Abstract
Over the last few years, some hemocomponents have been used advantageously in clinical neurosurgical practice, not systemically via transfusion but topically as a sealant (fibrin glue). This has diverted the attention of many authors to the role of platelets in the healing process. The combination of hyper-concentrated platelets and fibrin glue (fibrinogen, XIII factor, fibronectin) with activated thrombin produces a platelet gel that can be easily applied to “difficult” wounds. This topical use of hemocomponents has gained an important role in regenerative medicine. The authors have considered the possibility of using a preparation with a high autologous platelet concentration applied in addition to autologous bone during vertebral postero-lateral fusion. The aim of the procedure is to induce a higher rate of vertebral fusion. Between November 2007 and November 2008, 14 patients (9 men and 5 women, mean age 58.9) underwent laminectomy, vertebral stabilization and postero-lateral fusion. The number of vertebral levels involved in stabilization was: 1 in 2 patients, 2 in 5 patients, 3 in 5 patients, 4 in 1 patient and 5 in 1 patient. Platelet gel was obtained by taking 16 ml of peripheral venous blood from the patient. For this procedure two patented test tubes were used for each patient, with a capacity of 8 m each. These make up the REGEN-THT® (Thrombocyte Harvesting Tube) system that makes it possible to obtain 8 ml of autologous platelet gel in 40–45 min. The addition of Ca gluconate and ethanol at 95% makes it possible to obtain a preparation of plasma rich in platelets and activated thrombin with a platelet concentration five times superior to the haematic one. The platelet gel is combined with fragments of autologous bone and synthetic bone during surgical operation. To allow a comparative assessment of the degree of fusion achieved with and without application of the platelet preparation in each patient, it was arbitrarily decided to use it in only one half of the operative field. All patients underwent serial CT scans 3 and 6 months after surgery as well as plain X-rays to evaluate bone fusion. The reconstructed CT images, especially in sagittal and axial planes, permitted an evaluation of the degree of vertebral fusion and “bone growth”. The fusion rate was calculated measuring the increment of bone density on CT images, by means of an evaluation of the ROI (HU) in the newly formed bone, and comparing bone density within the bone callus formed by autologous and synthetic bone alone in the one to which the platelet preparation had been added. A good rate of fusion was observed in all patients. Furthermore, a comparative analysis of ROI at 3 and 6 months after surgery demonstrated a high increase in the fusion rate during the first 3 months after surgery. After 6 months the differences in ROI between the two sides had balanced out. However, at 6-month follow-up examination, bone density in the half of the surgical field in which platelet gel had been added to autologous–heterologous bone was higher in comparison to the contralateral one. Bony neoformation after posterior-lateral arthrodesis is well-evident 3 months after surgery and usually continues gradually for the following 18–24 months. The autologous platelet preparation used seems to accelerate bony deposition and to promote tissue healing, increasing bone density at the level of posterior–lateral arthrodesis. Moreover, this preparation has low production costs and is easy to apply.
Keywords: Platelet gel, AGF, Postero-lateral fusion, ROI
Introduction
During the last few years, platelet concentrate (platelet gel) has been increasingly used in lumbar spine surgery, in addition to both autologous bone and osteo-conducive materials such as hydroxyapatite, in order to enhance the density of bone fusion in postero-lateral and inter-transverse fusion procedures. Platelet gel is prepared as an ultra-concentrate of platelets associated with numerous growth factors that stimulate osteoblastic cell proliferation. Unfortunately, as pointed out by Vaccaro [24] at the present time there is an absence of controlled clinical trials evaluating how and to what extent platelet gel increases bone fusion in spinal surgery. We report our results regarding the use of platelet gel in postero-lateral fusion procedures in a consecutive series of 14 patients operated between November 2007 and November 2008, undergoing instrumental surgery for stenosis and thoracic or lumbar instability (Figs. 1, 2).
