Abstract
The study design is retrospective. The aim is to describe our experience about the treatment of patients with neuromuscular scoliosis (NMS) using Cotrel–Dubousset instrumentation. Neuromuscular scoliosis are difficult deformities to treat. A careful assessment and an understanding of the primary disease and its prognosis are essential for planning treatment which is aimed at maximizing function. These patients may have pelvic obliquity, dislocation of the hip, limited balance or ability to sit, back pain, and, in some cases, a serious decrease in pulmonary function. Spinal deformity is difficult to control with a brace, and it may progress even after skeletal maturity has been reached. Surgery is the main stay of treatment for selected patients. The goals of surgery are to correct the deformity producing a balanced spine with a level pelvis and a solid spinal fusion to prevent or delay secondary respiratory complications. The instrumented spinal fusion (ISF) with second-generation instrumentation (e.g., Luque–Galveston and unit rod constructs), are until 1990s considered the gold standard surgical technique for neuromuscular scoliosis (NMS). Still in 2008 Tsirikos et al. said that “the Unit rod instrumentation is a common standard technique and the primary instrumentation system for the treatment of pediatric patients with cerebral palsy and neuromuscular scoliosis because it is simple to use, it is considerably less expensive than most other systems, and can achieve good deformity correction with a low loss of correction, as well as a low prevalence of associated complications and a low reoperation rate.” In spite of the Cotrel–Dubousset (CD) surgical technique, used since the beginning of the mid 1980s, being already considered the highest level achieved in correction of scoliosis by a posterior approach, Teli et al., in 2006, said that reports are lacking on the results of third-generation instrumentation for the treatment of NMS. Patients with neuromuscular disease and spinal deformity treated between 1984 and 2008 consecutively by the senior author (G.D.G.) with Cotrel–Dubousset instrumentation and minimum 36 months follow-up were reviewed, evaluating correction of coronal deformity, sagittal balance and pelvic obliquity, and rate of complications. 24 patients (Friedreich’s ataxia, 1; cerebral palsy, 14; muscular dystrophy, 2; polio, 2; syringomyelia, 3; spinal atrophy, 2) were included. According the evidence that the study period is too long (1984–2008) and that in more than 20 years many things changed in surgical strategy and techniques, all patients were divided in two groups: only hooks (8 patients) or hybrid construct (16 patients). Mean age was 18.1 years at surgery (range 11 years 7 months–max 31 years; in 17 cases the age at surgery time was between 10 and 20 years old; in 6 cases it was between 20 and 30 and only in 1 case was over 30 years old). Mean follow-up was 142 months (range 36–279). The most frequent patterns of scoliosis were thoracic (10 cases) and thoracolumbar (9 cases). In 8 cases we had hypokyphosis, in 6 normal kyphosis and in 9 hyperkyphosis. In 8 cases we had a normal lordosis, in 11 a hypolordosis and in 4 a hyperlordosis. In 1 case we had global T4–L4 kyphosis. In 8 cases there were also a thoracolumbar kyphosis (mean value 24°, min 20°–max 35°). The mean fusion area included 13 vertebrae (range 6–19); in 17 cases the upper end vertebra was over T4 and in 11 cases the lower end vertebra was over L4 or L5. In 7 cases the lower end vertebra was S1 to correct the pelvic obliquity. In 5 cases the severity of the deformity (mean Cobb’s angle 84.2°) imposed a preoperative halo traction treatment. There were 5 anteroposterior and 19 posterior-only procedures. In 10 cases, with low bone quality, the arthrodesis was performed using iliac grafting technique while in the other (14 cases) using autologous bone graft obtained in situ from vertebral arches and spinous processes (in all 7 cases with fusion extended until S1, it was augmented with calcium phosphate). The mean correction of coronal deformity and pelvic obliquity averaged, respectively, 57.2% (min 31.8%; max 84.8%) and 58.9% (mean value preoperative, 18.43°; mean value postoperative, 7.57°; mean value at last follow-up, 7.57°). The sagittal balance was always restored, reducing hypo or hyperkyphosis and hypo or hyperlordosis. Also in presence of a global kyphosis, we observed a very good restoration (preoperatory, 65°; postoperatory, 18° kyphosis and 30° lordosis, unmodified at last f.u.). The thoracolumbar kyphosis, when present (33.3% of our group) was always corrected to physiological values (mean 2°, min 0°–max 5°). The mean intraoperative blood lost were 2,100 cc (min 1,400, max 5,350). Major complications affected 8.3% of patients, and included 1 postoperative death and 1 deep infection. Minor complications affected none of patients. CD technique provides lasting correction of spinal deformity in patients with neuromuscular scoliosis, with a lower complications rate compared to reports on second-generation instrumented spinal fusion.
Keywords: Neuromuscular scoliosis, Cotrel–Dubousset, Spinal fusion
Introduction
Neuromuscular scoliosis includes a wide spectrum of spinal deformities resulting from neuropathic and myopathic disorders. Kyphoscoliosis, lumbar hyperlordosis, and pelvic obliquity are characteristic features of these deformities. The treatment of these disorders remains a challenge for the spinal surgeon. The main goals of treatment are to maintain a balanced posture, maintain pulmonary function, facilitate hygiene care, and prevent painful spinal degeneration. A wide variety of disorders can lead to neuromuscular scoliosis. The Scoliosis Research Society has divided neuromuscular scoliosis into 2 types—neuropathic and myopathic. Examples of neuropathic scoliosis include upper motor neuron diseases, such as cerebral palsy, syringomyelia, and spinal cord injury, as well as lower motor neuron lesions, such as poliomyelitis and spinal muscular atrophy. Examples of myopathic scoliosis include Duchenne and Becker muscular dystrophy, arthrogryposis, and other congenital myopathies.
