Abstract
Insertion of an interspinous devices has became a common procedure for the treatment of different clinical picture of degenerative spinal disease. We present our experience in 1,575 patients with the use of two different interspinous spacers: Device for Intervertebral Assisted Motion (DIAM) and Aperius PercLID system. From 2000 through 2008, 1,315 consecutive patients underwent DIAM implantation and 260 had an Aperius PercLID procedure. The main surgical indications included: degenerative disc disease (478 patients), canal and/or foraminal stenosis (347 patients), disc herniation (283 patients), black disc and facet syndrome (143) and topping-off (64 patients). 1,100 patients underwent a single level implant and 475 had a multiple level implant. Mean operating time was 35 min for DIAM and 7 min for Aperius. Complications were detected in 20 patients (10 cases of infections, 10 fractures of the posterior spinous processes). 40 patients were subsequently treated with posterior arthrodesis (n = 30) or total disc replacement (n = 10). Patient’s postoperative clinical status was rated according to the modified Macnab criteria: symptoms resolution or improvement was achieved in 1,505 patients; and unchanged or unsatisfactory results in 70. Both techniques are safe, simple and less technically demanding. These approaches appear to be an effective alternative in selected cases, although conventional posterior lumbar decompression and fusion still may be required.
Keywords: Interspinous spacer, Degenerative spinal disease, Lumbar stenosis, Fusion
Introduction
Lumbar spinal stenosis (LSS) is a very common pathology of elderly population. Neurogenic intermittent claudication (NIC) represents the major complaint of these patients. LSS is often a position-dependent condition that is aggravated in extension and relieved in flexion. Interspinous spacers have been designed to treat NIC in patients with LSS by limiting extension [1, 2]. Preclinical studies demonstrated that distraction of the spinous processes allows increasing in the size of the spinal canal and neural foramina [3–5].
In recent years, extension limiting device usage in Europe has been rapidly increasing. A growing number of interspinous devices have been introduced to the market [6–8].
Implant designs vary from rigid to dynamic structure and material compositions include titanium, polyetheretherketone (PEEK) and elastomeric compounds. The surgical indications have been extended, ranging from degenerative spinal stenosis, discogenic low back pain, facet syndrome, disc herniations and low-grade instability. Interspinous spacers allow a good symptoms relief in a broad-spectrum pathologies, satisfying the modern concept of minimally invasive technique.
Concerns over the long-term effects of fusion on adjacent segments have led to the introduction of the concept of dynamic stabilization. Development of elastic interspinous stabilization systems follows the modern philosophy of motion preservation of the functional spinal unit (FSU).
Biomechanical studies reported that dynamic interspinous devices significantly unload the intervertebral disc at the instrumented level and do not significantly change the intradiscal pressures at the adjacent levels [9–13]. Clinical result in terms of symptoms relief is satisfying in a high percentage of cases and complications are few [14, 15].
We present our experience with two different interspinous devices over a period of 8 years.
Materials and methods
A total of 1,756 patients were treated with an interspinous device from 2000 to 2008. We gathered data from three different hospitals, where the senior author has been practicing during this period of time. Inclusion criteria were implant of an interspinous device and a minimum of 1 year follow-up available. 1,575 patients were suitable for this study.
DIAM® (Medtronic-Sofamor Danek) implant was performed in 1,315 patients for the treatment of degenerative disc disease (478 patients), canal and/or foraminal stenosis (347 patients), disc herniation (283 patients), black disc and facet syndrome (143) and topping-off (64 patients). Patients with high-grade congenital or degenerative spondylolisthesis were not considered candidates to any interspinous stabilization system at our centres. There were 736 females (56%) and 579 males (44%). The age of the patients ranged from 19 to 77 years; mean age at the time of surgery was 51 years.
Since 2007, we introduced the use of Aperius™ PercLID™ (Medtronic-Sofamor Danek) at our centres, for the treatment of degenerative LSS in elderly patients with high-risk general conditions. A total of 260 patients were treated with Aperius PercLID device. The age of the patients ranged from 52 to 91 years; mean age was 72 years.
All the patients underwent Device for Intervertebral Assisted Motion (DIAM) implantation using general anaesthesia. Surgical procedure for prosthesis application was performed in standard technique; differences between subjects were related to the underlying pathology: discectomy for herniated disc, decompression of nerve roots in foraminal stenosis, more extended neural decompression in central stenosis.