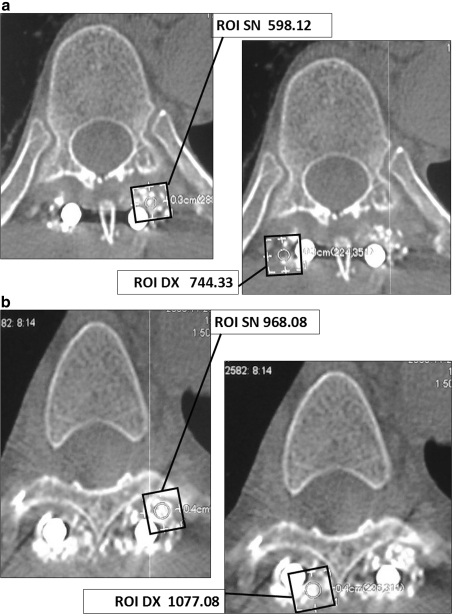
Fig. 1.
a Case 1: CT scans and ROI analysis of the PL fusion at 3 months: 52 years, T6 burst fracture involving posterior elements. Instrumentation with laminar and trasversal hooks at T3–T4–T5 and T7–T8–T9. PLF. b Case 1: CT scans and ROI analysis at 6 months
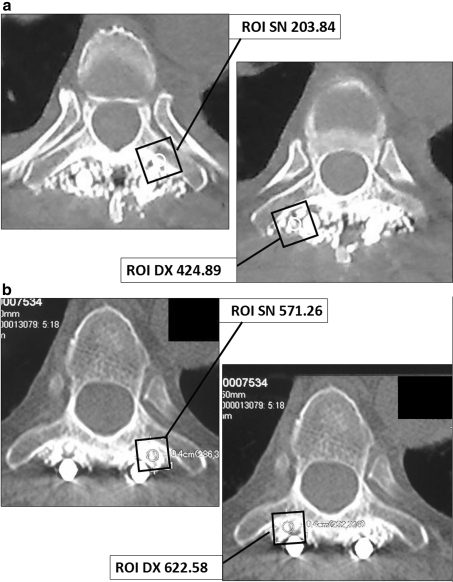
Fig. 2.
a Case 2: CT scans and ROI analysis of PL fusion at 3 months: 46 years, L1 burst fracture involving posterior elements. Instrumentation with L2 screws plus laminar and transversal hooks at T10 and T11. PLF. b Case 2: CT scans and ROI analysis at 6 months
Materials and methods
In the Department of Neurological and Neurosurgical Sciences of Rome University “Sapienza”, we evaluated 14 patients [average age 58.9 (range 35–89), 9 men and 5 women], who underwent laminectomy, vertebral fixation and postero-lateral fusion from November 2007 to November 2008. The number of levels involved in stabilization was: 1 in 2 patients, 2 in 5 patients, 3 in 5 patients, 4 in 1 patient and 5 in 1 patient. The demographic aspects of the study group are described in Table 1. Inclusion criteria included: traumatised patients with type B thoracic or lumbar fractures and patients with degenerative pathologies and lumbar instability documented by pre-operative dynamic radiographs.
Table 1.
Demographic aspects of our series
| No. | Sex | Age | Level | Etiology | Symptoms | Levels of fusion | Diabetes | Smoke | Fusion at 6 months |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 56 | THOR | Traumatic | Free | 5 | Ok | ||
| 2 | M | 67 | THOR | Traumatic | Paraplegia | 3 | Ok | ||
| 3 | F | 50 | LUMB | Degenerative | Free | 2 | y | y | Ok |
| 4 | F | 51 | LUMB | Degenerative | Weakness lower limbs | 2 | Ok | ||
| 5 | M | 71 | THOR | Traumatic | Free | 3 | Ok | ||
| 6 | M | 37 | LUMB | Degenerative | Low back pain | 1 | y | Ok | |
| 7 | F | 65 | LUMB | Degenerative | Claudicatio neurogena | 3 | Ok | ||
| 8 | M | 71 | LUMB | Degenerative | Weakness lower limbs | 1 | y | Ok | |
| 9 | M | 70 | LUMB | Degenerative | Weakness lower limbs | 2 | Ok | ||
| 10 | F | 89 | LUMB | Degenerative | Claudicatio neurogena | 3 | y | Ok | |
| 11 | M | 35 | THOR | Degenerative | Severe paraparesis | 2 | Ok | ||
| 12 | M | 45 | LUMB | Degenerative | Claudicatio neurogena | 3 | Ok | ||
| 13 | F | 58 | THOR | Traumatic | Paraplegia | 4 | Ok | ||
| 14 | M | 60 | LUMB | Degenerative | Weakness lower limbs | 2 | y | Ok |
THOR thoracic, LUMB lumbar, LL lower limbs
Surgical technique
Platelet gel was obtained by taking 16 ml of peripheral venous blood from each patient. For this procedure two patented test tubes were used for each patient, with a capacity of 8 ml. These make up the REGEN-THT® (Thrombocyte Harvesting Tube) system that makes it possible to obtain 8 ml of autologous Platelet gel in 40–45 min. The addition of Ca gluconate and ethanol at 95% provides a preparation containing plasma rich in platelets and activated thrombin with a platelet concentration five times superior to normal blood. The platelet gel is combined with fragments of autologous bone fashioned to the right size and to synthetic bone during surgical operation. To allow a comparative assessment of the degree of fusion achieved with and without application of the platelet preparation in each patient, it was arbitrarily decided to use it in only one half of the operative field, the right-sided one. All patients were informed in detail of the procedure involved before agreeing to take part in the study.
