Abstract
We conducted a randomized trial to evaluate effectiveness of Centchroman in control of mastalgia and compared it with Danazol. Research Question- Is proportion of pain relief achieved by Centchroman similar to or inferior to that achieved by Danazol? In a randomized controlled trial of Centchroman vs. Danazol in mastalgia, 81 patients with mastalgia were studied. Thirty-nine patients were randomized to Danazol arm and 42 in Centchroman arm. The treatment was given for 12 weeks, followed by observation for 12 weeks. The pain was measured by visual analogue scale (VAS) of 0–10. At 12 weeks 89.7% women achieved reduction in pain score to ≤3 in Centchroman group (pvalue 0.001). In Danazol group 69.44% women achieved reduction in pain score to ≤ 3 (p = 0.001). Three months after stopping therapy, Centchroman was more effective in pain score reduction at 24 weeks as compared to Danazol (p = 0.019). Centchroman is an effective, safe and inexpensive alternative to Danazol for treatment of mastalgia.
Electronic supplementary material
The online version of this article (doi:10.1007/s12262-010-0216-z) contains supplementary material, which is available to authorized users.
Keywords: Centchroman, Danazol, Randomized trial, Mastalgia, Breast pain, Antiestrogen, Non-inferiority trial
Introduction
Breast pain or mastalgia is one of the most common benign conditions of the breast. The pattern and severity of pain can be assessed by breast pain chart. Mastalgia may be cyclic or non-cyclic, intermittent or constant, localized or diffuse. Non cyclical mastalgia can be true i.e arising from breast tissue or it can arise from chest wall e.g Tietze’s syndrome and lateral chest wall pain [1].
Cyclical mastalgia is defined as breast pain with either only premenstrual exacerbation or pain throughout the month with premenstrual exacerbation. Non-cyclical mastalgia is defined as intermittent or continuous breast pain without premenstrual exacerbation and no obvious source of musculoskeletal disease.
During luteal phase of menstruation, cell proliferation of ductolobular tissue and interstitial fluid increase result in up to 15% increase in breast size and volume. This increase in breast tissue volume results in pressure on pain nerve endings causing premenstrual pain. Just prior to menstruation the estrogen and progesterone levels fall with reducing cellular proliferation in the early follicular phase and consequent relief of pain and engorgement [2].
Different hypotheses for mastalgia have led to use of different medical and non medical therapies. The mastalgia may be caused by either [3]:
Increased estrogen secretion from ovary
Deficient progesterone production
Increased Prolactin secretion
Many pharmacological agents have been tried in the therapy of mastalgia. The drug therapy includes agents inducing hormonal manipulation such as Danazol [4], Bromocriptine [5], Tamoxifen [6], and LH-RH [7] analogue like Goserelin. Some of the effective non-hormonal agents in mastalgia are Non- steroidal anti-inflammatory gels [8], reassurance [9] and breast support with sport’s bra [10].
There is considerable debate about drug of choice for management of mastalgia. We present our results of a trial of antiestrogen drug “Centchroman”. The objective of the study was to evaluate the effectiveness of Centchroman in control of mastalgia measured by visual analogue scale (VAS), and compare it with that of Danazol.
Patients and Methods
The study was approved by the Institute ethics committee of All India Institute of Medical Sciences. The patients were provided with a detailed printed information sheet ( in Hindi or English depending on the language understood by her) to explain about benign nature of breast pain, the currently available therapy with side effects, the potential benefits of Centchroman and its common use by Government of India as a contraceptive pill. We also informed patients about the possibility of scanty or delayed menstruation by Centchroman. Patients signed a consent form in Hindi or English after understanding this information.
We used a single blind (investigator assessing the response was blinded) two arm parallel design randomized controlled trial. The study was based on a non-inferiority hypothesis, with the aim of demonstrating an effectiveness of Centchroman either equal to or less than that of Danazol. Sample size consideration and details of method of randomization have been described in Appendix. Inclusion criteria: All women with mastalgia with VAS score ≥3 lasting for more than 7 days per cycle were included after signing a consent form. Exclusion criteria: 1. Past history of breast carcinoma or family history of breast carcinoma. 2. Patients with polycystic ovarian diseases and uterine cervical hyperplasia. 3. First six months of Lactation and 4. Pregnancy.