This kind of deformity is difficult to treat. A careful assessment and an understanding of the primary disease and its prognosis are essential for planning treatment which is aimed at maximizing function. These patients may have pelvic obliquity, dislocation of the hip, limited balance or ability to sit, back pain, and, in some cases, a serious decrease in pulmonary function. Spinal deformity is difficult to control with a brace, and it may progress even after skeletal maturity has been reached. Surgery is the main stay of treatment for selected patients. The goals of surgery are to correct the deformity producing a balanced spine with a level pelvis and a solid spinal fusion to prevent or delay secondary respiratory complications. Segmental instrumented spinal fusion with second-generation instrumentation (Luque–Galveston and unit rod constructs) has been considered the standard surgical treatment of neuromuscular deformity from 1970s to 1990s, despite major complication rates reaching 40–50% in treated patients [1–3].
Third-generation systems, including Cotrel–Dubousset (CD) and similar designs, have changed the approach to the surgical treatment of both adolescent and adult idiopathic scoliosis. They have virtually eliminated postoperative casts or braces and lowered pseudarthrosis rates in idiopathic spinal deformity surgery. Nevertheless in 2006, almost 25 years after the introduction of CD, Teli et al. underline that “there are few papers on the results of third-generation instrumented spinal fusion for the treatment of neuromuscular scoliosis” and in the same period Tsirikos et al. (2008), analyzing 287 consecutive patients with neuromuscular spine deformity, said that “the unit rod instrumentation is a common standard technique and the primary instrumentation system for the treatment of pediatric patients with cerebral palsy and neuromuscular scoliosis because it is simple to use, it is considerably less expensive than most other systems, and can achieve good deformity correction with a low loss of correction, as well as a low prevalence of associated complications and a low reoperation rate” [2, 4–6].
A literature review undertaken on neuromuscular scoliosis through the MEDLINE database, using “neuromuscular scoliosis” as the primary descriptor and limiting the sources to articles published between 2000 and 2011 evidenced 391 hits but only 19 papers were about the use of third-generation instrumented spinal fusion with a minimum 3 years follow-up.
Therefore, the purpose of this study was to present a consecutive series of patients with NMS (neuromuscular scoliosis) who were treated with the Cotrel–Dubousset instrumentation at a single institution. The goal was to report the incidence of surgical complications, degree of deformity correction, reoperation rate and prevalence of pseudarthrosis.
Materials and methods
Patients with neuromuscular disease and spinal deformity treated between 1984 and 2008 consecutively by the senior author (G.D.G.) with Cotrel–Dubousset instrumentation and minimum 36 months follow-up were reviewed, evaluating correction of coronal deformity, sagittal balance and pelvic obliquity, and rate of complications. The coronal and sagittal curves were measured by the Cobb method on full spine sitting or standing (before surgery, supine traction/bending) radiographs. Pelvic obliquity was the angle described by a line tangential to both iliac crests, and a line perpendicular to the line intersecting the middle of the pedicles of L4 and L5 on the posteroanterior view [7]. Exclusion criteria were incomplete clinical and/or radiologic documentation at follow-up. According the evidence that the study period is too long (1984–2008) and that in more than 20 years many things changed in surgical strategy and techniques, all patients were divided in two groups: only hooks (8 patients) or hybrid construct (16 patients).
Table 1 summarizes the main data of the study: 24 patients (Friedreich’s ataxia, 1; cerebral palsy, 14; muscular dystrophy, 2; polio, 2; syringomyelia, 3; spinal atrophy, 2) were included. Mean age was 18.1 years at surgery (range 11 years 7 months–max 31 years; in 17 cases it was between 10 and 20 years old; in 6 cases it was between 20 and 30 and only in 1 case was over 30 years old). Mean follow-up was 142 months (range 36–279). The most frequent patterns of scoliosis were thoracic (10 cases) and thoracolumbar (9 cases). In 8 cases we had a hypokyphosis, in 6 a normal kyphosis and in 9 a hyperkyphosis. In 8 cases we had a normal lordosis, in 11 a hypolordosis and in 4 a hyperlordosis. In 1 case we have a global T4–L4 kyphosis. In 8 cases there were also a thoracolumbar kyphosis (mean value 24°, min 20–max 35). In 8 cases we used only hooks (Fig. 1), in 2 cases only screws and in 14 cases hybrid instrumentation with hooks and screws (Figs. 2, 3). The mean fusion area included 13 vertebrae (range 6–19); in 17 cases the upper end vertebra was over T4 and in 11 cases the lower end vertebra was over L4 or L5. In 7 cases the lower end vertebra was S1 to correct the pelvic obliquity (Figs. 2, 3). Indications for fusion to the pelvis by iliac screws were given to nonambulatory patients with sitting unbalance and to patients who had curves including the pelvis and/or fixed pelvic obliquity. In 5 cases the severity of the deformity (mean Cobb’s angle 84.2°) imposed a preoperative halo traction treatment. There were 5 anteroposterior and 19 posterior-only procedures.