The Aperius implantation was conducted under spinal or local anaesthesia in 258 patients. Additional mild sedation was necessary in six of these 258 patients to control anxiety occurred during surgery. The remnant two patients ask general anaesthesia as personal preference.
Basically, the Aperius PercLID system is composed of a set of colour-coded distraction trocars of increasing sizes (8, 10, 12 and 14 mm). The 8 mm distraction trocar has a sharp pointy tip to pierce the interspinous ligament. Each trocar and each inserter have a curved shape which facilitates convenient access to the target level and optimal positioning of the implant. Definitive implant is preassembled on a single disposable inserter.
A small (1.5 cm) unilateral skin incision was made about 10 cm from the midline. Progressive distraction of the interspinous space and then selected implant size insertion were conducted under fluoroscopic guidance to achieve correct positioning.
Patients were evaluated at defined intervals: preoperative, 2, 6 and 12 months after surgery. Symptom severity, physical functioning, quality of life and self-rated pain were assessed using the Zurich Claudication Questionnaire (ZCQ), the EuroQol 5 Domain Questionnaire (EQ-5D) and the Visual Analog Scale (VAS). Overall clinical outcome was assessed using the modified Macnab criteria. Postoperative radiographs were obtained before hospital discharge, at 2 and 12 months follow-up to detect adverse occurrences.
Baseline severity of the disease is reported in Table 1.
Table 1.
Baseline (preoperative) severity of the disease
| VAS for low back pain, mean (SD) | 7.54 (±2.14) |
| EQ-5D score, mean (SD) | 32.87 (±11.40) |
| ZCQ score, mean (SD) | 42.31 (±21.53) |
To compare mean score improvements measured in continuous scales, such ZCQ and EQ-5D, we used analysis of covariance (ANCOVA) with the preoperative score as the covariate. For assessment of statistical significance of improvement in the outcome a paired t test was used.
Results
Considering the entire patient population, a single level implantation was performed in 1,100 patients and a multiple level in 475. A single level DIAM implantation was performed in 64% of patients (n = 839); a two-level implant in 33% (n = 435) and a three-level implant in 3% (n = 41).
In 260 patients treated with Aperius, a one-level procedure was performed in 72% of patients (n = 188); a two levels in the others.
The most commonly treated level was L4–L5 in both groups.
In DIAM group, single levels procedures were performed at: L4–L5 (502 cases), L5–S1 (267 cases), L3–L4 (57 cases), L2–L3 (12 cases) and L1–L2 (1 case). A double level DIAM implant at L4–L5 and L5–S1 was performed in 215 patients; at L3–L4 and L4–L5 in 158 patients and at L2–L3 and L3–L4 in 62 subjects. A three-level procedure was conducted in 23 cases in L3–L4, L4–L5 and L5–S1; in 18 cases at level L2–L3, L3–L4 and L4–L5.
Aperius single level implants were at L4–L5 (93 patients), L3–L4 (67 patients), L5–S1 (16 patients) and L2–L3 (12 patients), respectively. Two levels Aperius procedures were conducted at L4–L5 and L5–S1 levels in 23 patients, at L3–L4 and L4–L5 in 47 patients and at L2–L3 and L3–L4 in 2 patients.
Based on recordings available (in 72 and 95% of cases, respectively) mean operating time was 35 min (range 23–65 min) for a single level DIAM implantation and 7 min (range 5–10 min) for a single level Aperius procedure.
DIAM patients were allowed to ambulate on the first postoperative day. They were usually allowed to be discharged on postoperative day 2.
In case of percutaneous technique under spinal or local anaesthesia, patients were mobilized 6–12 h postoperatively.
Postoperative infection was reported in 10 patients undergone DIAM application (0.7%); they were treated with systemic antibiotic therapy and surgical debridement with prosthesis removal. No infections were detected after Aperius percutaneous technique.
Fracture of the spinous processes were detected on radiological follow-up images at 2 and 6 months, respectively, in 9 and 1 patients. Only three of the fractures of spinous processes were reported in Aperius group (1%).