Results
In all patients a good rate of fusion was observed 3 and 6 months after surgery. Furthermore, two aspects emerged from an analysis of bony density:
Comparative analysis of ROI 3 months after surgery demonstrated an enhanced fusion rate in the right site of the operative field, in which platelet gel had been added to autologous bone, in comparison to the other side of the field in which traditional bone fusion had been carried out. At 6-month follow-up, the differences observed in the ROI values among the two sides had balanced out. As far as bone density was concerned, after 3 months this was found to be higher on the side where the platelet gel had been applied with respect to the other side. The mean density evaluated by means of ROI in the left hemi-field was 345.70 HU, whereas the mean density of the right half-field, treated with platelet gel, was of 558.19 HU, demonstrating an increase of bone density of 212.49 HU (Table 2).
Comparative evaluation of the ROI at 6 months showed that the differences observed between the two sides had levelled out, with a mean density on the left of 634.14 HU, and on the right of 760.09 HU, namely an increase of bone density equivalent to 125.95 HU units (Table 2).
Table 2.
Comparative analysis of ROI and SD in left and right side at 3 and 6 months for each patient
| Levels of fusion | Diabetes | Smoke | ROI 3 months (right side, HU/SD) | ROI 3 months (left side, HU/SD) | ROI 6 months (right side, HU/SD) | ROI 6 months (left side, HU/SD) |
|---|---|---|---|---|---|---|
| 4 | N | N | 608.75/312.3 | 417.38/318.6 | 843.84/317.5 | 795.33/324.2 |
| 5 | N | N | 567.13/198.6 | 342.22/212.5 | 688.91/196.7 | 543.85/214.0 |
| 1 | N | Y | 361.60/203.15 | 194.22/199.3 | 599.09/207.5 | 412.82/215.10 |
| 3 | N | Y | 328.23/230.6 | 219.77/215.1 | 657.13/200.9 | 549.71/210.2 |
| 1 | Y | N | 434.05/160.16 | 110.59/179.5 | 677.12/170.6 | 535.55/189.20 |
| 3 | N | N | 424.89/450.3 | 203.84/401.56 | 622.58/429.0 | 571.26/380.22 |
| 2 | N | N | 744.33/200.12 | 598.12/223.11 | 1077.08/281.9 | 968.08/247.39 |
| 3 | N | N | 689.86/185.4 | 542.25/196.3 | 781.30/184.63 | 636.74/193.7 |
| 2 | Y | Y | 443.43/210.7 | 209.75/217.4 | 555.33/209.3 | 381.01/204.35 |
| 2 | Y | N | 447.78/268.3 | 197.34/266.12 | 629.13/275.6 | 555.65/261.45 |
| 3 | N | N | 743.46/288.1 | 461.80/277.7 | 949.22/280.3 | 773.37/279.57 |
| 3 | N | N | 721.88/178.89 | 492.34/190.7 | 989.55/187.5 | 778.01/192.1 |
| 2 | N | N | 523.09/214.17 | 441.19/220.81 | 770.98/207.7 | 666.77/215.24 |
| 2 | N | N | 776.03/256.2 | 409.09/225.7 | 800.12/210.39 | 709.90/234.8 |
| Mean | 558.19/238.35 | 345.70/238.88 | 760.09/239.96 | 634.14/240.10 |
Y yes, N no, ROI range of interest, HU hounsfield unit, SD standard deviation
CT scan at 3 and 6 months after surgery documented a modest increase of bone density in fusion stimulated by platelet gel compared to that stimulated by autologous/heterologous bone alone, demonstrating a faster bone deposition during the first 3 months after surgery. This aspect is also evident in patients who have risk factors for non-fusion such as smoking and diabetes. In our series, we studied two patients who smoked, two diabetic and one diabetic and smokers. The ROI analysis in these patients showed a faster rate of bone deposition during the first 3 months, which had levelled off at 6-month control examination, although the HU value of bone density was lower than in healthy patients (Table 2).