Table 1.
General data of the study
| Patient | Age at surgery | Gender | Stat. no. | Pattern | Form | Halo | Surgery time | Anterior approach | Note | Fusion area | f.u. months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| V.W. | 18.9 | F | 233–86 | T | C.P. | No | 16/02/1987 | zielke T10/L3 + hooks T7/L4 | 1–3 | T7–L4 | 279 |
| I.G. | 17.3 | M | 669–87 | TL | D.M. | No | 10/02/1988 | 1 | T2–L4 | 269 | |
| C.E. | 31.0 | F | 575–88 | L | P. | No | 21/11/1988 | 1 | T12–L5 | 268 | |
| F.M.E. | 15.4 | F | 79–89 | T | A.S. | No | 09/01/1989 | 1 | T2–L3 | 267 | |
| C.L. | 13.9 | F | 80–89 | T | C.P. | No | 23/01/1989 | 1–2 | T2–T10 | 247 | |
| R.A.L. | 23.1 | F | 304–90 | TL | C.P. | No | 11/09/1990 | zielke L3/S1 + hooks T8/L5 | 1–3 | T8–S1 | 232 |
| M.S. | 13.5 | F | 572–88 | T | A.F. | No | 02/12/1991 | 1 | T3–L4 | 210 | |
| B.G. | 20.1 | F | 398–92 | T | P. | No | 08/10/1993 | 1 | T3–L4 | 185 | |
| L.M. | 13.1 | F | 665–95 | T | C.P. | No | 18/10/1995 | T1–S1 | 148 | ||
| M.M. | 17.1 | M | 388–98 | TL | D.D. | Yes | 22/10/1998 | T3–S1 | 132 | ||
| R.A. | 24.6 | F | 464–99 | TL | A.S. | No | 21/02/2000 | T7/L2 | C7–S1 | 116 | |
| L.M. | 18.9 | M | 294–01 | TL | C.P. | No | 04/07/2001 | 6 | T2–S1 | 112 | |
| L.S. | 15.6 | M | 69–01 | T + L | C.P. | No | 24/10/2001 | T1–L4 | 111 | ||
| C.M.R. | 14.1 | F | 321–01 | TL | C.P. | No | 28/11/2001 | T7–L4 | 108 | ||
| R.I.† | 16.9 | F | 177–00 | T + L | C.P. | No | 26/02/2002 | T2–L4 | –† | ||
| F.C. | 15.3 | F | 505–02 | TL | C.P. | No | 07/03/2003 | T1–L5 | 95 | ||
| B.A. | 19.1 | M | 184/02 | T | S. | Yes | 03/12/2003 | T3–T12 | T1–L4 | 86 | |
| S.M.V. | 11.7 | F | 47/03 | T | S. | Yes | 14/01/2004 | t5–t11 | T2–L1 | 85 | |
| C.M.G. | 21.9 | F | 712–04 | L + T | C.P. | Yes | 20/12/2004 | 4 | T1–S1 | 73 | |
| P.I. | 15.2 | F | 186–04 | T | C.P. | No | 01/01/2005 | T2–S1 | 73 | ||
| M.L. | 21.5 | F | 729–06 | TL | C.P. | Yes | 23/10/2006 | T1–L4 | 51 | ||
| C.G. | 24.3 | F | 263–98 | L | C.P. | No | 28/02/2007 | 5 | T9–L3 | 47 | |
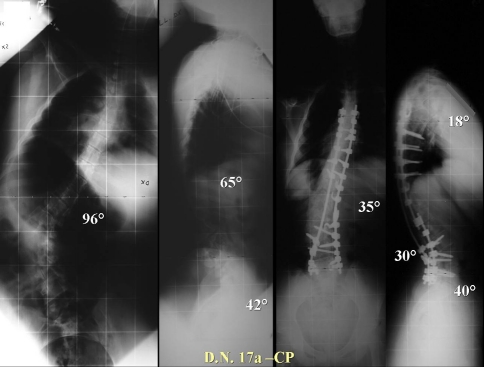
| D.N. | 17.0 | F | 125–07 | TL | C.P. | No | 26/07/2007 | T5–L3 | 42 | ||
| S.E. | 15.4 | F | 961/07 | T | S. | No | 07/01/2008 | T5–L1 | 36 |
1 only hooks, 2 in 1995 we would have had to extend caudally the fusion but we have not been able to make it for the worsening of the cardiological conditions, 3 Zielke + CD, 4 postoperative wound infection with removal of the instrumentation after 2 years, 5 postoperative hyperkyphosis, 6 Coffin Siris syndrome
† Died in the postoperative period
Fig. 1.
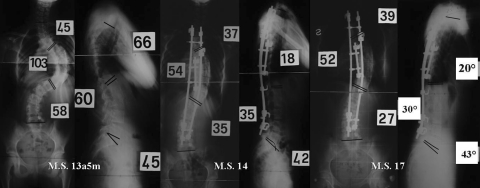
Only hooks implant in 13-year-old patient with Friedreich’s ataxia
Fig. 2.