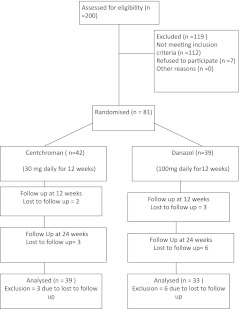
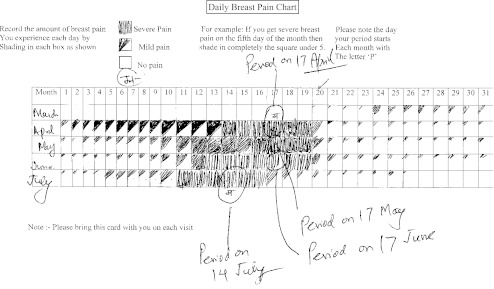
All women in reproductive age group with regular menses having mastalgia were recruited in the study (Fig. 1). After an initial clinical assessment and breast imaging with ultrasound (and mammogram for cases above age 35 years) to exclude any lump or mammary ductal disease, patients were asked to keep a record of their breast pain in a “pain diary”. In this diary patient filled the occurrence of pain on a day to day basis. The time of menses was also marked on pain diary. Figure 2 depicts a pain chart with cyclical pattern of breast pain and premenstrual exacerbation. The severity of mastalgia was assessed by visual analogue scale score ranging from 0–10, zero (0) indicating no pain and 10 indicating very severe pain. Ultrasound scan of pelvis and gynecological evaluation was performed to exclude the patients with polycystic ovarian disease and cervical hyperplasia, as Centchroman is known to worsen these conditions.
Fig. 1.
Consort flow diagram
Fig. 2.
Showing a daily breast pain chart note cyclical mastalgia with premenstrual exacerbation
Patients were randomized to get Centchroman 30 mg daily or Danazol 100 mg daily for 3 months. The patients were evaluated at one week to assess tolerance to the drug. Subsequently patients were followed at 4 weeks, 8 weeks, 12 weeks and 24 weeks and response to therapy was assessed by VAS score. The drug treatment was continued for a total of 12 weeks and then the patient was followed for another 12 weeks without medication to assess sustained response or recurrence of mastalgia.
Statistical Analysis
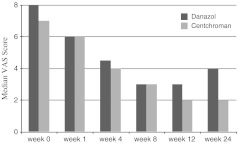
The data was analyzed by using SPSS version 15. Pain score was treated as continuous data. The VAS pain score was not normally distributed, hence data was analyzed by Nonparametric Mann Whitney U test and median pain score plotted against time to see its change with follow up ( Fig. 3) . The age, initial VAS score and duration of first menstrual cycle at start of study were normally distributed. Hence these were analyzed by Student’s t- test. Pattern of mastalgia either cyclical or non-cyclical were analyzed by Chi-square test.
Fig. 3.
Depicting the change in Median VAS pain score up to 24 weeks Follow up
The pain response over a period of time within same group was analyzed by using Repeated Measure Analysis of Variance (Repeated measure ANOVA). P-value less than 0.05 was considered significant. All p values are two sided.
Results
Of 81 patients, 39 were randomized in Danazol arm and 42 in Centchroman arm. Patient’s characteristics in terms of age, pre treatment VAS score and duration of cycle were similar in both arms. The type of mastalgia either cyclical or non-cyclical were also equally distributed in two groups. The baseline features are presented in Table 1.
Table 1.
