Abstract
This study aimed to assess the inhibitory activities of methanol extracts from the microalgae Ankistrodesmus convolutus, Synechococcus elongatus, and Spirulina platensis against Epstein-Barr virus (EBV) in three Burkitt’s lymphoma (BL) cell lines, namely Akata, B95-8, and P3HR-1. The antiviral activity was assessed by quantifying the cell-free EBV DNA using real-time polymerase chain reaction (PCR) technique. The methanol extracts from Ankistrodesmus convolutus and Synechococcus elongatus displayed low cytotoxicity and potent effect in reducing cell-free EBV DNA (EC50<0.01 µg/ml) with a high therapeutic index (>28 000). After fractionation by column chromatography, the fraction from Synechococcus elongatus (SEF1) reduced the cell-free EBV DNA most effectively (EC50=2.9 µg/ml, therapeutic index>69). Upon further fractionation by high performance liquid chromatography (HPLC), the sub-fraction SEF1’a was most active in reducing the cell-free EBV DNA (EC50=1.38 µg/ml, therapeutic index>14.5). This study suggests that microalgae could be a potential source of antiviral compounds that can be used against EBV.
Keywords: Microalgae, Ankistrodesmus convolutus, Synechococcus elongatus, Spirulina platensis, Lymphoblastoid cells, Epstein-Barr virus (EBV)
1. Introduction
Epstein-Barr virus (EBV) is a γ-herpes virus that infects 90% of the adult human population and is prevalent among Asians, especially the Chinese (Klein, 1994). The virus is able to coexist within its host almost asymptomatically. However, EBV infection may lead to the emergence of lymphoproliferative disorders, such as Burkitt’s lymphoma (BL) and Hodgkin’s lymphoma (HL) and many neoplasms, especially undifferentiated nasopharyngeal carcinoma (NPC) (Rickinson and Kieff, 1996; Rezk and Weiss, 2007). Infection of B lymphocytes by EBV may also cause infectious mononucleosis and post-transplantation lymphoproliferative disorder (PTLD) (Kurth et al., 2000; Krenauer et al., 2010).
Both the microalgae and macroalgae (seaweeds) are potential sources of antiviral compounds, with sulfated polysaccharides being the major active principles. For instance, calcium spirulan from the commercial alga Spirulina inhibits replication of viruses such as human cytomegalovirus, herpes simplex virus type 1 (HSV-1), human herpesvirus type 6 (HHV-6), and human immunodeficiency virus type 1 (HIV-1) (Rechter et al., 2006). The cell wall-sulfated polysaccharide of the red alga Porphyridium prevents the adsorptions of HSV-1, HSV-2, and varicella-zoster virus (VZV) into the host cells (Huleihel et al., 2001). Carrageenan, a type of sulfated polysaccharide from red seaweeds, is a potent inhibitor of human papillomavirus (HPV) that acts by preventing the binding of HPV virions to cells (Buck et al., 2006). In addition, the galactofucan sulfate extract from the brown seaweed Undaria pinnatifida is a potent inhibitor of the herpes viruses HSV-1, HSV-2, and human cytomegalovirus (HCMV) (Hemmingson et al., 2006).
Despite the many studies on antiviral compounds from algae, there have been few reports on such compounds used against EBV. The polysaccharide fractions from Spirulina showed only weak inhibition of EBV in B-lymphocytes (Rechter et al., 2006). Other studies focused on the anti-tumor promoting activity of EBV. For instance, dichloromethane extracts from the seaweeds Undaria pinnatifida and Laminaria inhibit the activation of EBV induced by tumor-promoters (Ohigashi et al., 1992).
There are no regulatory agency-approved drugs for the treatment of EBV-related diseases (Gershburg and Pagano, 2005). Most of the anti-EBV drugs inhibit replication of the virus by targeting the DNA polymerase. There has been increased interest in screening biological sources for bioactivity against EBV. For instance, a compound from licorice root, glycyrrhizic acid, inhibits EBV replication in superinfected Raji cells while acerogenin from Aceraceae inhibits the expression of EBV early antigen induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in mice (Lin, 2003; Akihisa et al., 2006). Ethanolic extract and andrographolid from Andrographis paniculata inhibit the expressions of Rta, Zta, and EA-D proteins of EBV in P3HR-1 cells (Lin et al., 2008).
The objective of the present study was to assess the antiviral activities of methanol extracts and fractions of the extracts from three microalgae from the University of Malaya Algae Culture Collection (UMACC), namely Ankistrodesmus convolutus, Spirulina platensis, and Synechococcus elongatus against EBV in lymphoblastoid cells. The three microalgae were chosen for this study as they were shown to have antineoplastic activity against NPC (Lai et al., 2008). To date, this is the first study involving the screening of the three microalgae for antiviral activity against EBV. Previous studies focused mainly on the screening of the microalgae for products such as fatty acids and pigments (Chu et al., 1995). The antiviral activities of the extracts were assessed based on their effects on the reduction of cell-free EBV DNA from the lymphoblastoid cells.
2. Materials and methods
2.1. Culture of microalgae
Three strains of microalgae from the UMACC, namely Spirulina (Arthrospira) platensis UMACC 161, Synechococcus elongatus UMACC 105, and Ankistrodesmus convolutus UMACC 101 were used for this study (Phang and Chu, 1999). Both Spirulina platensis and Synechococcus elongatus were grown in Kosaric Medium while Ankistrodesmus convolutus UMACC 101 was grown in Bold’s Basal Medium (BBM) (Phang and Chu, 1999). The cultures were placed on shelves illuminated with fluorescent lamps [42 µmol/(m2∙s), 12 h:12 h light-dark cycle] in temperature-controlled (28 °C) room.
2.2. Preparation of extracts from microalgae
The algae from stationary phase cultures were harvested by centrifugation (960×g) and the cell pellets were dried at 37 °C before extraction. The dried biomass (25–30 mg) was mixed with methanol (5 ml) and homogenized using a hand tissue grinder. The mixture was centrifuged (960×g) and the supernatant was transferred to a clean tube before being evaporated using a rotary evaporator (50 °C). The dried extract was dissolved again in a small volume of solvent and transferred into a small vial before use.