Discussion
In the literature, the majority of clinical studies have focused on platelet gel and important results have already been obtained in terms of osteo-induction [8, 9, 11, 16, 27]. The efficacy of platelet concentrate for stimulating bone growth has also been demonstrated by many maxillo-facial studies [18, 19, 21, 25] as well as in vitro studies [3, 4, 6, 7, 15, 22, 23], and in vivo in animals [13, 20]. In the neurosurgical field, the use of platelet gel has been employed in spinal fusion procedures. Lowery et al. [17] described a series of 19 patients in a retrospective review of autologous growth factors (AGF) combined with autograft and hydroxyapatite as an extender in posterior and anterior lumbar fusion. The authors reported a 100% fusion rate based on surgical exploration in 5 patients and on plain X-ray films in 14. In their retrospective study, Bose and Balzarini [1] described 60 cases of spinal fusion using AGF with autograft and reported a 96% fusion rate based on plain radiographic evidence.
Weiner and Wolker [26] reported on a retrospective study comprising two groups of patients who had undergone single- level inter-transverse fusion. A 62% fusion rate was observed in 32 patients in whom autogenous iliac crest graft augmented with AGF was used, compared to a 91% fusion rate in a group with bone graft alone. Their evaluation was based on flexion/extension radiographs. Hee et al. [12] in 2003 evaluated the effects of AGF combined with autograft in trans-foraminal lumbar interbody fusion performed in 23 patients: they compared these results with those obtained in a group of 111 patients treated by autograft alone, with a minimum follow-up of 2 years. Radiographic evaluation was performed at 4, 6 and 24 months, with more rapid incorporation of fusion at 4 and 6 months in AGF patients. At 24-month evaluation, no significant differences in fusion rate were detected. The authors concluded that AGF was capable of promoting graft incorporation, thus stimulating faster fusion.
In 2005, Jenis et al. [14] described a study in which 37 consecutive patients were submitted to anterior–posterior lumbar interbody fusion (ALIF-PLIF) with bone graft harvested from the iliac crest (22 patients) or allograft combined with AGF (15 patients). Patients were evaluated at 6 and 12 months by CT scan and at 24 months by plain X-rays. The results at 12 and 24 months demonstrated an 85% fusion rate in patients with autograft in comparison to an 89% rate with allograft and AGF. The authors concluded that allograft with AGF could represent a valid alternative to homologous fusion.
In the study published by Carreon et al. [5] in 2006, a series of 76 patients were treated with non-instrumental postero-lateral arthrodesis using autologous bone with AGF and the results were compared to those obtained in a group of patients treated with non-instrumental postero-lateral arthrodesis using autologous bone alone. A 25% non-fusion rate was observed in the AGF group compared to 17% in the control group. The authors concluded by recommending the use of autologous bone graft because it guarantees a higher rate of fusion.
In the present study, we decided to focus our attention to several aspects not taken into consideration in the studies previously published in the literature:
In previous studies the comparison was made between groups of patients who had undergone postero-lateral fusion on both sides of the operative field with autograft and allograft + AGF, subsequently evaluating the fusion rate and comparing it with a control group treated with autograft alone. In the patients of our study, a traditional postero-lateral fusion was performed in the left half of the operative field and a postero-lateral fusion with autograft/allograft + platelet gel in the right half. This technique made it possible to directly compare the two systems in each single patient, eliminating variability due to individual clinical conditions favouring non-fusion, such as smoking and diabetes [2, 10].