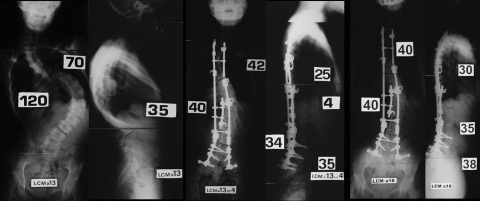
Hybrid CD implant until S1 in female patient with CP
Fig. 3.
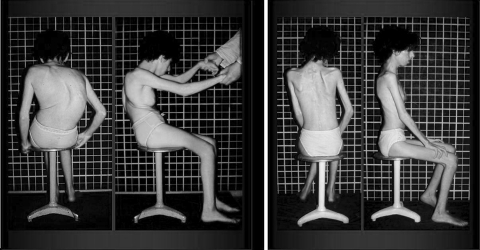
Preoperative and postoperative clinical images of the previous case
Indications for posterior-only instrumented spinal fusion were given to patients affected by flaccid NMS due to Duchenne muscular dystrophy (DMD), to those whose cardiopulmonary function was not deemed suitable for a double approach, and to those skeletally mature patients whose deformities were flexible (above 50% correction on traction/bending films). Indications for anteroposterior spinal fusion were given, with the above exceptions, to patients featuring rigid coronal curves (below 50% correction on traction/bending films) and/or fixed pelvic obliquity, deficiency of posterior elements, and skeletal immaturity. Anterior instrumentation was used to correct fixed thoracolumbar and lumbar coronal and/or sagittal deformities whenever bone was judged of sufficient quality by the surgeon.
In 10 cases, with low bone quality, the arthrodesis was performed using iliac grafting technique while in the other (14 cases) using autologous bone graft obtained in situ from vertebral arches and spinous processes (in all 7 cases with fusion extended until S1, it was augmented with calcium phosphate).
Clinical and radiologic outpatients’ follow-up was done at 3, 6, 12 months postoperatively and yearly thereafter with the modalities described above. Fusion and graft incorporation were routinely judged upon examination of posteroanterior and lateral radiographs. Oblique views and computed tomography scans were requested in cases of suspected pseudoarthrosis, loss of correction or implant failure.
Statistical analysis was performed by an SPSS program vers. 10 (Chicago, IL).
Results
Patients’ functional levels and etiologies of spinal deformity, according to WHO International classification of impairment, activity and participation, are displayed in Table 2 [8].
Table 2.
Patients’ functional level of spinal deformity according to World Health Organization-International classification of impairment, activity and participation. Geneva 2001
| Number of patients (n = 24) | |
|---|---|
| WHO functional level 1 (walkers without devices) | 3 |
| WHO functional level 2 (walkers with limitations in community walking) | 3 |
| WHO functional level 3 (walkers with mobility devices) | 8 |
| WHO functional level 4 (self-mobilized with either transport or power mobility outdoors) | 6 |
| WHO functional level 5 (self-mobilized with severe limitations) | 4 |
Tables 3 and 4 summarizes the main radiographic data of the study. The mean correction of coronal deformity and pelvic obliquity averaged, respectively, 57.2% (min 31.8%, max 84.8%, P < 0.05) and 58.9% (mean value preoperative, 18.43°; mean value postoperative, 7.57°; mean value at last follow-up, 7.57°, P < 0.05). After surgery, the average major curve correction was 58.4% (min 31.8%; max 84.8%, P < 0.05) in the hybrid group and 56% (min 34.1%; max 72.5%, P < 0.05) in the all-hooks group, even if the 31.8% value scored in the first group is connected to a wound infection that required removal of all metalwork at 23 months postoperatively with partial loss of correction (Fig. 4). No statistically significant difference about the major curve correction was according the different ages at surgery. The sagittal balance was always restored, reducing hypo or hyperkyphosis and hypo or hyperlordosis. Also in presence of a global kyphosis, we observed a very good restoration (preoperatory, 65°; postoperatory, 18° kyphosis and 30° lordosis, unmodified at last f.u. P < 0.05). The thoracolumbar kyphosis, when present (33.3% of our group) was always corrected to physiological values (mean 2°, min 0°–max 5°, P < 0.05).
Table 3.