2.3. Fractionation by column chromatography
The crude extracts were subjected to fractionation by way of silica gel column chromatography. The solid phase consisted of silica gel 60 (Merck, Germany) with a mesh size of 0.063–0.200 mm. Crude extracts of Ankistrodesmus convolutus and Synechococcus elongatus were eluted using a mixture of hexane and acetone with increasing polarity. The following solvent mixtures were used in sequence: 9:1, 8:2, 7:3, 6:4, 5:5, 3:7, and 1:9 (v/v) hexane-acetone, and the last fraction was eluted with 100% methanol. A total volume of 120 ml of each solvent mixture was applied to the column. Each elute was collected at a constant volume of 25 ml and spotted on a thin layer chromatography (TLC) plate and developed using petroleum ether-acetone (65:35, v/v). Elutes which gave similar TLC profiles were pooled as one fraction.
2.4. Fractionation by high performance liquid chromatography (HPLC)
After separation by column chromatography, the samples which showed activity were further fractionated by HPLC. The HPLC system consisted of a Perkin Elmer Series 2000 pump, a Gilson FC203B fraction collector, and a Perkin Elmer Series 200 UV/VIS detector set at 238 nm. The samples were first separated using a reversed-phase analytical column (Chromolith® performance RP-18 encapped, 4.6 mm×100 mm, pore size 2 µm to 13 nm, monolithic; Merck, Germany) equipped with a guard column (Chromolith® guard 5-4.6 mm cartridge, RP-18; Merck, Germany). In order to collect the fractions for further test, the samples were separated using a semi-preparative column (Chromolith® semi-prep RP-18 encapped 100-10 mm monolithic HPLC column, pore size 2 µm to 13 nm; Merck, Germany).
The mobile phase consisted of a mixture of acetonitrile:water operated on a gradient basis starting from a ratio of 9:1 to 100% acetonitrile within 30 min at a flow rate of 2 ml/min. The samples were dissolved in the mobile phase (50 mg/L) and 70 µl was injected into the semi-preparative column for each run. Fractions indicated by individual peaks or groups of peaks were collected from each run and fractions from several runs were pooled and concentrated before use.
2.5. Lymphoblastoid cell cultures
Three EBV-positive BL cell lines were used, namely Akata, B95-8, and P3HR-1. All cell lines were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 50 U penicillin-streptomycin, and were grown in a CO2-incubator.
2.6. Cytotoxicity assay
The cytotoxic effects of the extracts were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay (Mosmann, 1983). The cells (50 µl) were seeded into 96-well plates at an initial density ranging from 5×105 to 1×106 cells/ml. The microalgal extracts were dissolved in dimethyl sulfoxide (DMSO) and the concentrations tested ranged from 25 to 200 µg/ml. The final concentration of DMSO in the medium was not more than 0.03% (v/v) and did not cause any significant cytotoxic effect. The cultures treated with acyclovir and foscarnet were used for comparison. The negative control consisted of cells without extract but containing DMSO. The concentrations of the column chromatography fractions and HPLC sub-fractions ranging from 12.5 to 100.0 µg/ml and 2.5 to 20.0 µg/ml, respectively, were used for the test.
The cells were incubated at 37 °C for 72 h, after which the MTT solution (5 mg/ml) was added and the plates were incubated under 5% CO2 at 37 °C for 4 h in the absence of light. The excess supernatant was removed from each well and 100 µl of DMSO was added to dissolve the formazan crystals. The optical density (OD) was measured at 570 and 620 nm (reference wavelength). The cell viability (CV) was calculated using the following formula: CV=(OD570,TS/OD570,NC)×100%, where OD570,TS is the OD570 of treated samples, and OD570,NC is the OD570 of the negative control.
The median inhibitory concentration (IC50) was determined by finding the concentration of the test substance that inhibited 50% of the cell growth.
2.7. Effects of microalgal extracts on cell-free EBV viral load
The antiviral activities of the microalgal extracts were assessed based on their abilities to reduce the cell-free EBV load released from the lytic BL cells. The BL cells were incubated with the microalgal extracts or drugs (acyclovir and foscarnet), ranging from 0, 0.1, 1.0, 10.0, to 20.0 µg/ml. The concentrations of the extracts used were based on a preliminary range-finding test. The cultures were induced into the lytic cycle at the beginning of the experiment, immediately after adding the extracts or drugs. The lytic cycles of B95-8 and P3HR-1 cells were induced by growing the cultures in the presence of 0.5% (v/v) phorbol 12-myristate-13-acetate (PMA) (20 µg/ml) and 1% (v/v) sodium N-butyrate (0.5 µmol/L) for 24 h. The production of EBV in Akata cells was induced using 0.8% (v/v) rabbit antiserum to whole human IgG (Chang et al., 1999). The cell-free EBV DNA was then quantified using real-time quantitative polymerase chain reaction (PCR) after 72 h incubation and the amounts were compared with the control.
The chemically induced cells were pelleted by centrifugation (300×g, 5 min) and the supernatant was kept for DNA extraction after filtering through a 0.2 µm membrane filter. The DNA was extracted using QIAmp DNA blood mini kit (Qiagen, Germany) according to the manufacturer’s protocols. The DNA obtained was subjected to real-time PCR amplification based on fluorogenic PCR reactions set up in a reaction volume of 25 µl. The reaction mixture contained 12.5 µl of 2× TaqMan® universal PCR master mix, 2.5 µl of 10× primer and probe mixture made up of 3 µmol/L of each amplification primer and 0.25 µmol/L of the EBV probes, and 7.5 µl of ultrapure water. The DNA (2.5 µl) was amplified in a 96-well reaction plate format in a Bio-Rad iCycler. Each sample was analyzed in duplicate. Multiple negative water blanks were included in every analysis. The real-time PCR system was developed for EBV DNA detection toward the BamH1-W region. The BamH1-W system consisted of the amplification primers W-44F (5′-CCC AAC ACT CCA CCA CAC C-3′) and W-119R (5′-TCT TAG GAG CTG TCC GAG GG-3′), and the dual labeled fluorescent probe W-67T [5′-(FAM) CAC ACA CRA CAC ACA CCC ACC CGT CTC (TAMRA)-3′] (Lo et al., 1999). A standard curve was run in parallel using DNA extracted from the EBV-positive cell line Namalwa (American Type Culture Collection CRL-1432). The concentrations of cell-free EBV DNA were expressed as copies of EBV genome per ml of supernatant.
