Abstract
The purpose of this study was to isolate and characterize endophytic fungi from the stem tissue which can produce fragrant ingredients in Aquilaria sinensis (also called agarwood) to determine their antitumor and antimicrobial activities. Twenty-eight fungal endophytes were isolated from agarwood by strict sterile sample preparation and were classified into 14 genera and 4 taxonomic classes (Sordariomycetes, Dothideomycetes, Saccharomycetes, and Zygomycetes) based on molecular identification. Of the 28 isolates, 13 (46.4%) showed antimicrobial activity against at least one of the test strains by the agar well diffusion method, and 23 isolates (82.1%) displayed antitumor activity against at least one of five cancer cell lines by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The diameters of inhibition zones of YNAS07, YNAS14, HNAS04, HNAS05, HNAS08, and HNAS11 were equal to or higher than 14.0 mm against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, B. subtilis, Aspergillus fumigatus, and B. subtilis, respectively. The inhibition rates of YNAS06, YNAS08, and HNAS06 were not less than 60% to 293-T, 293-T, and SKVO3 cells, respectively. These results suggest that the endophytic fungi associated with agarwood will provide us with not only useful micro-ecological information, but also potential antimicrobial and antitumor agents.
Keywords: Agar diffusion method, Agarwood, Antimicrobial bioactivity, Antitumor bioactivity, Endophytic fungi
1. Introduction
In recent years, researchers have begun to realize that plants may serve as a repository of untold numbers of organisms known as endophytes (Bacon and White, 2000; Strobel, 2002). Most endophytes are capable of synthesizing bioactive compounds that may provide plants with a defense against pathogens, and some of these compounds have proven useful for novel drug discovery (Guo et al., 2008; Yan et al., 2011). Since the chemical constituents from medicinal plants were complex, more and more endophytic fungi with novel metabolites of pharmaceutical importance were isolated from medicinal plants, and a series of new and useful compounds were obtained (Huang et al., 2007; Guo et al., 2008; Kusari et al., 2009). No doubt, exploiting a variety of new natural products from endophytic fungi of medicinal plants has become a hot spot of new drug research.
Agarwood (this refer in particular to the stem tissue which can produce fragrant ingredients in Aquilaria sinensis) is a fragrant wood that has been widely used as traditional Chinese medicine and fragrance additive two thousand years ago (He et al., 2005). It is formed from A. sinensis (Lour.) Gilg. (Thymelaeaceae) which is the only origin species of agarwood in China (Liu, 1999; Qi, 1995). In recent years, many of the chemical ingredients have been isolated from agarwood (Yang and Chen, 1983; 1986; Yang et al., 1989a; 1989b; 1990; Yang, 1998; Yagura et al., 2003). By investigation of interrelated studies, agarwood has significant anticancer activities (Gunasekera et al., 1981), analgesic and anti-inflammatory activities (Zhou et al., 2008), and anti-depression activities (Okugawa et al., 1993; 1996). Endophytes from agarwood may be a rich source of antimicrobial and antitumor agents with novel mechanisms of action.
As one of the renowned traditional medicines, despite great progress toward understanding phytochemical constituents and pharmacological activities of agarwood, the micro-ecology and bioactivity of endophytic fungi associated with agarwood have not been exploited. For this reason, systematic investigation of endophytic fungi associated with agarwood and evaluation of their bioactive secondary metabolites were necessary. This can provide researchers with genetic information and may allow for new natural products with higher antimicrobial and antitumor activities to be found. The present study describes the endophytic fungi isolated from agarwood growing in two different rainforest areas, and reports their antimicrobial and antitumor activities.
2. Materials and methods
2.1. Source of endophytic fungi
Samples of agarwood were collected randomly from the tropical rainforests in Yunnan and Hainan Provinces of China in March 2009 and were identified. Plant samples were tagged, stored at 4 °C in a clean plastic bag, and taken to the laboratory for isolation of endophytic fungi.