At 1 year follow-up only 40 patients (2.5%) needed a second surgical intervention at the level previously operated. Failed back syndrome was treated with posterior arthrodesis (n = 30) or total disc replacement (n = 10). Posterior lumbar fusion was obtained with insertion of intersomatic cages (PLIF) in eight patients and with transpedicular screws placement in 22 patients, respectively.
Patients undergone total disc replacement were all young female patients (range 32–43 years) with a high-grade L5–S1 degenerative disc disease.
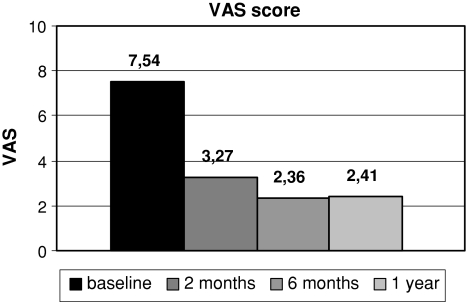
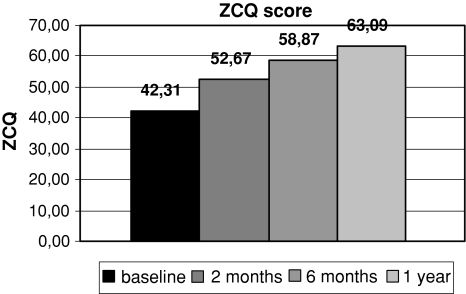
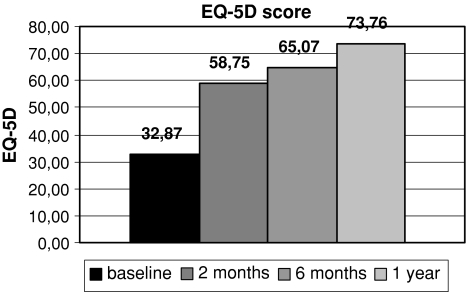
Significant improvements in all measured scores were noted at every follow-up interval. Results are shown in Figs. 1, 2 and 3.
Fig. 1.
Comparison of mean VAS score at baseline and at different follow-up intervals
Fig. 2.
Comparison of mean ZCQ score at baseline and at different follow-up intervals
Fig. 3.
Comparison of mean ED-5D score at baseline and at different follow-up intervals
Mean VAS score dropped from 7.54 ± 2.14 at baseline to 2.41 ± 1.78 at 1 year follow-up. The difference was statistically significant (P < 0.001).
Mean ZCQ score at baseline was 42.31 ± 21.53 and at 1 year follow-up 63.09 ± 17.37 (P < 0.001).
The average EQ-5D value improved significantly from 32.87 ± 11.40 at baseline to 73.76 ± 5.71 at 1 year follow-up (P < 0.001). The most important improvement was seen in mobility and ability for self-care.
Overall postoperative clinical status according to the modified Macnab criteria is shown in Table 2. Symptoms resolution or improvement was achieved in 1,505 patients; unchanged and/or unsatisfactory results were obtained in 70 patients.
Table 2.
Summary of modified Macnab criteria outcome
| Outcome | No. patients |
|---|---|
| Excellent | 924 |
| No pain | |
| No restriction of mobility | |
| Return to normal work and level of activity | |
| Good | 483 |
| Occasional nonradicular pain | |
| Relief of presenting symptoms | |
| Able to return to modified work | |
| Fair | 98 |
| Some improved functional capacity | |
| Still handicapped and/or unemployed | |
| Poor | 70 |
| Continued objective symptoms of root involvement | |
| Additional operative intervention needed at index level irrespective of length of postoperative follow-up |
Discussion
Increasing life expectancy has made LSS a very common pathology in the elderly population. An increasing number of old or high-risk patients ask neurosurgical intervention for the treatment of NIC.
Decompression and fusion have traditionally been used to manage different clinical pictures of degenerative lumbar spinal diseases. The concept of minimally invasive technique has been developing in recent years in a wide range of surgical disciplines, including spinal surgery. The theoretical goal of a minimal invasive approach is symptoms relief, minimizing anatomical modification and surgical-related complications. Introduction of these procedures allowed to extend surgical indications to an old or high-risk patients population.
