Abstract
Interspinous devices (IDs) were introduced in the 90s. Since then, they have rapidly become very popular for the minimally invasive treatment of lumbar pain disorders. They feature different shapes and biomechanical characteristics, and are used in the spine degenerative pathologies or as motion segment stabilizers (dynamic stabilization) or to obtain the decompression of neurological structures. The indications seem to be rather narrow and still to be verified in terms of their clinical efficacy. However, IDs are being extensively utilized beyond their classical indications with the inevitable risk of a clinical failure. The aim of the present work was to carry out a critical analysis of the causes of failure in a series of 19 patients. From January 2007 to March 2009, 19 patients with residual painful syndrome after the implantation of IDs were observed. The series includes 11 males and 8 females with a mean age of 53.6 years (range 38–84 years) who were operated on elsewhere and who underwent revision surgery at our hospital. The inclusion criteria were low back pain and/or radiculopathy after the device implantation without improvement of the painful symptomatology, radiculopathy with signs of sensory and motor deficit, intermittent neurogenic claudication, and infection. All patients were thoroughly re-assessed with new standard imaging examinations such as MRI and CT scans, considering the following image features: the position of the device with respect to the spinous processes (X-ray), the intervertebral disc disease of the level operated upon or of the adjacent levels (MRI), the segmental instability (dynamic X-rays), the severity of the canal stenosis (CT). The accurate evaluation of the clinical and imaging parameters revealed three main causes of failure: errors of indication, technical errors and the structural failure of the ID. The most frequent cause of failure was a wrong indication. The results of the study are presented and the causes of failure are discussed in detail.
Keywords: Lumbar interspinous devices, Failure, Lumbar spine stenosis
Introduction
The generic term “interspinous device” (ID) identifies a category of medical devices specifically designed to treat some painful diseases of the lumbar spine using a minimally invasive approach. The original idea was introduced by Senegas et al. [1]. He conceived a device to be implanted between two contiguous lumbar spinous processes specifically designed to act as a stabilizer. According to his theoretical assumption, the device was able to restore the physiologic mechanical conditions of a segment of motion affected by degenerative instability. The device has a central hard core that restricts the extension, while tension bands secured to the spinous processes limit the flexion of the spine. Following the first prototype of Senegas (first-generation Wallis implant), many other devices have been developed with different biomechanical characteristics [2–4]. Some of them are mere spacers able to induce an indirect decompression of neurological structures (dural sac and nerve roots) by means of distraction of the spinous processes. The possibility of treating lumbar spine pain syndromes with a minimally invasive approach has attracted the increasing attention of the scientific community, thus leading to a rapid and huge increase in the number of surgical procedures performed daily in the world.
As generally occurs with any new technique, the early contagious enthusiasm—resulting in an excessive and sometimes incorrect use of the device—has resulted in a rising number of failures and in a critical consideration about the indications and the true advantages of the technique.
The aim of the present work is to carry out a critical analysis of the causes of failure of the IDs in a series of 19 patients who were treated elsewhere and who underwent revision surgery at the spine Surgery Center of the Catholic University Hospital of Rome between 2007 and 2009.
Materials and methods
From January 2007 to March 2009, we observed 19 patients with a residual painful syndrome after implantation of IDs performed in other hospitals. The series includes 11 males and 8 females with a mean age of 53.6 years (range 38–84 years). The inclusion criteria were low back pain and/or radiculopathy after the device implantation without improvement of the painful symptomatology, radiculopathy with signs of sensory and motor deficit, intermittent neurogenic claudication, and infection. All patients were completely revalued with new standard imaging examinations such as MRI and CT scans. The following image features were considered: position of the device with respect to the spinous processes (X-ray), intervertebral disc disease of the level operated upon or of the adjacent levels (MRI), segmental instability (dynamic X-rays), and severity of canal stenosis (CT). The series was subdivided according to the preoperative diagnosis and to the type of the device as reported in Table 1. Twenty-six devices were used in 19 patients; two devices were implanted in 5 patients, two of which of the same type and three of different type. Only one patient received three devices of the same type (X-Stop). The implanted devices were 11 X-Stop, 5 DIAM, 3 U-Coflex, 2 BacJak, 2 Wallis, 1 Aperius, 1 Viking, 1 Superion. Based on the patients’ clinical history, we identified three groups of patients, those suffering from low back pain (7 pts), low back pain and radiculopathy (8 pts) and those with symptoms of neurogenic claudication (4 pts). The preoperative diagnosis was lumbar stenosis in 11 patients and intervertebral disc hernia in 4 patients. All patients but two (no. 10 and 13)—who refused the procedure—were re-operated (Table 1). The device was removed in sixteen patients. Four underwent only to the removal of the implant without other surgical procedure. Lumbar canal decompression was conducted in two patients and canal decompression and lumbar fusion as an additional procedure in another two. Fusion without decompression was performed in four patients, removal of the device and the intervertebral disc hernia resection was conducted in three patients and finally, removal of the device, neurological decompression, removal of intervertebral disc hernia, and fusion of the spine were simultaneously carried out during the same procedure in one patient. The device was left in situ at a segment below the adjacent fused levels in one patient.