Amplification data were collected by the iCycler iQ™ real-time PCR detection system and analyzed using the software provided. The mean quantity of each duplicate was used for further calculation. The concentration of EBV DNA in the supernatant was calculated using the following equation: 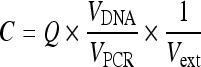 ,
where C (copies/ml) represents the concentration of EBV genome in the supernatant, Q (copies) represents the target quantity determined by a sequence detector of the PCR system, V
DNA represents the total volume of DNA obtained after extraction (typically 200 µl/Qiagen extraction), V
PCR represents the volume of DNA solution used for PCR (typically 2.5 µl), and V
ext represents the volume of sample extracted (typically 300 µl). The amounts of DNA of the treated samples were compared with the control and expressed on a percentage basis.
,
where C (copies/ml) represents the concentration of EBV genome in the supernatant, Q (copies) represents the target quantity determined by a sequence detector of the PCR system, V
DNA represents the total volume of DNA obtained after extraction (typically 200 µl/Qiagen extraction), V
PCR represents the volume of DNA solution used for PCR (typically 2.5 µl), and V
ext represents the volume of sample extracted (typically 300 µl). The amounts of DNA of the treated samples were compared with the control and expressed on a percentage basis.
2.8. Statistical analysis
Data were presented as mean with standard deviation (SD) derived from duplicate samples of two independent experiments. The data were analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test using Statistica software (Version 5). A P value of <0.05 was regarded as statistically significant.
3. Results
3.1. Yields of microalgal biomass, extracts, and fractions
Of the three microalgae, the highest biomass was obtained from Spirulina platensis (1.87 g dry weight (DW)/L) followed by Synechococcus elongatus (1.04 g DW/L) and Ankistrodesmus convolutus (1.01 g DW/L). The yields of crude methanol extracts from Ankistrodesmus convolutus (178 mg/g DW) and Synechococcus elongatus (178 mg/g DW) were similar and much higher than that from Spirulina platensis (87 mg/g DW). Bioassay-guided fractionation was performed on the methanol extracts of Ankistrodesmus convolutus and Synechococcus elongatus. A total of seven fractions were separated using silica gel column chromatography with yields ranging from 7.2 to 68.3 and 1.3 to 51.5 mg/g crude extracts of Ankistrodesmus convolutus and Synechococcus elongatus, respectively (Table 1). The fractions which showed bioactivity were separated using HPLC for further test. A total of 20 sub-fractions with yields ranging from 10 to 171 µg/mg dry fraction were obtained from Ankistrodesmus convolutus (Table 2). Another 10 sub-fractions were obtained from Synechococcus elongatus, with yields ranging from 21 to 325 µg/mg dry fraction.
Table 1.
Details of column chromatography fractions
| Fraction | Code | Mobile phase hexane:acetone | Yield (mg/g crude extract) |
| Ankistrodesmus convolutus | |||
| 1 | ACF1 | 9:1 | 7.2 |
| 2 | ACF2 | 8:2 | 20.4 |
| 7:3 | |||
| 3 | ACF3 | 7:3 | 23.2 |
| 4 | ACF4 | 7:3 | 9.4 |
| 6:4 | |||
| 5 | ACF5 | 6:4 | 8.3 |
| 6 | ACF6 | 5:5 | 22.8 |
| 3:7 | |||
| 1:9 | |||
| 7 | ACF7 | 100% methanol | 68.3 |
| Synechococcus elongatus | |||
| 1 | SEF1 | 9:1 | 12.0 |
| 2 | SEF2 | 8:2 | 17.0 |
| 7:3 | |||
| 3 | SEF3 | 7:3 | 3.9 |
| 4 | SEF4 | 7:3 | 1.6 |
| 6:4 | |||
| 5 | SEF5 | 6:4 | 1.3 |
| 5:5 | |||
| 6 | SEF6 | 5:5 | 37.6 |
| 4:6 | |||
| 1:9 | |||
| 7 | SEF7 | 100% methanol | 51.5 |
Table 2.