Following this new trend, X STOP® Interspinous Process Decompression System (Medtronic-Sofamor Danek) was introduced as an attractive alternative to conventional surgical procedures for patients with LSS with moderate to severe impairment. Without disrupting the normal anatomical structures, this rigid interspinous device could limits narrowing of the spinal canal and neural foramina [5, 16].
Siddiqui et al. performed different magnetic resonance imaging (MRI) studies to quantify the effect of the implant in vivo on the lumbar spine at the instrumented levels. Significant increase in the dimensions of the neural foramen and canal area were demonstrated after surgery. No significant changes were seen in disc heights, segmental and total lumbar spine movements postoperatively. Conclusions were that X-Stop device does not affect the sagittal kinematics of the lumbar spine and improves the degree of central and foraminal stenosis in vivo [3, 17, 18].
Biomechanical studies have been performed on cadavers looking at disc pressures and segmental range of movements. Swanson et al. reported that a rigid interspinous spacer does not significantly change the intradiscal pressures at adjacent levels, but it significantly unloads the intervertebral disc at the instrumented level in the neutral and extended positions [9].
Lindsey et al. described a reduction of flexion–extension range of motion at the instrumented level, but axial rotation and lateral bending were not affected. The range of motion in flexion–extension, axial rotation and lateral bending at the adjacent segments was not significantly affected by the implant [1].
Loading parameters of lumbar cadaver spines were measured in the facet joints of the implanted and adjacent levels by Wiseman et al. The implant significantly reduced the mean peak pressure, average pressure, contact area and force in the facet joints at the implanted level. The same parameters at the adjacent levels were not significantly different between the intact and implanted specimens [19].
Conclusions of these biomechanical studies were that interspinous implant does not significantly alter the kinematics and does not cause accelerated disc degeneration of the segments adjacent to the instrumented level. Interspinous process decompression will unlikely cause adjacent level facet pain or accelerated facet joint degeneration. Furthermore, pain induced from pressure originating in the facets and/or posterior anulus of the lumbar spine may be relieved by interspinous process decompression.
Clinical outcome in X-stop usage are satisfying, demonstrating extension limiting devices to be effective in relief of low back pain and NIC [2, 17, 20–22].
Results from prospective, randomized trials reported that rigid interspinous spacers offer a significant improvement over nonoperative therapies, at 1 and 2 years follow-up, with considerably lower morbidity than decompressive laminectomy [23–26].
Recently, a new rigid interspinous decompression system the Aperius™ PercLID™ has been introduced in the market. The implant core is manufactured of Titanium alloy (TiAl6V4 alloy), while the external shell is composed of commercially pure Titanium. This system, similarly to X-stop device, obtains neural element decompression through interspinous process distraction, limiting spinal motion in extension and enlarging spinal canal and neural foramina. Pain relief is achieved also unloading intervertebral disc and facet joints. In addition, it has the benefit of being a completely percutaneous technique. Hence, the procedure can be carried out under local or spinal anaesthesia which is a significant asset in cases where surgery is required; however, general anaesthesia represents a high risk due to the patient’s health status.
Since 2007, we introduced the use of Aperius at our hospital for the treatment of LSS in elderly patients with high-risk general conditions. Our clinical results were good, comparable with data reported in previous studies on outcomes with X-stop device.
We detected a very short duration of the procedure because surgical technique is easy. The percutaneous nature of the Aperius system allowed to reduce blood loss and risk of infections. The patient was mobilized a few hours after surgery, minimizing complications such deep venous thrombosis, particularly frequent in old bed-ridden patients. All of these advantages were reflected in a short hospital stay and a quick return to daily activities.
We support Aperius as a good option for the treatment of LSS in an old or high-risk population. Although this is not a comparative study, Aperius seems to represent an alternative to fusion techniques and to other interspinous devices requiring open-surgical technique.
In spinal surgery, the success of minimally invasive techniques was supported by advanced biomechanical knowledge. Every anatomical unit represents an important stabilizing component of the lumbar spine, so it deserves to be preserved whenever it is possible. Minimal modifications on the lumbar segmental anatomy allow maintenance of physiological kinematics and load repartition.
Surgical technique for interspinous devices application require minimal surgical exposure and satisfy the concept of anatomical preservation.