Table 1.
Patients’ clinical data
| Sex | Age (years) | Type | Level | Cause of failure | Surgical treatment | Preoperative syndrome/diagnosis | |
|---|---|---|---|---|---|---|---|
| 1 | M | 84 | 2 DIAM | L3–L4 L4–L5 | IE | DR + D | LBP + NC/LS |
| 2 | F | 79 | 2 X-Stop | L3–L4 L4–L5 | IE | DR + D | LBP + NC/LS |
| 3 | M | 47 | 1 Wallis 1 BacJak |
L3–L4 L4–L5 | IE | DR + D + F | LBP + NC/LS |
| 4 | M | 52 | Superion | L4–L5 | IE | DR + D + F | LBP + NC/LS |
| 5 | M | 45 | X-Stop | L4–L5 | IE | DR + HR | LBP + R |
| 6 | F | 51 | U-Coflex | L4–L5 | IE | DR + HR | LBP + R |
| 7 | M | 43 | DIAM | L4–L5 | IE | DR + F | LBP |
| 8 | M | 54 | U-Coflex | L4–L5 | IE | DR | LBP |
| 9 | F | 62 | X-Stop (3) | L2–L3 L3–L4 L4–L5 | IE | DR + F | LBP/AISA + LS |
| 10 | F | 73 | Wallis X-Stop |
L4–L5 L5–S1 |
IE | MT | LBP/AISA + LS |
| 11 | F | 67 | 1 DIAM/1 BacJak | L3–L4 L4–L5 |
I | DR | LBP + R/HDD |
| 12 | M | 43 | X-Stop | L5–S1 | IE + I | DR | LBP + R/HDD |
| 13 | M | 42 | X-Stop | L5–S1 | IE + I | MT | LBP + R/HDD |
| 14 | F | 59 | Aperius | L4–L5 | EI + TE + WL | DR + D + HR + F | LBP + R/LS |
| 15 | M | 51 | U-Coflex | L4–L5 | IE + WL | D + DS | LBP + R/HDD |
| 16 | M | 39 | X-Stop | L4–L5 | TE | DR | LBP/LS |
| 17 | F | 44 | X-Stop | L4–L5 | IE + TE | DR + HR | LBP/LS |
| 18 | M | 38 | Viking | L4–L5 | IE + WL + DB | DR + F | LBP + R/LS |
| 19 | F | 46 | DIAM | L4–L5 | DB | DR + F | LBP/LS |
D decompression, DB device breakage, DR device removal, DS dynamic stabilization, F fusion, HDD herniated disc disease, HR hernia removal, I infection, IE indication error, LBP low back pain, LS lumbar stenosis, MT medical therapy, NC neurogenic claudication, R radiculopathy, TE technique error, WL wrong level
Results
The analysis of our series allowed us to identify three major causes of failure of IDs: indication errors, technical errors, and failure of the implanted device. In some cases, more than one cause was recognizable as responsible of ID failure (no. 12, 13, 14, 15, 17, and 18).