Corrections of coronal deformity and pelvic obliquity
| Scoliosis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Curve | Pre | Bending test | Post | f.u. | Curve | Pre | Bending test | Post | f.u. | Curve | Pre | Bending test | Post | f.u. |
| V.W. | T4/T12 | 80 | 28 | 22 | 22 | X | X | X | X | X | X | X | X | X | X |
| I.G. | t7/l4 | 95 | 54 | 36 | 41 | X | X | X | X | X | X | X | X | X | X |
| C.E. | T11/L4 | 90 | 28 | 37 | 35 | X | X | X | X | X | X | X | X | X | X |
| F.M E. | T2/T6 | 45 | 24 | 26 | 27 | T7/T12 | 60 | 19 | 25 | 22 | L1/L4 | 48 | 4 | 16 | 15 |
| C.L. | T1/IL2 | 90 | 28 | 27 | 16 | X | X | X | X | X | X | X | X | X | X |
| R.A.L. | T10/L5 | 91 | 69 | 60 (30) | 38 | X | X | X | X | X | X | X | X | X | X |
| M.S. | T1/T5 | 45 | 43 | 37 | 39 | T6/T12 | 103 | 89 | 54 | 52 | L1/L5 | 58 | 38 | 35 | 27 |
| B.G. | T3/T10 | 100 | 68 | 60 | 62 | T11/L4 | 59 | 36 | 43 | 60 | X | X | X | X | X |
| L.M. | T1/T6 | 70 | 63 | 40 | 40 | T7/L3 | 120 | 70 | 40 | 40 | X | X | X | X | X |
| M.M. | T9/L4 | 75 | 68 | 36 | 36 | X | X | X | X | X | X | X | X | X | X |
| R.A. | T3/L3 | 63 | 56 | 39 | 39 | X | X | X | X | X | X | X | X | X | X |
| L.M. | T5/L4 | 64 | 15 | 36 | 34 | X | X | X | X | X | X | X | X | X | X |
| L.S. | T3/T11 | 79 | 60 | 43 | 48 | T11/L5 | 72 | 31 | 43 | 55 | X | X | X | X | X |
| C.M.R. | T9/L3 | 46 | 10 | 7 | 8 | X | X | X | X | X | X | X | X | X | X |
| R.I.† | T10/L4 | 67 | 53 | X | X | X | X | X | X | X | X | X | X | X | X |
| F.C. | T10/L3 | 93 | 72 | 56 | 41 | X | X | X | X | X | X | X | X | X | X |
| B.A. | T7/T12 | 81 | 45 | 42 | 43 | T12/L4 | 45 | 13 | 23 | 23 | X | X | X | X | X |
| S.M.V. | T4/T11 | 90 | 52 | 25 | 25 | T12/L5 | 55 | 2 | 27 | 25 | X | X | X | X | X |
| C.M.G. | T11/L5 | 66 | ? | 45 | 66 | T1/710 | 27 | ? | 22 | 22 | X | X | X | X | X |
| P.l. | T4/T11 | 85 | 47 | 33 | 33 | T12/L5 | 50 | 11 | 21 | 21 | X | X | X | X | X |
| M.L. | T4/T12 | 120 | 87 | 57 | 57 | L1/L5 | 65 | 19 | 30 | 30 | X | X | X | X | X |
| C.G. | T11/L3 | 58 | 15 | 13 | 13 | X | X | X | X | X | X | X | X | X | X |
| D.N. | T11/L4 | 96 | 62 | 35 | 38 | X | X | X | X | X | X | X | X | X | X |
| S.E. | T8/T12 | 50 | 11 | 19 | 35 | T3/T7 | 35 | X | 20 | 25 | L1/L4 | 30 | 3 | 3 | 3 |
† Died in the postoperative period
Table 4.
Corrections of sagittal curves
| Patient | Kyphosis | Lordosis | Sacral slope | Pelvic obliquity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | f.u. | Pre | Post | f.u. | Pre | Post | f.u | Pre | Post | f.u. | |
| V.W. | 42 | 40 (20) | 25 | 50 | 46 | 37 | 31 | 25 | 25 | X | X | X |
| I.G. | 12 | 3 | 3 | 15 | 42 | 38 | 27 | 45 | 45 | X | X | X |
| C.E. | 37 | 18 | 18 | 35 | 28 | 28 | 35 | 50 | 50 | X | X | X |
| F.M.E. | 25 | 27 | 34 | 80 | 52 | 65 | 46 | 44 | 60 | X | X | X |
| C.L. | 75 | 27 | 32 | 51 | 45 | 36 | 21 | 50 | 52 | X | X | X |
| R.A.L. | 30 | 34 | 27 | 40 | 20 | 20 | 28 | 30 | 41 | 28 | 12 | 12 |
| M.S. | 66 | 18 | 10 | 60 | 35 | 20 | 45 | 42 | 23 | X | X | X |
| B.G. | 47 | 41 | 25 | 51 | 44 | 40 | 38 | 34 | 35 | X | X | X |
| L.M. | 35 | 25 | 30 | 43 | 34 | 35 | 50 | 35 | 38 | 16 | 6 | 6 |
| M./M. | 6 | 4 | 2 | 3 | 28 | 30 | 25 | 47 | 45 | 26 | 26 | 8 |
| R.A. | 72 | 79 | 79 | 66 | 53 | 53 | 39 | 40 | 40 | 20 | 8 | 8 |
| L.M. | 18 | 10 | 10 | 35 | 30 | 30 | 47 | 47 | 47 | 8 | 5 | 5 |
| L.S. | 12 | 3 | 3 | 15 | 42 | 42 | 57 | 52 | 52 | X | X | X |
| C.M.R. | 1 | −6 | −6 | 20 | 38 | 38 | 40 | 40 | 40 | X | X | X |
| R.I.† | X | X | X | X | X | X | X | X | X | X | X | X |
| F.C. | 65 CT | 40 | 40 | 11 | 30 | 30 | 40 | 40 | 40 | X | X | X |
| B.A. | 69 | 49 | 53 | 53 | 48 | 46 | 47 | 49 | 40 | X | X | X |
| S.M.V. | 10 | 30 | 30 | 45 | 52 | 52 | 47 | 45 | 45 | X | X | X |
| C.M.G. | 60 | 53 | 57 | 20 | 58 | 42 | 60 | 53 | 40 | 14 | 6 | 6 |
| P.I. | 13 | 32 | 32 | 42 | 55 | 55 | 48 | 40 | 40 | 17 | 8 | 8 |
| M.L. | 65 | 28 | 28 | 62 | 55 | 55 | 50 | 53 | 53 | X | X | X |
| C.G. | 30 | 51 | 80 | 57 | 45 | 58 | 37 | 32 | 40 | X | X | X |
| D.N. | 65 CT | 18 | 18 | 15 | 30 | 30 | 42 | 40 | 40 | X | X | X |
| S.E | 17 | 25 | 25 | 31 | 53 | 53 | 47 | 40 | 40 | X | X | X |
† Died in the postoperative period
Fig. 4.