2.2. Isolation, identification, and phylogenetic analyses of endophytic fungi
Isolation of the endophytic fungi was performed based on the procedures described by Xu et al. (2008). The cleaned samples were cut into about 5 mm×5 mm×5 mm cubes and then surface-disinfected by washing in 75% ethanol for 1 min, sterile distilled water twice, 0.05 g/ml sodium hypochlorite solution for 3 min followed by two rinses in sterile distilled water. The surface-sterilized samples were cut into small pieces using a sterile blade and placed on plates with potato-dextrose agar (PDA) medium (the medium contained potato 200 g, glucose 20 g, and agar 15 g in 1 L of purified water) for incubation at 25 °C. Each fungus from plant tissue was removed and placed onto a new PDA petri plate until cultures were obtained for identification and fermentation.
Fungal identification methods were based on their internal transcribed spacer ribosomal DNA (ITS-rDNA) sequences. A pair of primers ITS1 (sequence: 5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) was used for ITS-rDNA amplification (Phongpaichit et al., 2006). The corresponding ITS-rDNA sequence of each endophytic fungus was then used for similarity analysis using BlastN algorithm against the public database at the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov). All of the fungal ITS-rDNA sequences were deposited in GenBank (accession Nos. GU355645–GU355672). Multiple sequence alignments were performed using the CLUSTAL X program (Thompson et al., 1994) and molecular evolutionary analyses were conducted using MEGA Version 4.0 (Kumar et al., 2008). The Kimura (1980)’s two-parameter model was used to estimate evolutionary distance. The phylogenetic tree was constructed using the neighbor-joining (NJ) algorithm (Naruya and Masatoshi, 1987) and maximum-parsimony (MP) analyses, with bootstrap values calculated from 1 000 replicate runs using the software routines included in the MEGA software (Li et al., 2008).
2.3. Preparation of fungal fermentation broth
The endophytic fungal isolates were cultured in potato dextrose liquid medium (the medium contained potato 200 g and glucose 20 g in 1 L of purified water) for 10 d at 25 °C on a shaker at 180 r/min. Crude fermentation broth was blended thoroughly and centrifuged at 4 000 r/min for 5 min. Liquid supernatant was extracted with an equal volume of ethyl acetate thrice. The organic solvent extract was then evaporated under reduced pressure to yield an ethyl acetate extract. The ethyl acetate extracts were dissolved in sterilized water to a final concentration of 0.5 mg/ml for antimicrobial and antitumor activity screening (Lv et al., 2010).
2.4. Antimicrobial activity assay
Gram-negative Escherichia coli and two Gram-positive species Bacillus subtilis and Staphylococcus aureus were used as test bacteria. Three pathogenic fungi Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus were used as indicator microorganisms to determine antifungal activity. All six indicator organisms were obtained from the Chinese Academy of Medical Sciences (CAMS). The agar well diffusion method was then employed to evaluate the antimicrobial activity (Rios et al., 1988). The bacteria were diluted with melted beef extract peptone (BEP) medium (beef extract 5 g, NaCl 5 g, peptone 10 g, and agar 15 g in 1 L of sterilized water; pH 7.2) to give 1×106 bacteria/ml of bacteria and poured into a 9-cm diameter petri plate containing 8 ml of solidified BEP. The concentration of fungal spores was adjusted to 1×105 spores/ml by diluting it with melted Sabouraud’s agar (SA) medium and poured into a 9-cm diameter petri plate containing 8 ml of solidified SA medium. After solidification, four circular equidistant wells (7.8 mm in diameter) were made in the BEP or SA layer using sterile cork borers. Then 80 μl of each fungal extract solution with a final concentration of 0.5 mg/ml was added into the wells. After incubation at 37 °C for 24 h for bacteria or at 25 °C for 48 h for fungi, the inhibition zones were observed, measured, and recorded. All tests were performed in triplicate.
2.5. Antitumor activity assay
Five human cancer lines (HepG2, MCF7, SKVO3, HL-60, and 293-T) were obtained from CAMS. HepG2, MCF7, HL-60, and 293-T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2 mmol l-glutamine, 100 U/ml of penicillin, and 100 μg/ml streptomycin. The SKVO3 cell line was maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 2 mmol/L l-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mmol/L (4-(2-hydroxyethyl)-1-piperazi-neethanesulfonic acid (HEPES), 1.0 mmol/L sodium pyruvate, 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml streptomycin. The tumor cell cultures were maintained at 37 °C in an atmosphere of 5% CO2 and 95% air with more than 95% relative humidity. Antitumor activity assay was conducted by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The MTT assay protocol was adapted from that described by Mosmann (1983) and Alley et al. (1988). The spectrophotometric absorbance at 578 nm was measured and the assay was performed in triplicate. Growth inhibition rate (IR) was calculated by the following formula: IR=(ODcon.−ODtreated)/ODcon.×100%, where ODcon. and ODtreated are ODs of control and treated wells, respectively.