Details of HPLC fractions*
| Fraction | Code | Yield (µg/mg dry fraction) |
| Ankistrodesmus convolutus | ||
| 1 | ACF1’a | 95 |
| 2 | ACF1’b | 38 |
| 3 | ACF1’c | 50 |
| 4 | ACF1’d | 22 |
| 5 | ACF1’e | 47 |
| 6 | ACF1’f | 129 |
| 7 | ACF1’g | 16 |
| 8 | ACF2’a | 171 |
| 9 | ACF2’b | 10 |
| 10 | ACF2’c | 144 |
| 11 | ACF2’d | 89 |
| 12 | ACF2’e | 50 |
| 13 | ACF2’f | 20 |
| 14 | ACF3’a | 73 |
| 15 | ACF3’b | 105 |
| 16 | ACF3’c | 109 |
| 17 | ACF3’d | 82 |
| 18 | ACF3’e | 96 |
| 19 | ACF3’f | 69 |
| 20 | ACF3’g | 109 |
| Synechococcus elongatus | ||
| 1 | SEF1’a | 149 |
| 2 | SEF1’b | 200 |
| 3 | SEF1’c | 315 |
| 4 | SEF1’d | 66 |
| 5 | SEF1’e | 34 |
| 6 | SEF4’a | 21 |
| 7 | SEF4’b | 325 |
| 8 | SEF4’c | 180 |
| 9 | SEF4’d | 29 |
| 10 | SEF4’e | 196 |
Only the column chromatography fractions which showed bioactivity were sub-fractionated by HPLC
3.2. Effects of microalgal extracts on cell-free EBV load from lymphoblastoid cells
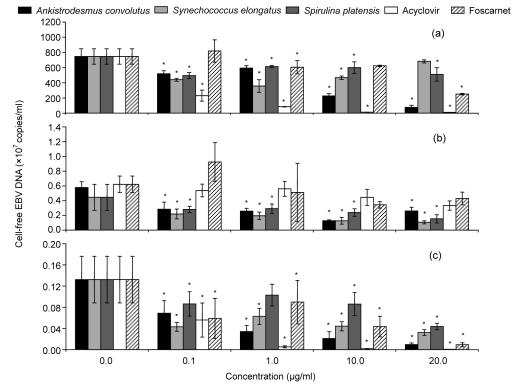
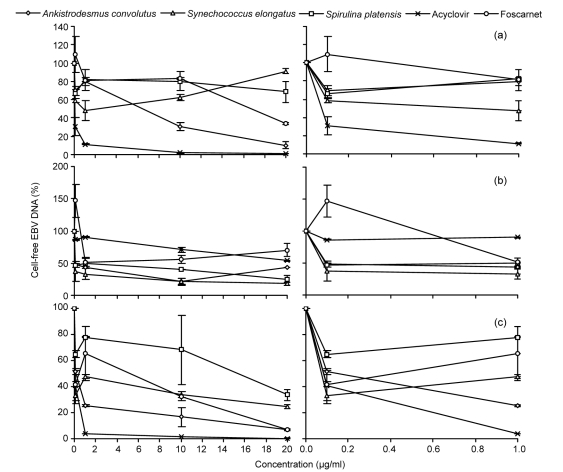
The effects of the microalgal extracts in reducing cell-free EBV load were assessed by determining the quantity of the EBV DNA in the supernatant using real-time PCR (Fig. 1). Akata cells produced the highest number of cell-free EBV DNA (6.7×109–8.2×109 copies/ml) (Fig. 1a). In general, the microalgal extracts reduced the amount of cell-free EBV DNA significantly (P<0.05) compared to the control. The percentage reduction of EBV DNA was compared to the negative control and the half maximal effective concentration (EC50) was determined. The antiviral effect of the crude methanol extracts varied with the cell lines tested (Fig. 2, Table 3). In Akata cells, only methanol extracts from Ankistrodesmus convolutus significantly reduced the percentage of cell-free EBV DNA compared to the control (Fig. 2a). In comparison, the percentage of cell-free EBV DNA decreased markedly in B95-8 and P3HR-1 cells incubated with the extracts from the three microalgae (Figs. 2b and 2c).
Fig. 1.
Cell-free EBV DNA load in the media of Akata (a), B95-8 (b), and P3HR-1 (c) cells treated with methanol extracts from microalgae or antiviral drugs
The Burkitt’s lymphoma (BL) cells were chemically induced into the lytic cycle before being exposed to the microalgal extracts or antiviral drugs for 72 h. DNA was extracted from the culture medium and BamH1-W LP fragment region of the EBV genome was quantified using real-time quantitative PCR. The data are shown as mean±SD of two independent experiments performed in duplicate. * P<0.05, significant difference from the control
Fig. 2.
Percentage of cell-free EBV DNA in the media of Akata (a), B95-8 (b), and P3HR-1 (c) cells treated with methanol extracts from microalgae or antiviral drugs, compared to the control
The Burkitt’s lymphoma (BL) cells were chemically induced into the lytic cycle before being exposed to microalgal extracts or antiviral drugs for 72 h. DNA was extracted from the culture medium and BamH1-W LP fragment region of the EBV genome was quantified using real time-PCR. The data are shown as mean±SD of two independent experiments performed in duplicate
Table 3.
Cytotoxicities of microalgal extracts and antiviral drugs and their effects on cell-free EBV DNA load in Akata, B95-8, and P3HR-1 cells
| Cell line | Microalgal extract/drug | IC50a (μg/ml) | EC50b (μg/ml) | Therapeutic indexc |
| Akata | Ankistrodesmus convolutus | 184.0±36.8 | 8.180±1.476 | 23 |
| Synechococcus elongatus | 190.0±14.1 | >20 | <10 | |
| Spirulina platensis | 89.0±1.4 | >20 | <4.5 | |
| Acyclovir | >200 | 1.190±0.809 | >168 | |
| Foscarnet | >200 | 0.690±0.085 | >290 | |
| B95-8 | Ankistrodesmus convolutus | 78.0±4.2 | 0.313±0.319 | 249 |
| Synechococcus elongatus | 116.5±6.3 | 0.021±0.026 | 5548 | |
| Spirulina platensis | 166.0±2.2 | 0.021±0.013 | 7905 | |
| Acyclovir | >200 | >20 | NDd | |
| Foscarnet | >200 | 0.900±0.081 | >222 | |
| P3HR-1 | Ankistrodesmus convolutus | >200 | 0.006±0.001 | >33333 |
| Synechococcus elongatus | >200 | 0.007±0.002 | >28571 | |
| Spirulina platensis | >200 | 13.750±4.052 | >15 | |
| Acyclovir | >200 | 0.114±0.058 | >1754 | |
| Foscarnet | >200 | 0.788±0.085 | >254 | |
IC50 is the cytotoxic concentration of the compound that decreased cell viability to 50% of untreated cells determined by MTT assay. Results represent means±SD of two independent experiments performed in duplicate
EC50 is the effective concentration of the compound needed to inhibit EBV genome copy numbers by 50% relative to the negative control. Results represent means±SD of two independent experiments performed in duplicate
Therapeutic index refers to the ratio of IC50 to EC50
ND: not determined
The extracts and drugs did not show a strong cytotoxic effect against the three cell lines, as indicated by the high IC50 values (Table 3). In general, the methanol extract from Spirulina platensis was less effective than the extracts from Ankistrodesmus convolutus and Synechococcus elongatus in reducing the cell-free EBV DNA load in Akata and P3HR-1 cells. Therefore, the extracts from the two microalgae, but not Spirulina platensis, were subjected to further fractionation. Methanol extracts from Ankistrodesmus convolutus and Synechococcus elongatus were most potent against P3HR-1 in reducing the EBV DNA load. The high therapeutic index showed that the extracts were active against EBV but not cytotoxic against BL cells. The crude extracts from all the three microalgae were least effective against Akata cells. The extracts of Ankistrodesmus convolutus and Synechococcus elongatus showed much higher activity than acyclovir and foscarnet against B95-8 and P3HR-1 cells based on the EC50 values.