Posterior dynamic devices have been developed to stabilize painful diseased lumbar segments while preserving motion. In a computational model, the elastic implant has shown a more physiological flexural stiffness respect to the titanium implant, which exhibited an excessive stiffness and permanent strains (plastic strains), even under physiological loads [27].
Biomechanical properties of elastic interspinous devices were confirmed in studies conducted on cadavers [9–13].
The DIAM is as a silicone interspinous shock absorber acting like a “bumper”. Its core provides stability in extension and two independent laces fastened to the spinous processes stabilize the segment in flexion acting like a tension band. The DIAM allows realignment of facets interface and restoring of the facets congruence. The distraction of the neural arch enlarges the neural foramen relieving neural compression.
Indications for use of the DIAM as they were originally formulated are: dynamic stenosis, foraminal stenosis and disk herniation. A further indication for the DIAM is adjacent segment disease after lumbar fusion. Elastic stabilization reduces stresses on the adjacent disc. The DIAM device can, therefore, be used in conjunction with a fusion when decompression or instrumentation compromises the adjacent facets, in multiple level fusions, and in cases of degeneration of segments adjacent to the fusion [11].
Biomechanics of the instrumented and adjacent levels due to the insertion of the DIAM spinal stabilization system were studied in a three-dimensional finite element model by Bellini et al. The implant caused a reduction in range of motion of the instrumented level by 17% in flexion and by 43% in extension, whereas at the adjacent levels no significant changes were predicted. Intradiscal pressure at the instrumented level was reduced in flexion, in extension and in axial rotation, while no variations in pressure were detected in lateral bending [13].
Philips et al. analyzed the effects of the DIAM device on cadaveric lumbar spine after discectomy. The DIAM device is effective in stabilizing the unstable segment, reducing the increased segmental flexion–extension and lateral bending motions observed after discectomy [28].
Preliminary clinical results with DIAM were satisfying in more than 90% of cases [14, 15, 29].
Randomized clinical trial is on the way.
To our knowledge, our series of 1,315 patients treated with DIAM is the largest available in literature to date. We reported good results in terms of pain relief and self-related evaluation of outcome in a large number of cases. High-grade spondylolisthesis patients were preliminary excluded from this study, as they can theoretically be not respondent to a dynamic stabilization system. A rigid interspinous distraction device has been previously showed an extremely high failure rate, defined as surgical re-intervention, in patients with degenerative spondylolisthesis [30].
We think that interspinous elastic device should be considered in any clinical picture, where a developed low-grade instability requires stabilization but at the same time the FSU deserves motion preservation.
In recent years, biological attempts to regenerate the disc are promising. Degenerative disc disease always includes mechanical alterations of disc height, intradiscal pressure, opening of neural foramina, load distribution and FSU motion. We think that interspinous dynamic devices could help restoring the physiological mechanical properties of a degenerated spinal segment.
We finally agree with Schnake et al. assertion that only dynamic stabilization systems currently offer the potential of a mechanical approach to intervertebral disc regeneration [31].
Last but not least we want to remind that interspinous implant represents a reversible surgical procedure. Any case of failed back syndrome could be surgically revisable. Anatomical preservation allow subsequent appliance of every other surgical technique, like decompression, anterior and posterior fusions, disc arthroplasty.
Conclusions
Clinical reports of patients who underwent interspinous stabilization are good. Interspinous spacers satisfy the concept of minimally invasive techniques.
Relief of low back pain and NIC is satisfactory in a relevant percentage of patients treated with Aperius device. A longer follow-up period is needed but clinical early results are good and promising. The surgical approach can be conducted under local or spinal anaesthesia, thanks to a percutaneous technique for implantation. Surgical technique is easy, duration of the procedure and time of hospitalization are short. Hence, this procedure can be proposed to an old or high-risk population.
DIAM represents a new philosophy in the treatment of degenerative lumbar spine pathology. It represents an alternative option to spinal fusion, providing an excellent pain control in the treatment of a broad spectrum of lumbar spine degenerative disease. It provides stability avoiding potential degenerations at the adjacent levels. DIAM represents a modern dynamic stabilization system allowing motion preservation and restoration of mechanical properties in a degenerate FSU. To date, there is no evidence that dynamic implants will lead to disc regeneration. Future scientific researches may be conducted to find such evidences. Modern treatment concepts should combine intradiscal biological therapy together with dynamic restoration of the affected spinal segment.