Comparison of baseline characteristics
| Danazol (mean) ( n = 39) | Centchroman (mean) (n = 41) | P-value | |
|---|---|---|---|
| Age | 32.05 | 31.35 | 0.65* |
| Visual Analogue Scale Score at base line Scale 0-10 | 6.92 | 6.69 | 0.57* |
| Duration of menstrual cycle in days | 28.89 | 28.87 | 0.97* |
| Number of cases with Cyclical mastalgia | 17 | 17 | 0.77** |
| Number of cases with Non-Cyclical mastalgia | 22 | 25 | 0.82** |
P values with * by t test, p values with ** by Chi- square test
There was significant relief of mastalgia in both arms. Patients in Danazol arm experienced early relief of mastalgia as compared to patients in Centchroman arm. VAS score of ≤3 was considered as relief from mastalgia. The proportion of patients achieving VAS score ≤3 was compared in the 2 groups at 12 and 24 weeks. In Centchroman group 35 of 39 patients (89.7%) and in Danazol group 25 of 36 patients (69.44%) were relieved of mastalgia at 12 weeks of therapy. At 24 weeks follow up ( i.e. 3 months after stopping drug) 27 of 38 patients (71.05%) in Centchroman group and 14 of 33 patients (42.42%) in Danazol remained relieved of mastalgia (Fig. 3).
The Relative Risk (RR) for significant pain relief ( i.e. VAS pain score of ≤3) with Centchroman as the “exposed” and Danazol as “reference” category, was calculated at 12 weeks and 24 weeks of follow up (Tables 2 and 3). The Relative risk(RR) of good pain relief at 12 weeks was 1.26 (95% CI; 1.01–1.64). The RR of good pain relief at 24 weeks was 1.67 (95% CI; 1.07–2.61) . The Risk difference between the two groups at 24 weeks ( 71%–42% = 29%) was 29% or 0.29 (95% Confidence interval 0.104 to 0.476). Since the response was 29% better with Centchroman which is greater than the delta of 15% (the clinically meaningful difference or Noninferiority margin) , we fail to reject the Null Hypothesis and conclude that the Centchroman is not inferior to Danazol.
Table 2.
Effect of Centchroman and Danazol on mastalgia
| Weeks | Danazol Median VAS (range in parenthesis) (n = 39) | Centchroman Median VAS (range in parenthesis) (n = 42) | P value Mann-Whitney U test comparing Danazol with Centchroman |
|---|---|---|---|
| 0 | 8 (3 to10) | 7 (2 to10) | 0.41 |
| 1 | 6 (2 to10) | 6 (2 to 8) | 0.177 |
| 4 | 4 (0 to 8) | 4 (0 to 7) | 0.97 |
| 8 | 3 (0 to 8) | 3 (0 to8) | 0.37 |
| 12 | 3 (0 to 5) | 2 (0 to 5) | 0.06 |
| 24 | 4 (0 to 9) | 2 (0 to 7) | 0.019 |
| P value ( to assess change in pain score over time with in a group Green House Geisser test) | 0.001 (Repeated measure ANOVA) | 0.001 (Repeated measure ANOVA) |
Table 3.
Relative risk of significant “Pain Relief” Centchroman Versus Danazol
| VAS less than 3 | Relative Risk (RR) | 95% Confidence interval (95% C.I.) |
|---|---|---|
| At 12 weeks | 1.26 | 1.01–1.64 |
| At 24 weeks | 1.67 | 1.07–2.61 |
Here Centchroman is considered as “Exposure” and Danazol as the “Reference” category. RR of 1.67 indicates that Centchroman is 1.67 times more effective in achieving pain relief when compared to Danazol
Relative risk of 1.67 means that the chance of achieving significant pain relief, was 1.67 times greater with Centchroman than with Danazol i.e. there was 67% greater chance of pain relief with Centchroman as compared to Danazol.
Three patients were lost to follow up in Danazol arm at 12 weeks and 6 patients at 24 weeks, similarly 2 patients at 12 weeks and 3 patients at 24 weeks were lost to follow up in Centchroman arm.
Side Effects
The side effects of treatment with Danazol and Centchroman were menstrual irregularities. Out of 39 patients on Danazol, 3 had delayed menses by 10 days, 1 had menorrhagia and 1 had allergic reaction in the form of urticaria. Of 41 patients in Centchroman arm the majority experienced scanty menses (n = 31), 6 had delayed menses by 10–15 days and 2 had menorrhagia. All these menstrual irregularities subsided after stopping medication and menses returned to normal pattern in all of them. No other side effect was reported by women receiving Centchroman.