In general, the column chromatography fractions were more cytotoxic than the crude extracts (Table 4). Of the fractions, ACF2 showed the highest activity (EC50=4.2 µg/ml) in reducing the cell-free EBV DNA from Akata cells, yet it showed high cytotoxicity, giving a low therapeutic index. In comparison, ACF3 showed low EC50 but high IC50 against Akata cells, giving a high therapeutic index. Of the fractions from Synechococcus elongatus, SEF3 showed low EC50 values (3.2–9.6 μg/ml) against the three cell lines.
Table 4.
Effects of silica gel column chromatography fractions of methanol extracts from microalgae against the release of EBV in Akata, B95-8, and P3HR-1 cell lines based on the amount of cell-free EBV DNA detected by real-time PCR
| Cell line | Silica gel fraction | IC50 (μg/ml) | EC50 (μg/ml) | Therapeutic index |
| Akata | Ankistrodesmus convolutus | |||
| ACF1 | >200 | >20 | ND | |
| ACF2 | 35±1 | 4.2±1.1 | 8.3 | |
| ACF3 | >200 | 6.0±1.2 | >33 | |
| ACF4 | 77±8 | >20 | <4 | |
| ACF5 | 87±14 | >20 | ND | |
| ACF6 | 51±2 | 19.7±0.8 | 3.0 | |
| ACF7 | >200 | >20 | ND | |
| Synechococcus elongatus | ||||
| SEF1 | >200 | >20 | ND | |
| SEF2 | >200 | 16.9±0.4 | >12 | |
| SEF3 | 22±2 | 9.6±0.4 | 2.0 | |
| SEF4 | >200 | >20 | ND | |
| SEF5 | >200 | >20 | ND | |
| SEF6 | >200 | >20 | ND | |
| SEF7 | >200 | >20 | ND | |
| B95-8 | Ankistrodesmus convolutus | |||
| ACF1 | 68±24 | 3.1±0.8 | 22.0 | |
| ACF2 | >200 | >20 | ND | |
| ACF3 | 94±12 | >20 | <5 | |
| ACF4 | >200 | >20 | ND | |
| ACF5 | >200 | 6.2±3.4 | >32 | |
| ACF6 | >200 | 4.4±2.2 | >46 | |
| ACF7 | >200 | >20 | ND | |
| Synechococcus elongatus | ||||
| SEF1 | 89±13 | 3.5±2.5 | 32.0 | |
| SEF2 | 91±6 | >20 | <5 | |
| SEF3 | 40±1 | 3.2±0.4 | 12.5 | |
| SEF4 | >200 | 15.7±4.7 | >13 | |
| SEF5 | >200 | >20 | ND | |
| SEF6 | >200 | >20 | ND | |
| SEF7 | >200 | >20 | ND | |
| P3HR-1 | Ankistrodesmus convolutus | |||
| ACF1 | >200 | 13.5±3.0 | >15 | |
| ACF2 | 55±1 | >20 | <3 | |
| ACF3 | >200 | >20 | ND | |
| ACF4 | >200 | >20 | ND | |
| ACF5 | >200 | 18.7±1.0 | >11 | |
| ACF6 | 70±2 | >20 | <4 | |
| ACF7 | >200 | >20 | ND | |
| Synechococcus elongatus | ||||
| SEF1 | >200 | 2.9±0.9 | >69 | |
| SEF2 | 76±4 | 13.1±4.3 | 6.0 | |
| SEF3 | 24±6 | 3.8±1.3 | 6.0 | |
| SEF4 | >200 | >20 | ND | |
| SEF5 | >200 | >20 | ND | |
| SEF6 | >200 | >20 | ND | |
| SEF7 | >200 | >20 | ND | |
Of the fractions tested against B95-8 cells, ACF6 showed the highest therapeutic index (>46) while ACF1 showed the lowest EC50 (3.1 µg/ml) in reducing the cell-free EBV load. On the other hand, SEF1 showed the highest inhibitory activity against EBV (EC50=2.9 µg/ml) in P3HR-1 cells and gave the highest therapeutic index (>69). Therapeutic index could not be determined for most of the fractions as IC50 and EC50 were much higher than the range of concentrations tested (>200 µg/ml and >20 µg/ml, respectively). Such fractions were not cytotoxic and did not show antiviral activity within the range of concentrations tested.
The column chromatography fractions (ACF1, ACF2, ACF3, SEF1, and SEF3) which showed bioactivity were separated using HPLC for further test (Tables 1 and 2). Only sub-fractions from ACF2 but not ACF3 were effective in reducing the cell-free viral DNA in Akata cells (Table 5) and of these sub-fractions, ACF2’d was most active (EC50=1.6 µg/ml). In general, the HPLC sub-fractions were more cytotoxic against B95-8 than against other cell lines as indicated by the low IC50 values.
Table 5.