Acknowledgments
Conflict of interest None.
References
- 1.Lindsey DP, Swanson KE, Fuchs P, Hsu KY, Zucherman JF, Yerby SA. The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine. 2003;28(19):2192–2197. doi: 10.1097/01.BRS.0000084877.88192.8E. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Hida K, Seki T, Iwasaki Y, Minoru A. An interspinous process distractor (X STOP) for lumbar spinal stenosis in elderly patients: preliminary experiences in 10 consecutive cases. J Spinal Disord Tech. 2004;17(1):72–77. doi: 10.1097/00024720-200402000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui M, Karadimas E, Nicol M, Smith FW, Wardlaw D. Influence of X stop on neural foramina and spinal canal area in spinal stenosis. Spine. 2006;31(25):2958–2962. doi: 10.1097/01.brs.0000247797.92847.7d. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui M, Nicol M, Karadimas E, Smith F, Wardlaw D. The positional magnetic resonance imaging changes in the lumbar spine following insertion of a novel interspinous process distraction device. Spine. 2005;30(23):2677–2682. doi: 10.1097/01.brs.0000187878.79676.26. [DOI] [PubMed] [Google Scholar]
- 5.Richards JC, Majumdar S, Lindsey DP, Beaupre GS, Yerby SA. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine. 2005;30(7):744–749. doi: 10.1097/01.brs.0000157483.28505.e3. [DOI] [PubMed] [Google Scholar]
- 6.Bono CM, Vaccaro AR. Interspinous process devices in the lumbar spine. J Spinal Disord Tech. 2007;20(3):255–261. doi: 10.1097/BSD.0b013e3180331352. [DOI] [PubMed] [Google Scholar]
- 7.Wilke HJ, Drumm J, Haussler K, Mack C, Steudel WI, Kettler A. Biomechanical effect of different lumbar interspinous implants on flexibility and intradiscal pressure. Eur Spine J. 2008;17(8):1049–1056. doi: 10.1007/s00586-008-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunzburg R, Szpalski M, Callary SA, Colloca CJ, Kosmopoulos V, Harrison D, Moore RJ. Effect of a novel interspinous implant on lumbar spinal range of motion. Eur Spine J. 2009;18(5):696–703. doi: 10.1007/s00586-009-0890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson KE, Lindsey DP, Hsu KY, Zucherman JF, Yerby SA. The effects of an interspinous implant on intervertebral disc pressures. Spine (Phila Pa 1976) 2003;28(1):26–32. doi: 10.1097/00007632-200301010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Senegas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur Spine J. 2002;11(Suppl 2):S164–S169. doi: 10.1007/s00586-002-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caserta S, La Maida GA, Misaggi B, Peroni D, Pietrabissa R, Raimondi MT, Redaelli A. Elastic stabilization alone or combined with rigid fusion in spinal surgery: a biomechanical study and clinical experience based on 82 cases. Eur Spine J. 2002;11(Suppl 2):S192–S197. doi: 10.1007/s00586-002-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitesides TE., Jr The effect of an interspinous implant on intervertebral disc pressures. Spine (Phila Pa 1976) 2003;28(16):1906–1907. doi: 10.1097/01.BRS.0000084662.02029.B2. [DOI] [PubMed] [Google Scholar]
- 13.Bellini CM, Galbusera F, Raimondi MT, Mineo GV, Brayda-Bruno M. Biomechanics of the lumbar spine after dynamic stabilization. J Spinal Disord Tech. 2007;20(6):423–429. doi: 10.1097/BSD.0b013e318031af6f. [DOI] [PubMed] [Google Scholar]
- 14.Mariottini A, Pieri S, Giachi S, Carangelo B, Zalaffi A, Muzii FV, Palma L. Preliminary results of a soft novel lumbar intervertebral prothesis (DIAM) in the degenerative spinal pathology. Acta Neurochir Suppl. 2005;92:129–131. doi: 10.1007/3-211-27458-8_28. [DOI] [PubMed] [Google Scholar]
- 15.Taylor J, Pupin P, Delajoux S, Palmer S. Device for intervertebral assisted motion: technique and initial results. Neurosurg Focus. 2007;22(1):E6. doi: 10.3171/foc.2007.22.1.6. [DOI] [PubMed] [Google Scholar]