3. Results
3.1. Endophytic fungi and their phylogenetic analyses
A total of 28 morphologically distinct fungal isolates were isolated and identified from the agarwood. They were classified into 14 genera, according to the results of molecular identification (Tables 1 and 2). Among the fungi isolated, 15 were from the agarwood collected at Yunnan and belonged to 12 different genera, 13 were from Hainan and belonged to 5 genera (Table 1). Fusarium and Phaeoacremonium were the dominant genera. Fusarium, Phaeoacremonium, and Phoma colonized both Yunnan agarwood and Hainan agarwood, while Chaetomium sp. and Pichia sp. were only found in Hainan agarwood. Nine other genera were only found in Yunnan agarwood.
Table 1.
Genera of endophytic fungi isolated from agarwood (A. sinensis) from Yunnan and Hainan
| Genus | Number of fungal isolates |
|
| Yunnan | Hainan | |
| Epicoccum | 1 | 0 |
| Cladosporium | 1 | 0 |
| Rhizomucor | 1 | 0 |
| Paraconiothyrium | 1 | 0 |
| Phaeoacremonium | 2 | 4 |
| Xylaria | 1 | 0 |
| Fusarium | 3 | 6 |
| Lasiodiplodia | 1 | 0 |
| Leptosphaerulina | 1 | 0 |
| Hypocrea | 1 | 0 |
| Phoma | 1 | 1 |
| Coniothyrium | 1 | 0 |
| Chaetomium | 0 | 1 |
| Pichia | 0 | 1 |
Table 2.
Antimicrobial and cytotoxic activities of endophytic fungi from agarwood (A. sinensis)
| Code | Closest identified relative | Similarity (%) | Antimicrobial activitya |
Cytotoxic activityb |
|||||||||
| EC | BS | SA | CA | CN | AF | HepG2 | MCF7 | SKVO3 | HL-60 | 293-T | |||
| YNAS01 | Epicoccum nigrum (FJ424262.1) | 100 | − | − | − | − | − | − | * | * | ** | − | * |
| YNAS02 | Cladosporium tenuissimum (FJ571446.1) | 100 | − | − | − | − | − | − | − | ** | ** | − | − |
| YNAS03 | Rhizomucor variabilis (EF583638.1) | 100 | − | − | − | − | − | ++ | * | ** | ** | − | − |
| YNAS04 | Paraconiothyrium variabile (EU295649.1) | 100 | − | − | − | − | − | − | − | − | − | − | − |
| YNAS05 | Phaeoacremonium rubrigenum (AB278173.1) | 100 | − | − | − | − | − | − | ** | ** | − | − | − |
| YNAS06 | Xylaria mali (AF163040.1) | 100 | − | − | − | − | − | − | − | − | *** | *** | **** |
| YNAS07 | Fusarium equiseti (FJ459976.1) | 100 | − | − | +++ | − | − | − | − | − | ** | − | ** |
| YNAS08 | Lasiodiplodia theobromae (FJ594752.1) | 100 | − | − | − | − | − | − | − | − | ** | * | **** |
| YNAS09 | Fusarium solani (AB258993.1) | 100 | − | + | + | + | − | − | − | * | ** | − | * |
| YNAS10 | Leptosphaerulina chartarum (EU272492.1) | 99 | ++ | − | + | ++ | − | − | − | * | *** | − | * |
| YNAS11 | Hypocrea lixii (EF596952.1) | 100 | − | − | − | − | − | − | − | − | ** | − | * |
| YNAS12 | Phoma herbarum (EU823313.1) | 99 | − | − | − | − | − | − | − | − | ** | − | − |
| YNAS13 | Fusarium oxysporum (GQ365156.1) | 100 | ++ | − | + | − | − | − | − | * | ** | − | ** |
| YNAS14 | Phaeoacremonium rubrigenum (AB278173.1) | 100 | +++ | − | − | + | − | − | − | − | ** | − | − |