Effects of HPLC sub-fractions of extracts from Ankistrodesmus convolutus and Synechococcus elongatus against the release of EBV in Akata, B95-8, and P3HR-1 cells based on the amount of cell-free EBV DNA detected by real-time PCR
| Cell line | HPLC sub-fraction | IC50 (μg/ml) | EC50 (μg/ml) | Therapeutic index |
| Akata | Ankistrodesmus convolutus | |||
| ACF2’a | >20 | 2.8±0.9 | >7.0 | |
| ACF2’b | >20 | >20 | ND | |
| ACF2’c | 7.9±0.9 | 3.5±0.7 | 2.3 | |
| ACF2’d | 4.0±1.3 | 1.6±0.2 | 2.6 | |
| ACF2’e | >20 | 1.7±0.1 | >12.1 | |
| ACF2’f | >20 | 2.2±0.5 | >9.0 | |
| ACF3’a | >20 | >20 | ND | |
| ACF3’b | >20 | >20 | ND | |
| ACF3’c | >20 | >20 | ND | |
| ACF3’d | >20 | >20 | ND | |
| ACF3’e | >20 | >20 | ND | |
| ACF3’f | >20 | >20 | ND | |
| ACF3’g | >20 | >20 | ND | |
| B95-8 | Ankistrodesmus convolutus | |||
| ACF1’a | 1.4±0.1 | 11.3±1.7 | 0.1 | |
| ACF1’b | >20 | 4.1±0.3 | >4.9 | |
| ACF1’c | 16.0±1.1 | >20 | <0.8 | |
| ACF1’d | >20 | >20 | ND | |
| ACF1’e | 13.0±1.4 | >20 | <0.7 | |
| ACF1’f | 9.8±2.6 | >20 | <0.5 | |
| ACF1’g | >20 | >20 | ND | |
| Synechococcus elongatus | ||||
| SEF3’a | >20 | >20 | ND | |
| SEF3’b | 1.4±0.1 | >20 | <0.1 | |
| SEF3’c | >20 | >20 | ND | |
| SEF3’d | 1.7±0.1 | >20 | <0.9 | |
| SEF3’e | >20 | >20 | ND | |
| P3HR-1 | Synechococcus elongatus | |||
| SEF1’a | >20 | 1.4±0.4 | >14.5 | |
| SEF1’b | >20 | >20 | ND | |
| SEF1’c | >20 | 1.5±0.2 | >13.1 | |
| SEF1’d | >20 | 1.4±0.1 | >14.0 | |
| SEF1’e | >20 | >20 | ND | |
| SEF3’a | >20 | 2.1±1.4 | >9.6 | |
| SEF3’b | >20 | >20 | ND | |
| SEF3’c | >20 | 4.2±0.2 | >4.8 | |
| SEF3’d | >20 | >20 | ND | |
| SEF3’e | >20 | >20 | ND | |
The sub-fractions from Synechococcus elongatus were not effective in reducing the cell-free EBV DNA in B95-8. However, most sub-fractions of this microalga (SEF1’a, SEF1’c, SEF1’d, SEF3’a, and SEF3’c) were effective in reducing the cell-free EBV DNA load in P3HR-1 cells. Of these, SEF1’a exhibited the strongest antiviral activity (EC50=1.4 µg/ml). All the sub-fractions from Synechococcus elongatus were not cytotoxic against P3HR-1 cells (IC50>20 µg/ml).
4. Discussion
In general, the results show that the antiviral activities of the extracts displayed high therapeutic index. This suggests that the extracts may act through different mechanisms rather than merely killing the cells or making them unhealthy. The antiviral activity varied with the BL cell lines tested. For instance, methanol extract from Synechococcus elongatus showed high antiviral activity (EC50<0.5 µg/ml) against B95-8 and P3HR-1 cell lines but not against Akata cells (EC50>20 µg/ml). Such variation could be due to the different origins and characteristics of the cell lines causing the EBV genome to be incorporated differently into the host cells. Akata cells originate from an EBV-positive BL of a Japanese patient with a unique productive cycle of the EBV genome (Takada et al., 1995). Akata cells are known to produce higher amounts of EBV than B95-8 and P3HR-1 cells. The high productivity of EBV virions by Akata cells may reduce the effectiveness of the microalgal extracts in inhibiting the release of the virus.
In general, the crude extracts were more effective in reducing the EBV DNA when compared to acyclovir and foscarnet. The EC50 obtained with foscarnet in P3HR-1 (0.79 μg/ml) and B95-8 cells (0.90 μg/ml) were much lower than the values reported by Ballout et al. (2007), which were 13.6 and 46.8 μg/ml, respectively. However, in the present study, the real-time PCR amplification was targeted at the BamH1-W gene while the study by Ballout et al. (2007) was based on the BXLF1 gene.
Of the column chromatography fractions, ACF1 and ACF2 from Ankistrodesmus convolutus showed the highest antiviral activities against EBV in B95-8 and Akata cells, respectively. On the other hand, SEF1 from Synechococcus elongatus inhibited the release of EBV from P3HR-1 cells most effectively. However, the antiviral activity of the column chromatography fractions was less potent than the crude extracts. Furthermore, the microalgal fractions showed higher cytotoxicity (lowest IC50=22 µg/ml) than the crude extracts (lowest IC50=78 µg/ml). Further fractionation by HPLC did not significantly improve the bioactivity. In fact, the fraction SEF3 lost its bioactivity after further fractionation by HPLC. Such a trend is commonly observed in the screening of biological resources for bioactive compounds. For instance, the crude acetone extract of Caulerpa racemosa showed bioactivity against the human melanoma cancer cell line but the isolated compound caulerpin lost such activity (Rocha et al., 2007). The synergistic effect of a mixture of components found in crude extracts might have been lost upon fractionation.
The inhibitory action of an antiviral compound can target at different stages of the life cycle of a virus. For instance, it may affect the infection stage by interfering with the adsorption of the virus to host cells. Sulfated polysaccharides from Porphyridium cruentum exerted their antiviral activities against HSV-1 and HSV-2 at this step by inhibiting the attachment of the virion to the host cell surface (Huleihel et al., 2001). A possible target action of an antiviral compound against EBV is by inhibiting the lytic cycle, which will prevent the virus from producing mature viral particles. For instance, andrographolide from a medicinal plant inhibits the transcriptions of BRLF1 and BZLF1, two immediate early genes involved in the lytic cycle of EBV (Lin et al., 2008).