- 16.Lauryssen C. Appropriate selection of patients with lumbar spinal stenosis for interspinous process decompression with the X STOP device. Neurosurg Focus. 2007;22(1):E5. doi: 10.3171/foc.2007.22.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine. 2007;32(12):1345–1348. doi: 10.1097/BRS.0b013e31805b7694. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui M, Karadimas E, Nicol M, Smith FW, Wardlaw D. Effects of X-STOP device on sagittal lumbar spine kinematics in spinal stenosis. J Spinal Disord Tech. 2006;19(5):328–333. doi: 10.1097/01.bsd.0000211297.52260.d5. [DOI] [PubMed] [Google Scholar]
- 19.Wiseman CM, Lindsey DP, Fredrick AD, Yerby SA. The effect of an interspinous process implant on facet loading during extension. Spine (Phila Pa 1976) 2005;30(8):903–907. doi: 10.1097/01.brs.0000158876.51771.f8. [DOI] [PubMed] [Google Scholar]
- 20.Brussee P, Hauth J, Donk RD, Verbeek AL, Bartels RH. Self-rated evaluation of outcome of the implantation of interspinous process distraction (X-Stop) for neurogenic claudication. Eur Spine J. 2008;17(2):200–203. doi: 10.1007/s00586-007-0540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchta J, Sobottke R, Eysel P, Simons P. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur Spine J. 2009;18(6):823–829. doi: 10.1007/s00586-009-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondrashov DG, Hannibal M, Hsu KY, Zucherman JF. Interspinous process decompression with the X-STOP device for lumbar spinal stenosis: a 4-year follow-up study. J Spinal Disord Tech. 2006;19(5):323–327. doi: 10.1097/01.bsd.0000211294.67508.3b. [DOI] [PubMed] [Google Scholar]
- 23.Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, Skidmore GA, Vessa PP, Dwyer JW, Puccio S, Cauthen JC, Ozuna RM. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13(1):22–31. doi: 10.1007/s00586-003-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4(6):463–471. doi: 10.3171/spi.2006.4.6.463. [DOI] [PubMed] [Google Scholar]
- 25.Hsu KY, Zucherman JF, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, Skidmore GA, Vessa PP, Dwyer JW, Cauthen JC, Ozuna RM. Quality of life of lumbar stenosis-treated patients in whom the X STOP interspinous device was implanted. J Neurosurg Spine. 2006;5(6):500–507. doi: 10.3171/spi.2006.5.6.500. [DOI] [PubMed] [Google Scholar]
- 26.Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, Skidmore GA, Vessa PP, Dwyer JW, Puccio ST, Cauthen JC, Ozuna RM. A multicenter, prospective, randomized trial evaluating the X STOP Interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30(12):1351–1358. doi: 10.1097/01.brs.0000166618.42749.d1. [DOI] [PubMed] [Google Scholar]
- 27.Vena P, Franzoso G, Gastaldi D, Contro R, Dallolio V. A finite element model of the L4–L5 spinal motion segment: biomechanical compatibility of an interspinous device. Comput Methods Biomech Biomed Eng. 2005;8(1):7–16. doi: 10.1080/10255840500062914. [DOI] [PubMed] [Google Scholar]
- 28.Phillips FM, Voronov LI, Gaitanis IN, Carandang G, Havey RM, Patwardhan AG. Biomechanics of posterior dynamic stabilizing device (DIAM) after facetectomy and discectomy. Spine J. 2006;6(6):714–722. doi: 10.1016/j.spinee.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Kim KA, McDonald M, Pik JH, Khoueir P, Wang MY. Dynamic intraspinous spacer technology for posterior stabilization: case–control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg Focus. 2007;22(1):E7. [PubMed] [Google Scholar]
- 30.Verhoof OJ, Bron JL, Wapstra FH, Royen BJ. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17(2):188–192. doi: 10.1007/s00586-007-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnake KJ, Putzier M, Haas NP, Kandziora F. Mechanical concepts for disc regeneration. Eur Spine J. 2006;15(Suppl 3):S354–S360. doi: 10.1007/s00586-006-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]