| YNAS15 | Coniothyrium nitidae (EU552112.1) | 95 | ++ | − | + | + | − | − | − | * | *** | − | − |
| HNAS01 | Fusarium solani (EU214559.1) | 100 | − | − | − | − | − | − | − | * | − | − | − |
| HNAS02 | Fusarium solani (AY633746.1) | 99 | − | − | − | − | − | − | − | − | ** | * | − |
| HNAS03 | Fusarium solani (EU214559.1) | 100 | − | − | − | − | − | − | − | − | − | * | − |
| HNAS04 | Fusarium avenaceum (FJ478097.1) | 99 | + | +++ | − | + | − | − | * | − | ** | − | ** |
| HNAS05 | Phaeoacremonium rubrigenum (AB278173.1) | 100 | − | +++ | − | + | − | − | * | ** | ** | − | * |
| HNAS06 | Phaeoacremonium rubrigenum (AB278173.1) | 100 | − | − | − | − | − | − | − | − | **** | − | − |
| HNAS07 | Fusarium solani (EU214559.1) | 100 | − | − | − | − | − | − | ** | * | − | * | *** |
| HNAS08 | Chaetomium globosum (GQ337423.1) | 100 | − | − | − | ++ | − | ++++ | * | − | ** | − | ** |
| HNAS09 | Phoma medicaginis (EF017209.1) | 100 | − | − | − | − | − | − | − | − | *** | − | − |
| HNAS10 | Phaeoacremonium rubrigenum (AB278173.1) | 100 | − | + | + | − | − | − | − | − | − | − | − |
| HNAS11 | Fusarium equiseti (FJ459976.1) | 100 | − | ++++ | − | − | − | − | − | − | − | − | − |
| HNAS12 | Pichia guilliermondii (GQ280287.1) | 100 | − | − | − | − | − | − | − | − | − | − | − |
| HNAS13 | Phaeoacremonium rubrigenum (AB278173.1) | 100 | − | − | − | ++ | − | − | − | − | − | − | − |
EC: Escherichia coli; BS: Bacillus subtilis; SA: Staphylococcus aureus; CA: Candida albicans; CN: Cryptococcus neoformans; AF: Aspergillus fumigatus
The antimicrobial activity is expressed by the diameter (d) of inhibition zone: −, d<8.0 mm; +, 8.0 mm≤d<11.0 mm; ++, 11.0 mm≤d<14.0 mm; +++, 14.0 mm≤d<17.0 mm; ++++, d≥17.0 mm
The cytotoxic activity is expressed by the inhibition rate (IR), −, IR<10%; *, 10%≤IR<20%; **, 20%≤IR<40%; ***, 40%≤IR<60%; ****, 60%≤IR<80%; *****, IR≥80%
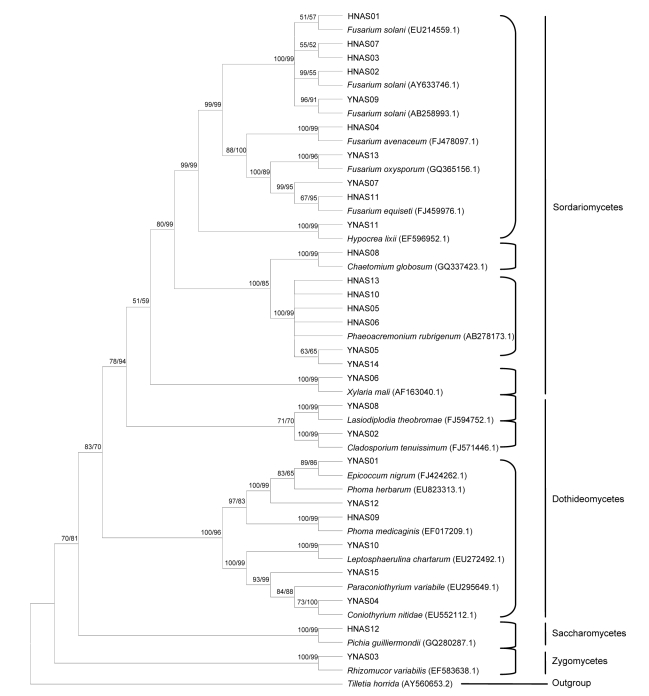
Aside from Rhizomucor variabilis (Zygomycota) all the endophytes were Ascomycota or anamorphic fungi that belonged to three classes (Sordariomycetes, Dothideomycetes, and Saccharomycetes) (Fig. 1). Sordariomycetes, the biggest group, contained 18 independent isolates that belonged to four orders (Hypocreales, Sordariales, Calosphaeriales, Xylariales) and five genera (Fusarium, Hypocrea, Chaetomium, Phaeoacremonium, and Xylaria). Eight isolates spanning seven genera (Lasiodiplodia, Cladosporium, Epicoccum, Phoma, Leptosphaerulina, Paraconiothyrium, and Coniothyrium) belonged to the Dothideomycetes. This class contained three taxonomicorders (Botryosphaeriales, Capnodiales, Pleosporales). Isolate HNAS12, sharing 100% similarity with Pichia guilliermondii, was the only member of the Saccharomycetes group, and it formed subgroup 8, corresponding to the order Saccharomycetales. Isolate YNAS03 formed subgroup 9 with Rhizomucor variabilis and belonged to the Zygomycetes (Fig. 1).