The focus of the present study was not on the inhibition of viral attachment or incorporation of viral genome into host cell DNA. Instead, the microalgal extracts reduced the cell-free EBV DNA, indicating that the lytic cycle or the release of the virions (secretory phase) could be inhibited. This antiviral approach is different from other therapeutic strategies for EBV-positive neoplasms, and aims to induce a switch from latent to lytic cycle to cause cytolysis of the infected cells (Daibata et al., 2005). However, the role of lytic cycle in EBV-associated diseases is still under debate (Gershburg and Pagano, 2005). The inhibition of the lytic cycle could be beneficial from another point of view. For instance, drugs such as ganciclovir and cidofovir which inhibit EBV DNA replication and lytic infection have been shown to be effective in treating oral hairy leukoplakia in immunosuppressed individuals (Walling et al., 2003). The inhibition of the lytic cycle may restrict the spread of EBV from latently-infected cells and prevent the tumor-promoting effect on the neighbouring healthy cells. The virions of EBV, which are produced by B lymphocytes, are known to exhibit tropism by which they preferentially infect epithelial cells, which may promote oncogenesis of such cells (Schwarzmann et al., 1998; Feng et al., 2000). Recurrent EBV reactivation may also result in the accumulation of genome instability and promote tumor progression of NPC (Fang et al., 2009). It has been reported that induction of the EBV lytic phase during malaria infection may increase BL development in a malaria-endemic area (Chêne et al., 2007). Thus, inhibition of the lytic cycle of EBV could be a useful strategy to aid in the reduction of the risk of such diseases. A similar study focusing on the potential of andrographolide as an anti-EBV drug based on its inhibition of the lytic cycle of EBV was reported by Lin et al. (2008). However, that study was based on the inhibitions of the lytic genes encoding Rta, Zta, and EA-D rather than assessing the amounts of cell-free EBV DNA.
There are several possible mechanisms by which the microalgal extracts inhibit the lytic cycle or release of the virus. The microalgal extracts could inhibit the egression of EBV virions by interfering with the destruction of the microtubules and microfilaments induced by the virus. However, ongoing studies based on an immunofluorescence technique showed that the extracts did not affect the cytoskeleton of the host cells (data not shown). Thus, inhibition of the EBV release could be modulated through the effect on the expressions of EBV genes involved in the lytic cycle such as BZLF1 and BRLF1 (Lin, 2003). The extracts may also interfere with the signaling pathways in the lytic cycle involving p38 and c-Jun terminal kinases (Adamson et al., 2000).
The active principles of the microalgal extracts were likely to be pigment components as colored compounds were observed when the fractions were separated with TLC. The absorption spectral data further indicate that the compounds absorbed light within the visible light range. Liquid chromatography-mass spectrophotometry (LC-MS) analysis showed that the active fractions SEF1 and ACF2 contained several compounds with absorption maxima ranging from 300 to 660 nm. Further studies to elucidate the structure of the active principles are in progress.
5. Conclusions
The present study demonstrated that the methanol extracts from Ankistrodesmus convolutus, Synechococcus elongatus, and Spirulina platensis inhibited the release of EBV from BL cells. The bioactivities against EBV of the extracts from Ankistrodesmus convolutus and Synechococcus elongatus increased after fractionation by column chromatography but the fractions were more cytotoxic against the host cells than the crude extracts. However, further fractionation by HPLC did not cause a significant increase in bioactivity. The results showed that the microalgae could be a potential source of antiviral compounds against EBV.
Acknowledgments
We thank Prof. Kenzo TAKADA (Institute of Genetic Medicine, Hokkaido University, Japan) and Prof. Sam Choon KOOK (Institute of Biological Sciences, University of Malaya) for providing the cell lines. The technical support from the NPC Laboratory, University of Malaya and Cancer Research Initiatives Foundation (CARIF) is gratefully acknowledged. We wish to thank the Director General of Health of Malaysia for his permission to publish this paper and the Director of the Institute for Medical Research for her support.
Footnotes
Project (IRPA: 06-05-01-0000-PR0054105-04) supported by the Ministry of Science, Technology, and Innovation (MOSTI) of Malaysia
References
- 1.Adamson AL, Darr D, Holley-Guthrie E, Johnson RA, Mauser A, Swenson J, Kenney S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74(3):1224–1233. doi: 10.1128/JVI.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akihisa T, Taguchi Y, Yasukawa K, Tokuda H, Akazawa H, Suzuki T, Kimura Y. Acerogenin M, a cyclic diarylheptanoid, and other phenolic compounds from Acer nikoense and their anti-inflammatory and anti-tumor-promoting effects. Chem Pharm Bull. 2006;54(5):735–739. doi: 10.1248/cpb.54.735. [DOI] [PubMed] [Google Scholar]