Female with CP. Deep wound infection with associated pseudoarthrosis that required removal of all metalwork at 23 months postoperatively with partial loss of correction
Hypotensive anesthesia and autologous blood retrieval via cell-saver were used in all patients. Average preoperative hematocrit was 39.4% (range 30.3–47.7%, P < 0.05). The mean intraoperative blood lost were 2,100 cc (min 1,400, max 5,350, P < 0.05) and the average operative time was 552 min (range 315–685 min).
No statistically significant difference about intraoperative blood lost and operative time were according the different ages at surgery. The average hospital stay was 25 days (range 12–65 days), and the median stay was 18 days. The average hospital stay for patients undergoing posterior versus anteroposterior spinal fusion was 20 days (range 14–71 days) versus 40 days (range 24–65 days), respectively, with a median hospital stay of 16 days versus 32 days, respectively.
Major complications affected 8.3% of patients, and included 1 postoperative death, 1 failure due to a wrong choice of the fusion area that required a caudal extension of the arthrodesis 6 years later, and 1 deep wound infection with associated pseudoarthrosis that required removal of all metalwork at 23 months postoperatively with partial loss of correction. Minor complications affected none of patients (Fig. 4).
Discussion
The surgical management of neuromuscular scoliosis has often, in the past, been looked at with trepidation because of potentially lethal complications. Surgical procedures are difficult and not free of complications. With the introduction of segmental spinal instrumentation and improved postoperative care facilities, surgery is now considerably safer, and results are better and far more consistent. The deformity frequently presents as a long “C” shaped progressive curve incorporating pelvic obliquity and the management is complicated by numerous medical co-morbidities including poor bone quality especially in the pelvis. Other concerns include mobility and seating issues, skin condition, nutritional and elimination problems and respiratory and cardiac dysfunction. These issues must all be carefully considered in the preoperative evaluation of the patient as well as later in the course of treatment. They ideally involve a multi-disciplinary team approach for effective management. The indication for surgery is a deformity that is causing functional problems, such as loss of sitting balance, difficulties with elimination, impingement of the rib cage on the pelvis or a progressive deformity that can be reasonably anticipated to cause such problems. Extensive surgery is often required to improve the deformity and to stabilize the vertebral column to the pelvis. Fixation to the lumbo-sacral junction remains a surgical challenge.
In this study, in stiff scoliosis, an additional anterior multilevel discectomies were performed to maximize the flexibility of the curve; anterior and posterior approaches can be performed under 1 or 2 anaesthetic sessions. These combined procedures can achieve better curvature correction, decrease the incidence of pseudarthrosis, and prevent recurrent deformity through a circumferential fusion. However, the one-stage anterior–posterior procedures are associated with increased morbidity, a higher incidence of technical complications and a higher perioperative mortality rate. Therefore, patients with very severe deformities in the presence of concomitant medical problems, may have a lower risk of complications if the anterior surgery is performed a 7–10 days before the posterior procedure. The long-term outcome of spine deformity correction in NMS remains debatable.
Segmental instrumented spinal fusion with second-generation instrumentation (Luque–Galveston and unit rod constructs) has been considered the standard surgical treatment of neuromuscular deformity until 1990s, despite major complication rates reaching 40–50% in treated patients. Wimmer and colleagues reviewed their experience with the Luque–Galveston and the Isola instrumentations in 52 patients. They found comparable results in terms of correction. The Luque group had a scoliosis correction of 54 and a 64% correction in pelvic tilt, while the Isola group had correction of 57 and 63% improvement in pelvic tilt. They found greater correction in smaller curves (<60°) than in larger curves (>100°) in both groups [9].
Gaine and colleagues [10] have also reported similar corrections with the Luque-unit rod and Isola instrumentations in patients with DMD.
Vialle and colleagues [11], using a hybrid system, reported a mean scoliosis correction of 62% in patients with CP.
Westerlund and colleagues [12] reported a final correction of 66% using Luque–Galveston instrumentation.
Onimus in 1992 [13] reported comparable correction with Cotrel–Dubousset instrumentation and pelvic fixation with iliosacral screws underling that Cotrel–Dubousset (CD) and similar designs instrumentations, have changed the approach to the surgical treatment of all kind of scoliosis. They have virtually eliminated postoperative casts or braces and lowered pseudarthrosis rates in neuromuscular spinal deformity surgery.
We also achieved 57.2% improvement in Cobb’s angle and 58.9% correction in pelvic obliquity, which is similar to published reports; our pseudoarthrosis rate (4.1%; 1 of 24 patients) detected at an average follow-up of 142 months (range 36–279), in this series, is in the lower range compared with previous reports on second-generation ISF, possibly due to the increased stability provided by third-generation instrumentation.