Fig. 1.
Neighbour-joining (NJ) phylogenetic tree based on ITS-rDNA sequences of 28 endophytic fungi isolated from the agarwood (A. sinensis) and the closest identified relatives from GenBank
Numbers above branches indicate bootstrap values of NJ/MP (>50%, right) from 1 000 bootstrap replicates. Isolates YNAS came from Yunnan Province and isolates HNAS came from Hainan Province
3.2. Antimicrobial activity
The fermentation broths of the 28 endophytic fungal isolates from agarwood were screened for antimicrobial activity against six microbial pathogens by the agar well diffusion method (Table 2). Thirteen isolates (46.4%) showed promising growth inhibitory activity against at least one of the test strains, but no endophyte had antimicrobial activity against all six pathogenic microbes (Table 2). A high proportion of fungi (28.6%) had activity against C. albicans. The numbers of fungal isolates displaying antimicrobial activity against E. coli, B. subtilis, S. aureus, and A. fumigatus were five, five, six, and two, respectively. No isolate displayed antagonistic activity against C. neoformans. YNAS14 showed promising growth inhibitory activity against E. coli. The isolate HNAS11 exhibited a high antimicrobial activity against B. subtilis. HNAS04 and HNAS05 showed a similar, but slightly lower than HNAS11, degree of antagonistic activity against B. subtilis. Isolate YNAS07 was most high antagonistic to S. aureus, while HNAS08 had a high inhibitory activity against A. fumigatus.
3.3. Antitumor activity
The fermentation broths from the 23 fungi displayed antitumor activity against at least one of five cancer cell lines. Fourteen were from the agarwood of Yunnan and nine were from Hainan (Table 2). Among them, 7 isolates (25.0%) displayed activity against HepG2 cells, 11 (39.3%) against MCF7 cells, 19 (67.9%) against SKVO3, 5 (17.9%) against HL-60 cells, and 12 (42.9%) against 293-T cells. Therefore, the highest proportion of active isolates was that from the test of SKVO3 cells, followed by 293-T, and MCF7. In contrast, all isolates showed low cytotoxic effect toward HepG2 and HL-60. The isolates YNAS06 and YNAS08 displayed a high antitumor activity on 293-T, and the isolate HNAS06 showed a high antitumor activity on SKVO3 cells. They may have potential practical value.
4. Discussion
Endophytes are presumably ubiquitous in plants, with populations dependent on host species and location (Tan and Zou, 2001). Not all endophytes may have been isolated since some may not grow under laboratory conditions, some may grow too slowly to be seen, and some fungi may be missed because their morphology characteristics were very similar to others. Endophytes from a particular host usually include one to several taxa that are adapted to that host (Schulz and Boyle, 2005). For example, Discula umbrinella is primarily found in Fagus sylvatica (Sieber and Hugentobler, 1987), Physalospora vaccinii in Vaccinium oxycoccus (Schulz et al., 1993), and in current study more than 50% of the isolates were Fusarium spp. and Phaeoacremonium rubigenum-like isolates.