Critical analysis of previous published studies attracted our interest to comparative evaluation of the fusion rate. On the basis of CT images, a map of bone densities can be obtained using a variety of methods, so that a comparative assessment of the fusion rate at the instrumented level with and without platelet preparation is possible. The most simple and widely used method, that we use too, is a system of electronic targeting which delimitates the region of interest (ROI), a surface varying in size and shape (generally a square-shaped area 0.4 cm2 in size), that the operator can trace directly on the CT images. The computer is then able to perform an immediate calculation of the mean pixel content (bi-dimensional unit of measurement) in the perimeter traced by the operator over the diagnostic region of interest. In order to express the densitometric value of pixels, a scale of numbers has been introduced in which the value 0 corresponds to water, −1,000 to air and +1,000 to cortical bone. The unit of measurement employed is the Hounsfield Unit (HU) scale. It is important to emphasize that the numerical value obtained from such measurements is the average of the densitometric values of the pixels contained in the area studied. Together with the ROI measurement, the computer also gives us the standard deviation (SD) of the pixel concentration in the measured area, whose numerical value is inversely proportionate to tissue homogeneity. It is clear that in an area as large as 0.4 cm2 the SD will be extremely variable. A retrospective analysis of the SD values measured and recorded at the same time as ROI showed that all the SD values remained below 450.3, with a median value on the right side of 238.5 at 3-month follow-up and 239.96 at 6 months, and in the left side of 238.88 at 3 months and 240.1 at 6 months. In order to obtain a control value of bone tissue homogeneity, a series of ROI measurements were made in an area of homogenous cortical bone measuring 0.4 cm2 in another site: results showed that the value of SD remained within a range of 40–150. If we consider that PLF is performed on a composite of fragmented autologous–heterologous bone, which is far more dishomogenous than cortical bone, satisfactory values of bone fusion have been suggested as SD <500. A comparison between the SD values measured on the two opposite sides at 3 and 6 months showed a good homogeneity of bone fusion, with values always below 450.03 (median 239), and confirmed that the use of platelet gel does not influence the homogeneity of the newly formed bone callus.
In this context, it is important to point out that in previous studies it was the radiologist who evaluated fusion by direct visual appraisal of follow-up CT or plain X-ray images whereas in the present study evaluation of axial CT images, ROI and SD provided concrete numeric values regarding bone density and homogeneity.
In our study the fusion rate at 6 months was 100%. Bone density in the right half of the operative field was higher by 125.95 HU (760.09 vs. 634.14 HU) in comparison to the left one in which AGF was not used. Moreover, a higher velocity of bone apposition was observed during the first 3 months after surgery in the half-field where the gel was employed: this subsequently normalized in the following 6 months, so that only slight differences existed between the two halves at 6-month follow-up. This feature has also been observed in diabetic patients and smokers, in which it was observed, similar to healthy subjects, an increase in the rate of bone growth during the first 3 months, with subsequent levelling of the rate of bone deposition at 6 months, while maintaining a level of fusion lower than in healthy subjects. This is a very important aspect because it suggests that the gel could be used to speed up post-operative recovery, in terms of mobility, thus reducing the duration of rehabilitation and the need for orthopaedic devices, especially in diabetic and smoker patients. Consequently, patients are able to make an early return to normal working activities.
Another aspect which must not be underestimated is the low cost of gel preparation owing to the fact that the gel is prepared in the Institute of Haematology of our university structure, using the patient’s own blood. It should be pointed out that the entire cost of this method does not exceed 140,00 Euros The cost of each antiseptic “pastette” is 0,026 Euro each plus VAT. The capsules of Petri cost 0.151 each plus VAT, whereas the vials of calcium glutamate cost 0.25 cents each. The vials of ethyl alcohol cost 2.5 Euros each. These are the real costs and as far as the cost of centrifuge is concerned it can be quantified in 50 cents/1 Euro per procedure.
Conclusion
The use of platelet gel in postero-lateral fusion increases the rate of fusion and represents a valid support for the surgeon in the treatment of pathologies that require stabilization and fusion. Moreover, its wide availability and low cost makes it easy and cheap to use. Enhanced bone deposition means that patients recover faster and have less need of orthesis protection.
Acknowledgments
Conflict of interest None.