Indication errors
Wrong indications may be explained by the following considerations: the devices belong to two major types with different biomechanical behaviour. They can be classified as semi-constrained—with bands that have to be looped and tensioned around the adjacent spinous processes—and as non-constrained. The first type of devices is designed to reduce the mobility of the instrumented segment in order to restore stiffness of the unstable degenerated segments. Therefore, the so-called “dynamic stabilization” is intended to relieve instability-induced low back pain, thus postponing the need for more invasive surgical treatments [1]. The second type of device, without tension bands, is unable to influence the flexion of the spine in anyway. But, it significantly limits the extension of the segment of motion. This type of device is mainly used to expand the intervertebral space, stretch the ligamenta flava and the posterior fibres of the anulus fibrosus thus enlarging both the central canal and the neuroforamina and indirectly decompressing neurological structures [5]. Therefore, the best indication for this device seems to be radiculopathy and neurogenic claudication due to lumbar spine degenerated disc disease (DDD), which improves with lumbar spine flexion and deteriorates with lumbar spine extension. The encouraging initial results of these simple minimally invasive techniques have led to expand the number of indications to other lumbar painful conditions such as herniated disc disease treated conservatively or surgically, degenerative spondylolisthesis, severe stenosis and low back pain without neurologic symptoms. Based on the previous considerations, we have identified the following errors of indication.
Severe lumbar stenosis
We observed four cases of severe stenosis implanted with the device without neurological decompression. Two devices were implanted at contiguous levels in three patients. In two, very old patients (no. 1, and 2), 84- and 79-year-old, respectively, devices of the same type were used (DIAM and X-Stop, respectively) while in another patient (no. 3) the devices implanted were of a different type (Wallis at the level L3–L4 and BackJack at L4–L5). The fourth patient (no. 4) was implanted with a Superion at L4–L5. All patients were affected by a severe neurogenic claudication that did not improve after the procedure. One patient (no. 4) developed a painful scoliosis soon after the operation. In the older patients, the IDs were removed and decompressive laminectomy without stabilisation was carried out with a satisfactory improvement of the walking capacity. In the younger patients, a stabilisation of the spine was performed in addition to the removal of the devices and to decompressive laminectomy so as to prevent the deterioration of painful lumbar instability.
Herniated disc disease
Patients (no. 5, 6) were affected by sciatica due L4–L5 herniated disc disease. The patients were implanted with the X-Stop and U-Coflex devices, respectively, without removal of the herniated disc. They did not improve after the operation and therefore required a new surgical procedure in order to remove the herniated disc.
Error of diagnosis
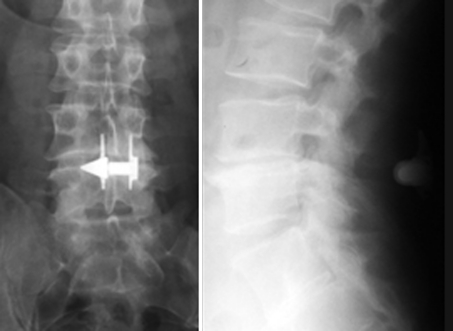
In our series, we observed some cases of wrong indication due to the misdiagnosed aetiology of the painful syndrome. Two patients were affected by HDD at a different level with respect to the one where the device was implanted. In one case (no. 17), the device was implanted at L4–L5 while the HDD was at the level of L5–S1; in the other, the implanted level was L4–L5 while the HDD was at L2–L3 (no. 14). In another patient (no. 15), the device was implanted in L4–L5 after removing a calcified hernia. After a very short period of relief from symptoms, a recurrence occurred and the patient was re-operated upon at the same level and at the one above (L3–L4) without any improvement. One year later, a CT investigation clearly showed the presence of degenerated disc disease at two more cephalic levels. At L2–L3 and L3–L4, two large and largely calcified medial masses protruded from the disc space and caused cauda equine compression (Fig. 1). The revaluation of the patient’s MRI before and after device implantation showed that the disc protrusions were already evident, although the real size and the extension of the masses were difficult to identify because of the low capacity of MRI to perfectly visualize the calcified tissues. On the basis of the CT scan, the decompression and the rigid stabilisation of L2–L3, L3–L4 was carried out without removal of the ID in L4–L5.
Fig. 1.