We also assessed the regression in nodularity and breast tenderness with therapy. Both the drugs were equally effective in regression of breast tenderness and nodularity. At 12 weeks 90% women in Danazol and 100% women in Centchroman group achieved regression in nodularity. In general women enjoying good pain relief also got relieved of their breast tenderness and nodularity.
Discussion
Severe breast pain interferes with the daily routine life of women and raises fear of breast cancer.
In most patients with mild pain, reassurance (that the symptoms are not due to cancer) is all that is required. A Brazilian study [9] verified overall success rate of 70.2% with reassurance in a study of 85 patients with mastalgia. The other non medical means are dietary measures like fat restriction and avoidance of methylxanthines [11]. Breast support with sport’s brassier [10] in a randomized trial of 200 patients relieved the pain in 89% of patients. The support garments provide relief of pain by reducing the tension on overstretched Cooper’s ligament especially in women endowed with large mammary glands. Hence support garments should be advised in these patients.
The drugs available for the treatment of mastalgia are Danazol, Tamoxifen, Bromocriptine, Evening Primrose Oil, Gamolenic acid [12], LHRH analogue Goserline, oral contraceptive pills, diuretics and topical NSAIDs gels with varying efficacy and side effects.
We have performed a meta-analysis of randomized trials on mastalgia and found Tamoxifen, Danazol, and Bromocriptine to be significantly effective in treatment of mastalgia when compared to placebo [13]. However the response to Evening Primrose oil was no better than placebo. This meta-analysis suggested that antiestrogen, Tamoxifen may be the drug of choice. Bromocriptine and Danazol are best avoided because of their side effects and high cost. Evening primrose oil should not be used because of lack of benefit over placebo. Similarly there is no evidence of any benefit with Vitamin E preparations (like EVION, Merck India Ltd).
Greenblat and co-workers introduced Danazol in 1971. They suggested that it might have a role in mastalgia. Danazol is unique in its action on the pituitary ovarian axis. In monkeys it was shown to act as anti-gonadotrophin, as it depressed serum levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH) and prevented ovulation. Its action in humans is not so clearly defined because it only interferes with FSH and LH levels at higher doses [4]. The usual dose of Danazol for treatment of mastalgia is 100–400 mg per day. Danazol is a very effective agent for severe breast pain and nodularity, with an overall improvement rate of 70%. It is superior to bromocriptine in treatment of cyclical mastalgia [14, 15]. In our study Danazol at dose of 100 mg once a day was effective in 69% cases at 12 weeks. After stopping the medication, the response rate was sustained only in 42% of cases at the end of 24 weeks. In a meta-analysis of 4 randomized trials we found a highly significant relief of mastalgia using Danazol, as compared to placebo [13]. The side effects of Danazol are mainly amenorrhea, the incidence of which increases with dose up to 100% at 600–800 mg per day. It causes weight gain, acne, amenorrhea and hirsutism. All the side effects of Danazol are dose dependent. Presently Danazol is not commonly used in most breast clinics because of several side effects and is reserved as a second line drug for mastalgia.
Tamoxifen is now considered the drug of choice in most breast clinics in the West for treatment of mastalgia (under supervision for 3 months, as drug is not licenced for this use)[Professor Robert E Mansel, Chief Cardiff mastalgia clinic U.K, personal communication]. Tamoxifen is a steroidal antiestrogen and has been found to be effective in relief of mastalgia in various randomized trials [16–18]. It has been used in dosage ranging from 5 mg–20 mg/ day for a period of 12 weeks. In a meta-analysis on 3 published randomized trials of tamoxifen, the overall relief of pain achieved with Tamoxifen compared to placebo had a relative risk (RR) of 1.92 (95% CI; 1.42–2.58) which was highly significant with p < 0.0001 [13]. Tamoxifen is a very well tolerated drug at low dosage of 10 mg daily for 3 months, with minimal side effects. However its use may be associated with hot flashes, vaginal dryness, low libido, mood swings, nausea and rarely fluid retention.
