versus true-positive neuromonitoring changes in adolescent idiopathic scoliosis surgery. Spine. 2002;27(8):825–830. doi: 10.1097/00007632-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Seyal M, Mull B. Mechanisms of signal change during intraoperative somatosensory evoked potential monitoring of the spinal cord. J Clin Neurophysiol. 2002;19(5):409–415. doi: 10.1097/00004691-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Luk KDK, Hu Y, Wong YW, Leong JCY. Variability of somatosensory-evoked potentials in different stages of scoliosis surgery. Spine. 1999;24(17):1799–1804. doi: 10.1097/00007632-199909010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ku ASW, Hu Y, Irwin MG, et al. Effect of sevoflurane/nitrous oxide versus propofol anaesthesia on somatosensory evoked potential monitoring of the spinal cord during surgery to correct scoliosis. Br J Anaesth. 2002;88:502–507. doi: 10.1093/bja/88.4.502. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z. The effects of isoflurane and propofol on intraoperative neurophysiological monitoring during spinal surgery. J Clin Monit Comput. 2004;18:303–308. doi: 10.1007/s10877-005-5097-5. [DOI] [PubMed] [Google Scholar]
- 21.Pelosi L, Stevenson M, Hobbs GJ, et al. Intraoperative motor evoked potentials to transcranial electrical stimulation during two anaesthetic regimens. Clin Neurophysiol. 2001;112:1076–1087. doi: 10.1016/S1388-2457(01)00529-6. [DOI] [PubMed] [Google Scholar]
- 22.Sloan TO, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19(5):430–443. doi: 10.1097/00004691-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Wiedemayer H, Fauser B, Sandalcioglu IE, et al. The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg. 2002;96:255–262. doi: 10.3171/jns.2002.96.2.0255. [DOI] [PubMed] [Google Scholar]
- 24.Devlin VJ, Schwartz DM. Intraoperative neurophysiologic monitoring during spinal surgery. J Am Acad Orthop Surg. 2007;15:549–560. doi: 10.5435/00124635-200709000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440–2449. doi: 10.2106/JBJS.F.01476. [DOI] [PubMed] [Google Scholar]
- 26.Ofiram E, Lonstein JE, Skinner S, Perra JH. The disappearing evoked potentials: a special problem of positioning patients with skeletal dysplasia. Case report. Spine. 2006;31:E464–E470. doi: 10.1097/01.brs.0000222122.37415.4d. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz DM, Drummond DS, Hahn M, et al. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000;13(2):178–182. doi: 10.1097/00002517-200004000-00015. [DOI] [PubMed] [Google Scholar]