(Case no. 15) The CT scan performed after implantation of an interspinous device shows wide calcified masses above the instrumented level responsible for the narrowing of the vertebral canal and for the compression of the cauda equina nerve roots
Degenerative disc disease
Two cases were treated for low back pain syndrome without radiating pain to the leg (no. 7, 8). In one of the two patients, an additional nucleoplasty was performed during the same operative session. None of them improved after 6 months of medical therapy and physical rehabilitation.
Scoliosis
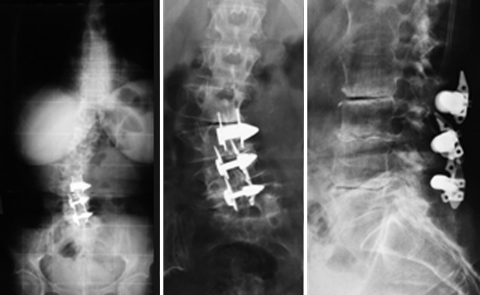
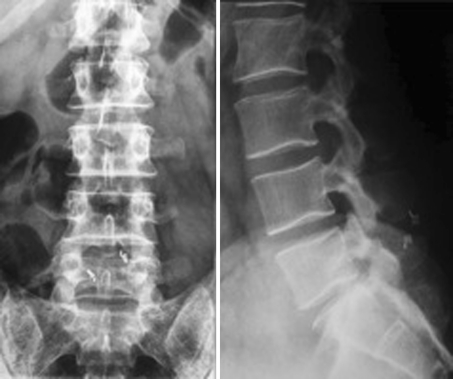
In our series, we observed two patients who were suffering from low back pain due to adult scoliosis (no. 9, 10) treated with ID implantation. In one patient, three X-Stops were positioned at three consecutive levels from L2–L3 to L4–L5 (Fig. 2); in the other patient, the X-Stop at L5–S1 and the Wallis at L4–L5 were used. The comparison of the preoperative and post-operative X-rays did not show any changes as to the sagittal and coronal balance or the Cobb angle. The patient did not experience any pain relief and improvement of claudication after the device implantation.
Fig. 2.
(Case no. 9) Three interspinous devices were implanted at the level of L2–L3, L3–L4 and L4–L5 in a painful adult scoliosis. The upright position X-ray of the spine shows the coronal misalignment of the spine (left) while standard images of the lumbar tract allow for a better visualization of the degenerative features typical of adult degenerative scoliosis at the instrumented levels (middle, right)
Infection and choice of the wrong level
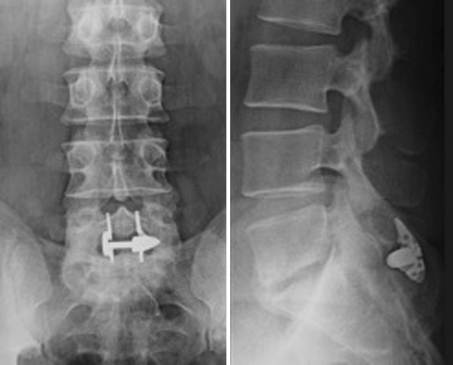
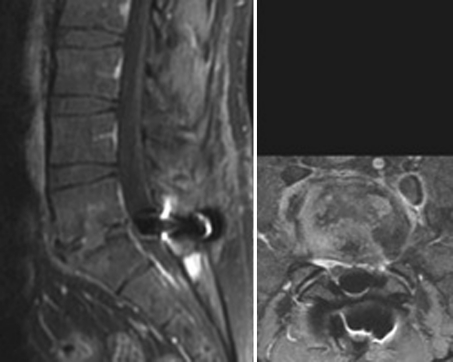
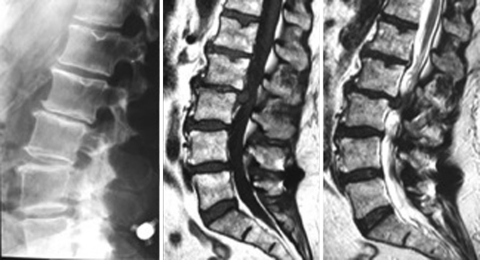
Another two important aspects are the increasing number of devices implanted in case of HDD as an additional procedure and the use of the device at the level L5–S1. With regard to the first aspect, many surgeons use an ID at the level affected by herniated disc disease with the aim to prevent the recurrence of the hernia. They think that the restriction of motion at the affected level together with the unloading effect of the spacer can avoid or at least reduce the incidence of recurrences. Considering now the implantation of the device at L5–S1, this segment is generally reported as not suitable for this purpose, because of anatomical constraints (frequent absence of an adequate spinous process of the sacrum) and of its particular biomechanical behaviour. Although we do not agree with the indications suggested above, we decided to leave aside the patients implanted following the removal of the hernia without complications; instead, we considered every case of complications. We observed three cases (no. 11, 12, and 13) who developed discitis after the hernia removal and the implantation of the device at the same level. In one case (no. 11) the discitis developed in the L3–L4 intervertebral disc and two different devices were implanted. The DIAM was used at L3–L4 and the BackJack at the level below. In another two cases (n. 12, 13), the infected level was L5–S1 where the X-Stop had been used (Fig. 3, 4). In two patients, the disc curettage and the device removal were performed; the third patient refused the operation and underwent a prolonged antibiotic therapy and cast immobilization.