Centchroman (Ormeloxifene–C30H35NO3) is a novel non-steroidal, selective estrogen receptor modulator, anticancer and anti-osteoporotic drug formulated by the Central Drug Research Institute, Lucknow, India. It was included in the National Family Welfare Program in 1995 as a “once a week pill”.
Centchroman [19] has weak estrogen agonistic activity in some tissues like bones, and potent anti-estrogenic action in uterus and breast. It is devoid of progesterone, androgenic and anti-androgenic activities.
Centchroman is free from side effects like nausea, vomiting, weight gain and dizziness. Centchroman does not delay return of fertility (after stopping) as it does not disturb ovulation. It has only one adverse effect, delayed menses in less than 10% of cycles. It maintains normal ovulatory cycles. Centchroman has no apparent adverse effects on endocrine, hematologic, liver and lipid function and, to date, has not been associated with any serious complications viz. heart attack, stroke or thrombosis [20–22].
Centchroman is well absorbed when given orally. Single 30 mg dose results in maximum concentration in serum of 30 to 78 ng/mL in 3 to 8 hours. The drug is well absorbed in nursing mothers with maximum serum concentration of 50 to 79 ng/ ml achieved in 6 hours. The drug is widely distributed throughout the body due to high lipid solubility. The mean residence time was found to be 128 days. It binds strongly with serum albumin. The drug does not compete with cortisol, oestradiol, progesterone, diethylstilbosterol, testosterone and tamoxifen. In target tissues such as endometrium and breast, it competes with estradiol for binding to estrogen receptors and shows an anti-estrogenic activity. The drug is demethylated and about 26% is excreted unchanged in feces [23].
Centchroman is an economic and easily available drug for therapy of mastalgia (Trade name SAHELI by Hindustan Latex Ltd. usual price Rs 2 per 30 mg tablet per day as compared to Danazol costing Rs 15 or more for 100 mg per day). We used it in a pilot study for mastalgia at the All India Institute of Medical Sciences New Delhi, and with its encouraging result the present RCT was conducted after obtaining permission from Drug controller of India for this use and approval of our Institute ethics committee [24]. In the initial part of this study Centchroman was given as 30 mg on alternate days to 19 patients with significant pain relief. The frequency of dosage was later increased to once daily, as there were no major side effects seen and some women missed doses with alternate day therapy. Hence to improve compliance and effect, in latter part of study it was given as 30 mg once a day to 23 patients. The initial response was more pronounced when Centchroman was administered on daily basis as compared to alternate day regimen [24].
In our randomized trial combining both alternate and daily dosage, Centchroman was found to have response rate of 89.7 % (reduction of pain to less than or equal to 3 on VAS) at the end of 12 weeks. Danazol achieved 69.44% response rate at 12 weeks. A greater proportion of women in Centchroman group continue to enjoy pain free life even after stopping the drug, suggesting a longer carry-over effect. Thus the probability of remaining pain free at 6 months was 71% with Centchroman and 42% with Danazol. The response of pain relief was 29% better with Centchroman at 24 weeks (71%-42% = 29%). The trial has demonstrated that this drug is not inferior to Danazol. However, we can not claim the superiority of Centchroman, because our study was based on a null hypothesis of non-inferiority. There is now a need to launch a larger trial in many centres to control this commonest breast symptom of our sisters, based on a Superiority hypothesis.
Centchroman scores over Tamoxifen in being non-steroidal molecule hence devoid of steroid like side effects in the long term therapy. It is also cheaper than Tamoxifen and Danazol. Moreover, there are no reports of endometrial carcinoma or thromboembolic side-effects with long term use of Centchroman as compared to Tamoxifen. It is a pride molecule for Mother India as it is developed, manufactured and marketed by Indian Government.
Conclusion
This trial has demonstrated that Centchroman therapy offers a safe, at least equally effective and inexpensive alternative to Danazol for treatment of mastalgia.