- 3.Ballout M, Germi R, Fafi-Kremer S, Guimet J, Bargues G, Seigneurin J, Morand P. Real-time quantitative PCR for assessment of antiviral drug effects against Epstein-Barr virus replication and EBV late mRNA expression. J Virol Methods. 2007;143(1):38–44. doi: 10.1016/j.jviromet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Buck CB, Thompson CD, Roberts JN, Müller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathogens. 2006;2(7):e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y, Tung CH, Huang YT, Lu J, Chen JY, Tsai CH. Requirement of cell to cell contact in EBV infection of NPC and keratinocytes. J Virol. 1999;73(10):8857–8866. doi: 10.1128/jvi.73.10.8857-8866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chêne A, Donati D, Guerreriro-Cacais AO, Levitsky V, Chen Q, Falk KI, Orem J, Kironde F, Wahlgren M, Bejarano MT. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathogens. 2007;3(6):e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu WL, Phang SM, Goh SH. Influence of carbon source on the growth, biochemical composition and pigmentation of Ankistrodesmus convolutus . J Appl Phycol. 1995;7(1):59–64. doi: 10.1007/BF00003551. [DOI] [Google Scholar]
- 8.Daibata M, Bandobashi K, Kuroda M, Imai S, Miyoshi I, Taguchi H. Induction of lytic Epstein-Barr virus (EBV) infection by synergistic action of rituximab and dexamethasone renders EBV-positive lymphoma cells more susceptible to ganciclovir cytotoxicity in vitro and in vivo. J Virol. 2005;79(9):5875–5879. doi: 10.1128/JVI.79.9.5875-5879.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang CY, Lee CH, Wu CC, Chang YT, Yu SL, Chou SP, Huang PT, Chen CL, Hou JW, Chang Y, et al. Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int J Cancer. 2009;124(9):2016–2025. doi: 10.1002/ijc.24179. [DOI] [PubMed] [Google Scholar]
- 10.Feng P, Ren EC, Liu D, Chan SH, Hu H. Expression of Epstein-Barr virus lytic gene BRLF1 in nasopharyngeal carcinoma: potential use in diagnosis. J Gen Virol. 2000;81(Pt 10):2417–2423. doi: 10.1099/0022-1317-81-10-2417. [DOI] [PubMed] [Google Scholar]
- 11.Gershburg E, Pagano JS. Epstein-Barr virus infections: prospects for treatment. J Antimicrob Chemother. 2005;56(2):277–281. doi: 10.1093/jac/dki240. [DOI] [PubMed] [Google Scholar]
- 12.Hemmingson JA, Falshaw R, Furneaux RH, Thompson K. Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta) J Appl Phycol. 2006;18(2):185–193. doi: 10.1007/s10811-006-9096-9. [DOI] [Google Scholar]
- 13.Huleihel M, Ishanu V, Tal J, Arad S. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J Appl Phycol. 2001;13(2):127–134. doi: 10.1023/A1011178225912. [DOI] [Google Scholar]
- 14.Klein G. Epstein-Barr virus strategy in normal and neoplastic B cells. Cell. 1994;77(6):791–793. doi: 10.1016/0092-8674(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 15.Krenauer A, Moll A, Ponisch W, Schmitz N, Niedobitek G, Niederwieser D, Aigner T. EBV-associated post-transplantation B-cell lymphoproliferative disorder following allogenic stem cell transplantation for acute lymphoblastic leukaemia: tumor regression after reduction of immunosuppression—a case report. Diagn Pathol. 2010;5(1):21. doi: 10.1186/1746-1596-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurth J, Spieker T, Wustrow J, Strickler GJ, Hansmann LM, Rajewsky K, Küppers R. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity. 2000;13(4):485–495. doi: 10.1016/S1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 17.Lai PJ, Chu WL, Naidu R, Khoo ASB, Kok YY, Shar MM, Ling SN, Mak JW, Lim KC, Balraj P, et al. Antiproliferative activity of microalgal extracts on nasopharyngeal carcinoma (NPC) cells. Malaysian J Sci. 2008;27(2):19–31. [Google Scholar]
- 18.Lin JC. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antiviral Res. 2003;59(1):41–47. doi: 10.1016/S0166-3542(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 19.Lin TP, Chen SY, Duh PD, Chang LK, Liu YN. Inhibition of the Epstein-Barr virus lytic cycle by andrographolide. Biol Pharm Bull. 2008;31(11):2018–2023. doi: 10.1248/bpb.31.2018. [DOI] [PubMed] [Google Scholar]
- 20.Lo DYM, Chan LYS, Lo KW, Leung SF, Zhang J, Chan ATC, Lee JCK, Hjelm NM, Johnson PJ, Huang DP. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59:1188–1191. [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Ohigashi H, Sakai Y, Yamaguchi K, Umezaki I, Koshimizu K. Possible anti-tumor promoting properties of marine algae and in vivo activity of Wakame seaweed extract. Biosci Biotechnol Biochem. 1992;56(6):994–995. doi: 10.1271/bbb.56.994. [DOI] [PubMed] [Google Scholar]
- 23.Phang SM, Chu WL. University of Malaya Algae Culture Collection (UMACC): Catalogue of Strains. Kuala Lumpur, Malaysia: Institute of Postgraduate Studies and Research, University of Malaya; 1999. p. 77. [Google Scholar]
- 24.Rechter S, König T, Auerochs S, Thulke S, Walter H, Dörnenburg H, Walter C, Marschall M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antiviral Res. 2006;72(3):197–206. doi: 10.1016/j.antiviral.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Rezk S, Weiss L. Epstein-Barr virus-associated lymphoproliferative disorders. Human Pathol. 2007;38(9):1293–1304. doi: 10.1016/j.humpath.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Rickinson AB, Kieff E. Epstein-Barr Virus. In: Fields BN, Knipe BN, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Williams and Wilkins; 1996. pp. 2397–2476. [Google Scholar]
- 27.Rocha FD, Soares AR, Houghton PJ, Pereira RC, Kaplan MAC, Teixeira VL. Potential cytotoxic activity of some Brazilian seaweeds on human melanoma cells. Phytother Res. 2007;21(2):170–175. doi: 10.1002/ptr.2038. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzmann F, Jager M, Horner M, Prang N, Wolf H. Epstein-Barr viral gene expression in B-lymphocytes. Leuk Lymphoma. 1998;30(1-2):123–129. doi: 10.3109/10428199809050935. [DOI] [PubMed] [Google Scholar]
- 29.Takada K, Shimizu N, Tanabe-Tochikura A, Kuroiwa Y. Pathogenic role of Epstein-Barr virus in human cancer. Intervirology. 1995;38(3-4):214–220. doi: 10.1159/000150435. [DOI] [PubMed] [Google Scholar]
- 30.Walling DM, Flaitz CM, Nicholas CM. Epstein-Barr virus replication in oral hairy leukoplakia: response, persistence, and resistance to treatment with valacyclovir. J Infect Dis. 2003;188(6):883–890. doi: 10.1086/378072. [DOI] [PubMed] [Google Scholar]