Teli and colleagues reviewed 56 patients operated using hybrid pedicle screw and hook systems. The pseudoarthrosis rate was 1.8%. That compares to rates varying from 1.5 to 10% for the unit rod and Luque–Galveston systems. The loss of correction of scoliosis, kyphosis, and lordosis in their series was 0–6% at follow-up. However, the loss of lordosis was more in patients who had posterior-only surgery [14].
In NMS like in idiopathic scoliosis, the use of pedicle screw in lumbar curves allows to obtain better correction results versus conventional hook instrumentation and using also thoracic pedicle screws is possible to obtain also a shorter fusion length in thoracic curves.
Pedicle screws have the disadvantage of limited purchase in an osteoporotic spine. Larger diameters should be used and, preferably, all levels should be instrumented, which allows greater points of fixation as well as application of greater corrective forces, as underlined in Figs. 5, 6. Pedicle screws also provide three-column fixation, which helps achieve greater correction of rotation. In our experience, halo traction is poorly tolerated and not so useful.
Fig. 5.
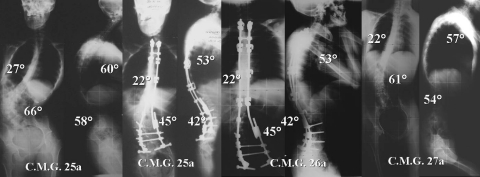
Mainly pedicular screws implant in 17-year-old patient with CP. Thoracic pedicle screws allow to obtain better correction results
Fig. 6.
Preoperative and postoperative clinical images of the previous case. Notice the residual imbalance
Sarwahi, Sarwark and colleagues, in comparing children with neuromuscular to idiopathic scoliosis, found frequent problems with the development of postoperative pneumonia (3.5 vs. 0.7%), respiratory failure (24.1 vs. 9.2%), urinary tract infections (5.3 vs. 0.7%), and surgical wound infections (1.3 vs. 0.3%). Complications of scoliosis surgery in children with neuromuscular scoliosis also include implant failure, gastrointestinal complications, and death. The complication rates vary from 44 to 80%, with a perioperative death rate of 0–7% [15, 16].
Szoke and colleagues [17] described an 8.7% rate of postoperative wound infection; 7 of 15 were deep infections with 1 late infection that needed implant removal.
In our series major complications affected 8.3% of patients, and included 1 postoperative death, 1 deep wound infection with associated pseudoarthrosis, that required removal of all the instrumentation with lost of the correction, and 1 implant failure due to a wrong choice of the fusion area.
Our study shows that children with neuromuscular scoliosis have greater intraoperative blood loss than children with idiopathic scoliosis. Blood loss is correlated with the number of levels operated and the degree of pelvic obliquity. Multiple reasons are attributed by other studies to this increased bleeding, including poor nutritional status, venous pooling, impaired connective tissue function, and antiseizure medication, such as valproic acid which is known to decrease platelet count and factor VIII levels [18, 19].
Brenn and colleagues studied 17 patients with cerebral palsy and compared them with 17 patients with idiopathic scoliosis. They found that children with cerebral palsy develop significant alterations in coagulation parameters early, although they had normal coagulation profiles. The baseline prothrombin time and partial prothrombin time, although within normal limits, were significantly higher in patients with neuromuscular scoliosis than in patients with idiopathic scoliosis. After 15% loss of blood volume, investigators found differences between prothrombin time, partial prothrombin time, maximum amplitude on thromboelastography, ionized calcium, and serum magnesium levels. Their report indicated that increased bleeding caused an apparent coagulopathy instead of transient hypercoagulable state seen in normal states. Children with cerebral palsy have increased bleeding that starts earlier in the procedure despite a normal coagulation profile [20].
In our series average preoperative hematocrit was 39.4% (range 30.3–47.7%, P < 0.05). The mean intraoperative blood lost were 2,100 cc (min 1,400, max 5,350, P < 0.05) and the average operative time was 552 min (range 315–685 min).
Intraoperative complications related to pelvic fixation of the rod occurred in five patients. According to literature preoperative lumbar hyperlordosis was correlated with increased morbidity and a greater incidence of technical complications associated with the pelvic fixation.
Our present study supports these findings and we find using pedicle screw instrumentation could also be helpful obtaining better coronal as well as sagittal balance.
Limitations of the present study include the retrospective design and absence of an internal control group treated with second-generation implants, making comparison with other investigators’ series of patients an absolute necessity from the point of view of operating time, blood loss, hospital stay, deformity correction, and complications.
Conclusion
The correction of severe and complex spinal deformity in patients with neuromuscular scoliosis has always been challenging and not without complications. Obtaining adequate fixation with the Luque–Galveston technique or unit rod technique can be difficult in patients with neuromuscular scoliosis who often have a porotic bone. These difficulties can be minimized by CD instrumentation that appears to provide good correction of deformity along with maintenance of correction at follow-up. Implant loosening has not been seen in our series and this may well be due to the increased construct stiffness produced by pedicle screws. The results from this study support our view that the CD technique is able to offer NMS patients improved quality of life through satisfactory and lasting correction of their spinal deformity, at the expense of a lower complication rate than that generally reported for second-generation segmental instrumented spinal fusion.