Future Recommendation
The cost-effectiveness and quality of life with Centchroman therapy should be assessed in comparison with other drugs on a larger multi centre randomized trial, based on superiority hypothesis.
Funding Support
NIL.
Presentation
None.
(PDF 84 kb)
Appendix
Sample Size Consideration
We put forth the assumption that, Centchroman therapy would achieve pain relief similar to or slightly less than that achieved by Danazol. Hence in this calculation one tailed hypothesis was considered. The clinically meaningful difference in the proportion of cases getting pain relief in between the two arms is represented by delta-(δ) which is the non-inferiority margin in calculating the sample size with the following null hypothesis (H0).
H0: ε ≤ δ, and alternative hypothesis HA: ε > δ , where ε is the difference in the proportion of success between two therapies. (ε = p1–p2 ; where p1 = pain response proportion with Centchroman therapy,
p2 = pain response proportion with Danazol therapy).
Allowing the 15% of difference between results of two therapies as the clinically meaningful difference, the value of δ has been chosen as 0.15.
Assuming: p1 = 50% .
The literature showed that 60% cases get benefit in mastalgia by Danazol therapy, hence value of p2 = 60% or 0.6.
The values of other factors were taken as follows:-Zœ = 1.645 for œ = 0.05 for one tailed hypothesis
Zβ = 0.84 for power = 80% for one or two tailed hypothesis
With all these values the formula used in calculation of sample size for one group was as follows: -
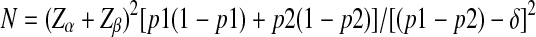 |
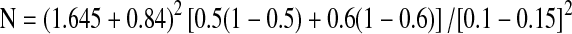 |
The value of N (i.e. the sample size for one group) was calculated to be 48. Expecting 10% of withdrawal of the volunteers from the groups, the total numbers of patients to participate were considered 110. The study presents the result on 81 subjects.
Method of Randomization
Block randomization with a block size of 4 was employed to generate a list of random numbers on web site www.randomization.com. The sealed numbered opaque brown envelopes were prepared with the label of case number e.g “ case 1” . The envelopes were opened after ensuring inclusion criteria and signing the consent form. The envelope contained a folded slip of paper stating the assigned treatment, i.e Centchroman or Danazol. The assigned treatment was given to the patient. No randomization violation or contamination occurred.
References
- 1.Preece PE, Mansel RE, Bolton PM, Hughes LE, Baum M, Gravelle IH. Clinical syndromes of mastalgia. Lancet. 1976;2:670–3. doi: 10.1016/S0140-6736(76)92477-6. [DOI] [PubMed] [Google Scholar]
- 2.Potten CS, Watson RJ, Williams GT, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988;58:163–70. doi: 10.1038/bjc.1988.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes LE, Mansel RE, Webster DJT. Problems of concept and nomenclature of benign breast disease. Benign disorders and diseases of the breast. 2. London: Saunders; 2000. p. 15. [Google Scholar]
- 4.Asch RH, Greenblatt RB. The use of an impeded androgen Danazol in the management of benign breast disorders. Am J Obstet Gynecol. 1977;127:130. doi: 10.1016/s0002-9378(16)33237-9. [DOI] [PubMed] [Google Scholar]
- 5.Mansel RE, Preece PE, Hughes LE. A double blind trial of the prolactin inhibitor bromocriptine in painful benign breast disease. Brit J Surg. 1978;65:724–727. doi: 10.1002/bjs.1800651015. [DOI] [PubMed] [Google Scholar]
- 6.Fentiman IS, Caleffi M, Hamed H. Dosage and duration of tamoxifen for mastalgia. A controlled trial. Br J Surg. 1989;76:901–904. doi: 10.1002/bjs.1800760909. [DOI] [PubMed] [Google Scholar]