It was reported that endophytic fungi were isolated from the leaves, stems, and roots of A. sinensis (Gong and Guo, 2009; Wang et al., 2009). Gong and Guo (2009) found that the dominant genus was mycelia sterilia sp., and Wang et al. (2009) reported that it was Colletotrichum sp. However, the results of the current study indicated that the dominant genus was Fusarium sp. from agarwood, which indicated that the composition and structure of fungal species in agarwood were different to those that do not produce fragrant ingredients in A. sinensis.
Gong and Guo (2009) obtained four isolates (3.1%) which could inhibit at least one of the tested fungi and bacteria by the same screening process of 128 endophytic fungi from healthy tissues of A. sinensis. The inhibition rates of the above endophytic fungi were lower than those from agarwood in the present research.
The bioactive abilities of endophytic fungi were not identical, even though they were isolated from the same host species, and belonged to the same genus. For example, Fusarium solani (YNAS09) displayed antimicrobial activity to B. subtilis, S. aureus, and C. albicans, and showed antitumor activity against SKOV3. On the other hand, F. solani (HNAS07) had no antimicrobial or antitumor activity against SKOV3, but displayed antitumor activity against the other four tumor cells. The other example was that for both YNAS05 and HNAS05 belonging to Phaeoacremonium rubrigenum, the former displayed no antimicrobial activity and showed cytoactivity against HepG2 and MCF7 cells, while the latter had antimicrobial activity to B. subtilis and C. albicans, but inhibited growth of HepG2, MCF7, SKVO3, and 293-T cells. These results demonstrated that those endophytic fungi with diverse bioactivities in the study were potential drug candidates. We are currently undertaking research on naturally bioactive chemicals putatively produced by the endophytic fungi identified in the present study. In the future, studies should focus on whether the endophytes attribute to the formation mechanism of agarwood in A. sinensis.
Acknowledgments
The authors are grateful to Dr. David BASTIN (University of Sydney, Australia) and Dr. Wendy LUO (University of Michigan, USA) in critically reading the manuscript.
Footnotes
Project supported by the National High-Tech R & D Program (863) of China (No. 2008AA09Z405), and the International Science and Technology Cooperation Projects of China (No. 2009DFA32250)
References
- 1.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48(3):589–601. [PubMed] [Google Scholar]
- 2.Bacon CW, White JF. Microbial Endophytes. New York: Marcel Dekker; 2000. pp. 85–117. [Google Scholar]
- 3.Gong LJ, Guo SX. Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afr J Biotechnol. 2009;8(5):731–736. [Google Scholar]
- 4.Gunasekera SP, Kinghorn AD, Cordell GA, Farnsworth NR. Plant anticancer agents. XIX. Constituents of Aquilaria Malaccensis . J Nat Prod. 1981;44(5):569–572. doi: 10.1021/np50017a010. [DOI] [PubMed] [Google Scholar]
- 5.Guo B, Wang Y, Sun X, Tang K. Bioactive natural products from endophytes: a review. Appl Biochem Microbiol. 2008;44(2):136–142. doi: 10.1134/S0003683808020026. [DOI] [PubMed] [Google Scholar]
- 6.He ML, Qi SY, Hu LJ. Rapid in vitro propagation of medicinally important Aquilaria agallocha . J Zhejiang Univ-Sci B. 2005;6(8):849–852. doi: 10.1631/jzus.2005.B0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang WY, Cai YZ, Hyde KD, Corke H, Sun M. Endophytic fungi from Nerium oleander L. (Apocynaceae): main constituents and antioxidant activity. World J Microbiol Biotechnol. 2007;23(9):1253–1263. doi: 10.1007/s11274-007-9357-z. [DOI] [Google Scholar]
- 8.Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Dudley J, Nei M, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9(4):299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusari S, Zuhlke S, Spiteller M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J Nat Prod. 2009;72(1):2–7. doi: 10.1021/np800455b. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Zhao GZ, Chen HH, Wang HB, Qin S, Zhu WY, Jiang CL, Li WJ. Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainfrest. Lett Appl Microbiol. 2008;47(6):574–580. doi: 10.1111/j.1472-765X.2008.02470.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu CH. Chen-Xiang. In: Liu CH, editor. Encyclopedia of Medicine of Li Shi Zhen, Ben Cao Gang Mu, Wood Part. Beijing: Chinese Medicine publishing Inc; 1999. pp. 1116–1118. (in Chinese) [Google Scholar]