Conflict of interest
None.
References
- 1.Benson ER, Thomson JD, Smith BG, et al. Results and morbidity in a consecutive series of patients undergoing spinal fusion for neuromuscular scoliosis. Spine. 1998;23:2308–2318. doi: 10.1097/00007632-199811010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Teli M, Cinnella P, Vincitorio F, Lovi A, Grava G, Brayda-Bruno M. Spinal fusion with Cotrel–Dubousset instrumentation for neuropathic scoliosis in patients with cerebral palsy. Spine. 2006;14:E441–E447. doi: 10.1097/01.brs.0000221986.07992.fb. [DOI] [PubMed] [Google Scholar]
- 3.Drummond DS. Neuromuscular scoliosis: recent concepts. J Pediatr Orthop. 1996;16:281–283. doi: 10.1097/01241398-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Neustadt JB, Shufflebarger HL, Cammisa FP. Spinal fusions to the pelvis augmented by Cotrel–Dubousset instrumentation for neuromuscular scoliosis. J Pediatr Orthop. 1992;12:465–469. doi: 10.1097/01241398-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Miladi LT, Zeller RD. Iliosacral screw fixation for pelvic obliquity in neuromuscular scoliosis: a long term follow-up study. Spine. 1997;22:1722–1729. doi: 10.1097/00007632-199708010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Tsirikos AI, Lipton G, Chang WN, Dabney KW, Miller F. Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine. 2008;33(10):1133–1140. doi: 10.1097/BRS.0b013e31816f63cf. [DOI] [PubMed] [Google Scholar]
- 7.Alman BA, Kim HK (1999) Pelvic obliquity after fusion of the spine in Duchenne muscular dystrophy. J Bone Jt Surg 81-B:821–824 [DOI] [PubMed]
- 8.International classification of impairment, activity and participation. Geneva: World Health Organization; 2001. [Google Scholar]
- 9.Wimmer C, Wallnöfer P, Walochnik N, et al. Comparative evaluation of Luque and Isola instrumentation for treatment of neuromuscular scoliosis. Clin Orthop Relat Res. 2005;439:181–192. doi: 10.1097/01.blo.0000173252.95130.cb. [DOI] [PubMed] [Google Scholar]
- 10.Gaine WL, Lim J, Stephenson W, et al. Progression of scoliosis after spinal fusion in Duchenne’s muscular dystrophy. J Bone Jt Surg. 2004;86B:550–555. [PubMed] [Google Scholar]
- 11.Vialle R, Delecourt C, Morin C. Surgical treatment of scoliosis with pelvic obliquity in cerebral palsy. The influence of intraoperative traction. Spine. 2006;31(13):1461–1466. doi: 10.1097/01.brs.0000219874.46680.87. [DOI] [PubMed] [Google Scholar]
- 12.Westerlund LE, Gill SS, Jarosz TS, et al. Posterior only unit rod instrumentation and fusion for neuromuscular scoliosis. Spine. 2001;26(18):1984–1989. doi: 10.1097/00007632-200109150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Onimus M, Manzone P, Lornet JM, et al. Surgical treatment of scoliosis in bed-ridden patients with cerebral palsy. Rev Chir Orthop Reparatrice Appar Mot. 1992;78:312–318. [PubMed] [Google Scholar]
- 14.Teli M, Elsebaie H, Biant L, Noordeen H. Neuromuscular scoliosis treated by segmental third-generation instrumented spinal fusion. J Spinal Disord Tech. 2005;18:430–438. doi: 10.1097/01.bsd.0000171873.99803.9f. [DOI] [PubMed] [Google Scholar]
- 15.Sarwahi V, Sarwark JF, Schafer MF, et al. Standards in anterior spine surgery in pediatric patients with neuromuscular scoliosis. J Pediatr Orthop. 2001;21:756–760. doi: 10.1097/00004694-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Tsirikos AI, Chang W, Dabney K, et al. Life expectancy in pediatric patients with cerebral palsy and neuromuscular scoliosis who underwent spinal fusion. Dev Med Child Neurol. 2003;45:677–682. doi: 10.1111/j.1469-8749.2003.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 17.Szoke G, Lipton G, Miller F, et al. Wound infection after spinal fusion in children with cerebral palsy. J Pediatr Orthop. 1998;18(6):727–733. doi: 10.1097/00004694-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Chambers HG, Weinstein CH, Mubarak SJ, et al. The effect of valproic acid on blood loss in patients with cerebral palsy. J Pediatr Orthop. 1999;19:792–795. doi: 10.1097/00004694-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Winter SL, Kriel RL, Novacheck TF, et al. Perioperative blood loss: the effect of valproate. Pediatr Neurol. 1996;15:19–22. doi: 10.1016/0887-8994(96)00124-5. [DOI] [PubMed] [Google Scholar]
- 20.Brenn BR, Theroux MC, Dabney KW, et al. Clotting parameters and thromboelastography in children with neuromuscular and idiopathic scoliosis undergoing posterior spinal fusion. Spine. 2004;29(15):E310–E314. doi: 10.1097/01.BRS.0000132513.88038.64. [DOI] [PubMed] [Google Scholar]