References
- 1.Bose B, Balzarini M. Bone graft gel: autologous growth factors used with autograft bone for lumbar spine fusion. Adv Ther. 2002;19:170–175. doi: 10.1007/BF02848692. [DOI] [PubMed] [Google Scholar]
- 2.Brown CW, Orme TJ, Richardson HD. The rate of pseudoarthrosis (surgical non-union) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1986;11:942–943. doi: 10.1097/00007632-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Canalis E, et al. effect of growth factors on bone cell replication and differentiation. Clin Orthop. 1985;193:246–263. [PubMed] [Google Scholar]
- 4.Canalis E, et al. Effects of platelet derived growth factor on bone formation in vitro. J Cell Physiol. 1989;140:530–537. doi: 10.1002/jcp.1041400319. [DOI] [PubMed] [Google Scholar]
- 5.Carreon LY, et al. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine V. 2005;30(9):E243–E246. doi: 10.1097/01.brs.0000160846.85397.44. [DOI] [PubMed] [Google Scholar]
- 6.Centrella M, et al. Human platelet derived transforming growth factor-b stimulates parameters of bone growth in fetal rat calvariae. Endocrinology. 1986;119:2306–2312. doi: 10.1210/endo-119-5-2306. [DOI] [PubMed] [Google Scholar]
- 7.Centrella M, et al. Platelet derived growth factor enhances deoxyribonucleic acid and collagen synthesis in osteoblast-enriched cultures from fetal rat parietal bone. Endocrinology. 1989;125:13–19. doi: 10.1210/endo-125-1-13. [DOI] [PubMed] [Google Scholar]
- 8.Dohan E, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Eppley B, Woodell J, Higgins J. Platelet quantification and growth factor analysis from platelet rich plasma (PRP): implications in wound healing. Plastic Reconstruct Surg. 2004;114:1502–1508. doi: 10.1097/01.PRS.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 10.Glassman SD, Anagnost SC, Parker A. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25:2608–2615. doi: 10.1097/00007632-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Hannon T, Polston G, Pekarske W. Determination of platelet yields from platelet rich plasma for autotransfusion machines. Anesth Analg. 1996;88:104–109. [Google Scholar]
- 12.Hee H, Madj M, Holt R, Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12:400–407. doi: 10.1007/s00586-003-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howes R, et al. Platelet derived growth factor enhances demineralised bone matrix induced cartilage and bone formation. Calcif Tissue Int. 1988;42:34–38. doi: 10.1007/BF02555836. [DOI] [PubMed] [Google Scholar]
- 14.Jenis LG, Banco RJ, Kwon B. A prospective study of autologous growth factors (AGF) in lumbar interbody fusion. Spine J. 2006;6:14–20. doi: 10.1016/j.spinee.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Kasperk CH, et al. Interactions of growth factors present in bone matrix with bone cells effects on DNA synthesis and alkaline phosphatase. Growth Factors. 1990;3:147–158. doi: 10.3109/08977199009108277. [DOI] [PubMed] [Google Scholar]
- 16.Kevy S, Jacobson M. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corp Technol. 2004;36:28–35. [PubMed] [Google Scholar]
- 17.Lowery GL, Kulkarny S, Pennisi AE. Use of autologous growth factors in lumbar spinal fusion. Bone Aug Suppl. 1999;25(2):47S–50S. doi: 10.1016/s8756-3282(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 18.Marx RE, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 19.Marx RE, et al. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Noda M, et al. In vivo stimulation of bone formation by transforming growth factor B. Endocrinology. 1998;124:2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- 21.Oprea WE, karp JM, Hosseini MM, Davies JE. Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac surg. 2003;14(3):292–300. doi: 10.1097/00001665-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Pfeilschifter J, et al. Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet derived growth factor, and transforming growth factor B. Endocrinology. 1990;127:69–75. doi: 10.1210/endo-127-1-69. [DOI] [PubMed] [Google Scholar]
- 23.Slater M, et al. Involvement of platelets in stimulating osteogenic activity. J Orthop Res. 1995;13:655–663. doi: 10.1002/jor.1100130504. [DOI] [PubMed] [Google Scholar]
- 24.Vaccaro AR, Sharan AD, Tuan RS, et al. The use of biologic materials in spinal fusion. Orthopaedics. 2001;24:191–197. doi: 10.3928/0147-7447-20010201-25. [DOI] [PubMed] [Google Scholar]
- 25.Van Den Dolder J, Mooren R et al (2006) Platelet rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng 12 (11) [DOI] [PubMed]
- 26.Weiner BK, Wolker M. Efficacy of autologous growth factors in lumbar intertransverse fusion. Spine. 2003;28(17):1968–1971. doi: 10.1097/01.BRS.0000083141.02027.48. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann R, Jakubietz R, Jakubietz M, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41:1217–1224. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]