Fig. 3.
(Case no. 12) A 43-year-old man with herniated disc disease of the L5–S1 intervertebral disc, who underwent removal of the hernia and implantation of an interspinous device at the same level as an additional procedure. Shortly after the operation, he complained symptoms of discitis
Fig. 4.
(Case no. 12) The MRI of the lumbar spine clearly shows the features of an infection of the L5–S1 intervertebral disc
Technical error
We found only three cases in which the implant was incorrectly positioned (no. 14, 16, and 17). In one case (no. 14), the APERIUS was implanted while in the others (no. 16, and 17) the X-Stop was used.
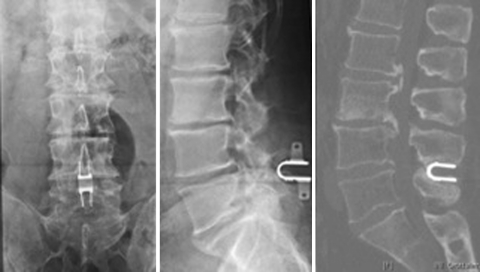
According to the technical specifications of the manufacturer and to the biomechanical behaviour of the lumbar spine, it is mandatory to implant the device deeply into the interspinous space, close to the zygapophyseal joints. Frequently, this correct position is difficult to achieve because of the hypertrophy of the joints with pronounced osteophites, especially in case of advanced degenerative disc disease or canal stenosis. According to us, a device is incorrectly positioned when it is implanted externally with respect to a vertical line which, on a lateral view X-ray, crosses the mid part of the space between two contiguous spinous processes. In two cases (X-Stop), the device was positioned inside the supraspinous ligament but too superficially, in fact, it was possible to find it with a superficial palpation and it acted as a painful ledge under the skin (Fig. 5). During revision surgery, the devices were easily recognized after a skin incision; they were kept inside the space by a thinned and elongated supraspinous ligament. The devices were removed. In the other patient, the device (APERIUS) was improperly implanted at L4–L5, outside the supraspinous ligament. This was also a case of wrong indication because of the presence of a large extruded herniated disc two levels above, which was responsible for the patient’s neurological impairment (Fig. 6).
Fig. 5.
(Case no.16) Post-operative standard X-rays after implantation of an interspinous device at L4–L5. In the A–P view (left), the device seems to be in a correct position while on the lateral view (right), the device is not close to the zygapophyseal joints, but positioned too superficially outside the influence of the spinous processes
Fig. 6.
(Case no.14) Post-operative lateral view of the lumbar spine shows an interspinous device positioned outside the interspinous and supraspinous ligament at L4–L5 level and a coexisting significant narrowing of the L2–L3 disc (left). The T1 and T2 weighted MRI sagittal view of the lumbar spine shows a wide hernia of the L2–L3 intervertebral disc (middle, right)
Device failure
In our case series, two patients showed breakage of the device (no. 18, and 19). These cases seemed to be quite similar because the implant failure was misdiagnosed until revision surgery was performed and the breakage became evident. Both patients referred no improvement in the painful symptomatology after the procedure, despite medical and physical therapies. In one patient (DIAM), we found a foreign body reaction to the device material. In particular, at surgery, we identified a major local tissue reaction surrounding the device. The polyethylene lining of the silicone core was broken. The microscopic examination of tissue samples showed the classical features of foreign body reaction. Our observations are similar to the ones reported by Jerosch and Moursi [6]. In the preoperative phase, no specific imaging feature was indicative of the diagnosis apart from a slight hyperintensity around the device in T2-weighted MRI. This finding, completely non-specific, was probably related to the inflammatory reaction around the device.