- 7.Mansel RE, Goyal A, Preece P, Leinster S, Maddox PR, Gateley C, Kubista E, Fournier D. European randomized, multicenter study of goserline (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191(6):1942–9. doi: 10.1016/j.ajog.2004.06.100. [DOI] [PubMed] [Google Scholar]
- 8.Colak T, Ipek T, Kanik A, Ogetman Z, Aydin S. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525–30. doi: 10.1016/S1072-7515(02)01893-8. [DOI] [PubMed] [Google Scholar]
- 9.Barros AC, Mottola J, Ruiz CA. Reassurance in the treatment of mastalgia. Breast J. 1999;5:162. doi: 10.1046/j.1524-4741.1999.98089.x. [DOI] [PubMed] [Google Scholar]
- 10.Hadi MS. Sports Brassiere; Is it a solution for mastalgia? Breast J. 2000;6:407. doi: 10.1046/j.1524-4741.2000.20018.x. [DOI] [PubMed] [Google Scholar]
- 11.Minton JP, Foeking MK, Webster DJT, et al. Response of fibrocystic disease to caffeine withdrawal and correlation with cystic nucleotides with breast disease. Am J Obstet Gynecol. 1979;135:157. [PubMed] [Google Scholar]
- 12.Goyal A, Mansel RE. A randomized multicenter study of gamolenic acid with and without, antioxidants, vitamins and minerals in the management of mastalgia. Breast J. 2005;11:41–47. doi: 10.1111/j.1075-122X.2005.21492.x. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A, Mansel RE, Arvind N, Prasad K, Dhar A, Chabra A. Evidence based management of Mastalgia: a meta-analysis of randomized trials. Breast. 2007;16:503–12. doi: 10.1016/j.breast.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Hughes LE, Mansel RE, Webster DJT. Problems of concept and nomenclature of benign breast disease. Benign Disorders and Diseases of the Breast. 2. London: Saunders; 2000. p. 108. [Google Scholar]
- 15.Mansel RE, Preece PE, Huges LE. A double blind trial of prolactin inhibitor bromocriptine in painful benign breast disease. Br J Surg. 1978;65:724–727. doi: 10.1002/bjs.1800651015. [DOI] [PubMed] [Google Scholar]
- 16.Messinis LE, Lolis D. Treatment of Premenstrual Mastalgia with Tamoxifen. Acta Obstet Gynecol Scand. 1988;67:307–309. [PubMed] [Google Scholar]
- 17.Fentiman IS, Caleffi M, Brame K, Chaudary MA, Hayward JL. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1(8476):287–8. doi: 10.1016/S0140-6736(86)90825-1. [DOI] [PubMed] [Google Scholar]
- 18.Kontostolis E, Stefanidis K, Navrozoglou I, Lolis D. Comparison of Tamoxifen with Danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393–397. doi: 10.3109/09513599709152566. [DOI] [PubMed] [Google Scholar]
- 19.Singh MM, Centchroman A selective estrogen receptor modulator, as a contraceptive and for the management of hormone-related clinical disorder. Med Res Rev. 2001;21(4):302–347. doi: 10.1002/med.1011. [DOI] [PubMed] [Google Scholar]
- 20.Kamboj VP, Setty BS, Chandra H, Roy SK, Kar AB. Biological profile of Centchroman—a new post-coital contraceptive. Indian J Exp Biol. 1977;15:1144–1150. [PubMed] [Google Scholar]
- 21.Vaidya R, Joshi U, Meherji P, Rege N, Betrabet S, Joshi L, Sheth A, Devi PK. Centchroman in healthy female volunteers. Indian J Exp Biol. 1977;15:1173–1176. [PubMed] [Google Scholar]
- 22.Multicentric trial with biweekly cum weekly dose. Lucknow: Central Drug Research Institute; 1991. [Google Scholar]
- 23.Lal J. Clinical pharmacokinetics and interaction of Centchroman- a mini review. Contraception. 2010 doi: 10.1016/j.contraception.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Dhar A, Srivastava A. Role of centchroman in regression of Mastalgia and Fibroadenoma. World J Surg. 2007;31:1178–84. doi: 10.1007/s00268-007-9040-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 84 kb)