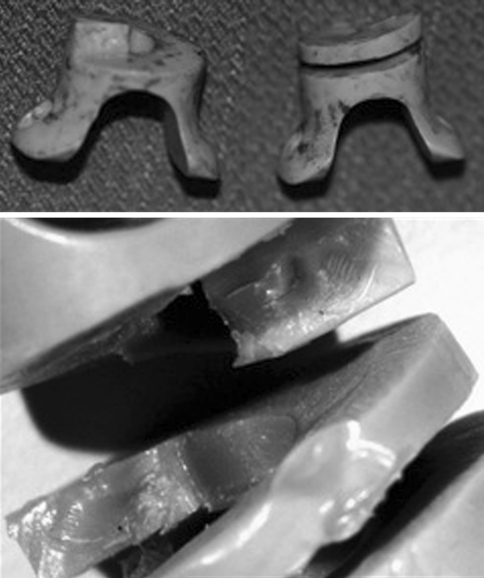
In the second case, the rupture of the device was discovered only at surgery due to the radio transparency of the implant, which did not permit to appreciate its morphology preoperatively. The device was fractured in two parts with the fracture line located at the base of the central core, where the spiral begins (Figs. 7, 8). This ID was originally designed and built as a spring and its final shape was obtained by milling a single piece of PEEK.
Fig. 7.
(Case no.18) An interspinous device was implanted at L4–L5 level in a patient who complained sciatica. The radio transparency of the device does not allow us to visualize the morphology of the device and its position. A new operation was performed, because of the worsening of the symptoms after the first operation
Fig. 8.
(Case no. 18) The device was noticed broken at surgery and was removed. The device as it appeared soon after the removal (up) and after microscope magnification of the opposite surfaces of fracture (down)
Conclusions
To date, the management of lumbar spine DDD with interspinous devices remains a challenging matter. Although the modern trend is to use even less invasive spinal instrumentation systems, no guidelines exist in the literature about the proper selection of patients suitable for their use. As a consequence, the extensive use of IDs in spondylotic patient can lead to clinical failure. The present retrospective study was able to recognize some causes of clinical failure among which the most frequent was a wrong indication. We focused on this aspect and tried to distinguish the right indications from the wrong ones although this crucial point is not clearly analyzed in the literature. In reviewing the literature on this subject, we found that the most frequent indication was spinal stenosis. Biomechanical studies showed that flexion and extension of the lumbar spine induce changes in the volume of the vertebral canal [7]. This is particularly true for a healthy subject without the typical morphologic changes of a degenerated spine. Instead, the situation can be quite different in cases of stenosis according to the type and the degree of the degenerative processes. On the basis of this biomechanical consideration, it is possible to speculate that the pathogenesis of the painful syndrome is of more important than its aetiology in choosing the type of surgical procedure. Therefore, in our opinion, the ID is indicated in case of mild or moderate stenosis of one or two levels only when the patient refers a clear improvement of symptoms in a bending forward position. Since it is unlikely to have an improvement in the bending forward position when the central canal is severely narrowed, severe stenosis is a contraindication for an ID implant. In our series, we observed four cases of severe stenosis with no improvement after surgery required another surgical procedure to remove the devices and decompressive laminectomy. Another aspect concerning the ID indication is its use in lumbar spine deformity. It has been suggested that the ID is not suitable in case of major instability and severe deformity. We certainly agree with this view and for this reason we consider the use of IDs to be contraindicated to treat adult scoliosis as in the cases reported. There are other important considerations related to ID indications such as the increasing tendency to implant a device at the affected level combined to the removal of a disc herniation and to implant it at L5–S1. So far, there is no evidence in the literature that the ID can reduce the incidence of disc herniation recurrence. Therefore, the suggested benefits of the ID to treat disc herniation remain a hypothesis [8]. Furthermore, considering the cost of the device, its use does not seem to be justified unless randomized controlled studies demonstrate its efficacy. In fact, in our series, we considered only three cases with herniated disc disease and with an ID implanted at the level where spondylodiscitis developed. In 2 of the 3 cases above, the device was implanted at the L5–S1 level. In these cases, we criticize not only the use of the device but also the decision to implant it at a level generally considered not suitable for this procedure.
Finally we reported some brief considerations regarding technical errors and some cases of device failure.
As to technical errors, it is difficult to diagnose the correct position of a device whose material cannot be detected by a routine imaging examination. In fact, the reliability of the imaging examination is significantly impaired by the radio transparency of some devices, such as those made of PEEK, whose morphological characteristics are difficult to recognize. On the contrary, the X-Stop and the APERIUS are easily identifiable as in the cases reported in this paper, because they are made of titanium alloy. We think that technical errors occur mostly during the learning curve of a new procedure. However, Barbagallo et al. [9] demonstrated that the careful evaluation of the spine anatomical features before implantation is important to avoid late complications such as the dislocation of the device or fractures of the spinous process.
Device failures seem to be rare complications as proven by the literature review. We observed two cases of device failure. Although the devices were different in terms of biomechanical features and manufacturing characteristics, we could draw similar conclusions for both devices. In both cases, in fact, the implant design may play a role in its failure or its failure may be due to inappropriate manoeuvres during its positioning.
In conclusion, our study demonstrated that there are several factors that can lead to an ID implant failure, incorrect indication apparently being the most common. Despite the lack of reliable guidelines on interspinous implant use, the study suggests that the patients likely to benefit from this simple surgery should be more carefully selected, thus minimizing the risk of post-operative ID failure.
Conflict of interest
None.
References
- 1.Senegas J, Vital JM, Pointillart V, Mangione P. Long-term actuarial survivorship analysis of an interspinous stabilization system. Eur Spine J. 2007;16:1279–1287. doi: 10.1007/s00586-007-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucherman JF, Hsu KY, Hartjen CA, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13:22–31. doi: 10.1007/s00586-003-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips FM, Voronov LI, Gaitanis IN, et al. Biomechanics of posterior dynamic stabilizing device (DIAM) after facetectomy and discectomy. Spine J. 2006;6:714–722. doi: 10.1016/j.spinee.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Gunzburg R, Szpalski M, Callary SA, et al. Effect of a novel interspinous implant on lumbar spinal range of motion. Eur Spine J. 2009;18(5):696–703. doi: 10.1007/s00586-009-0890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey DP, Swanson KE, Fuchs P, et al. The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine. 2003;28:2192–2197. doi: 10.1097/01.BRS.0000084877.88192.8E. [DOI] [PubMed] [Google Scholar]
- 6.Jerosch J, Moursi MG. Foreign body reaction due to polyethylene’s wear after implantation of an interspinal segment. Acta Orthop Trauma Surg. 2008;128:1–4. doi: 10.1007/s00402-007-0354-3. [DOI] [PubMed] [Google Scholar]
- 7.Dai LY, Xu YK, Zhang WM, et al. The effect of flexion-extension motion of the lumbar spine on the capacity of the spinal canal: an experimental study. Spine. 1989;14:523–525. doi: 10.1097/00007632-198905000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Floman Y, Millgram MA, Smorgick Y, et al. Failure of the Wallis interspinous implant to lower the incidence of recurrent lumbar disc herniations in patients undergoing primary disc excision. J Spinal Disord Tech. 2007;20:337–341. doi: 10.1097/BSD.0b013e318030a81d. [DOI] [PubMed] [Google Scholar]
- 9.Barbagallo GMV, Olindo G, Corbino L, et al. Analysis of complications in patients treated with the X-Stop interspinous process decompression system: proposal for a novel anatomic scoring system for patient selection and review of the literature. Neurosurgery. 2009;65:11–120. doi: 10.1227/01.NEU.0000346254.07116.31. [DOI] [PubMed] [Google Scholar]