- 13.Lv YL, Zhang FS, Chen J, Cui JL, Xing YM, Li XD, Guo SX. Diversity and antimicrobial activity of endophytic fungi associated with the alpine plant Saussurea involucrata . Biol Pharm Bull. 2010;33(8):1300–1306. doi: 10.1248/bpb.33.1300. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann F. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Naruya S, Masatoshi N. The Neighbor-joining method: a new method for reconstructing phylogenetic tree. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 16.Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato A. Effects of agarwood extracts on the central nervous systems in mice. Planta Med. 1993;59(1):32–36. doi: 10.1055/s-2006-959599. [DOI] [PubMed] [Google Scholar]
- 17.Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato A. Effects of jinkoh-eremol and agarospirol from agarwood on the central nervous systems in mice. Planta Med. 1996;62(1):2–6. doi: 10.1055/s-2006-957784. [DOI] [PubMed] [Google Scholar]
- 18.Phongpaichit S, Rungjindamai N, Rukachaisirikul N, Sakayaroj J. Antimicrobial activity in cultures of endophytic fungi isolated from Garcinia species. FEMS Immunol Med Microbiol. 2006;48(3):367–372. doi: 10.1111/j.1574-695X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 19.Qi SY. III Aquilaria Species: In Vitro Culture and the Production of Eaglewood (Agarwood) In: Bajaji YPS, editor. Biotechnology in Agriculture and Forestry. Medicine and Aromatic Plant VIII. Vol. 33. Berlin Heidelberg, Germany: Springer-Verlag; 1995. pp. 36–46. [Google Scholar]
- 20.Rios JL, Recio MC, Villar A. Screening methods for natural products with antimicrobial activity: a review of the literature. J Ethnopharmacol. 1988;23(2-3):127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 21.Schulz B, Boyle C. The endophytic continuum. Mycol Res. 2005;109(6):661–686. doi: 10.1017/S095375620500273X. [DOI] [PubMed] [Google Scholar]
- 22.Schulz B, Wanke U, Draeger S, Aust HJ. Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res. 1993;97(12):1447–1450. doi: 10.1016/S0953-7562(09)80215-3. [DOI] [Google Scholar]
- 23.Sieber VT, Hugentobler C. Endophytic fungi in leaves and twigs of healthy and diseased beech trees (Fagus sylvatica L.) Eur J Forest Pathol. 1987;17(7):411–425. doi: 10.1111/j.1439-0329.1987.tb01119.x. [DOI] [Google Scholar]
- 24.Strobel GA. Rainforest endophytes and bioactive products. Crit Rev Biotechnol. 2002;22(4):315–333. doi: 10.1080/07388550290789531. [DOI] [PubMed] [Google Scholar]
- 25.Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep. 2001;18(4):448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Zhang WM, Pan QL, Li HH, Tao MH, Gao XX. Isolation and molecular identification of endophytic fungi from Aquilaria sinensis . J Fungal Res. 2009;7(1):37–42. (in Chinese) [Google Scholar]
- 28.Xu L, Zhou L, Zhao J, Li J, Li X, Wang J. Fungal endophytes from Dioscorea zingiberensis rhizomes and their antibacterial activity. Lett Appl Microbiol. 2008;46(1):68–72. doi: 10.1111/j.1472-765x.2007.02264.x. [DOI] [PubMed] [Google Scholar]
- 29.Yagura T, Ito M, Kiuchi F, Honda G, Shimada Y. Four new 2-(2-phenylethyl) chromone derivatives from withered wood of Aquilaria sinensis . Chem Pharm Bull. 2003;51(5):560–564. doi: 10.1248/cpb.51.560. [DOI] [PubMed] [Google Scholar]
- 30.Yan XN, Sikora IR, Zheng JW. Potential use of cucumber (Cucumis sativus L.) endophytic fungi as seed treatment agents against root-knot nematode Meloidogyne incognita . J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(3):219–225. doi: 10.1631/jzus.B1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang JS. Review of the chemical constituents isolated from Chen-Xiang. Nat Prod Res Develop. 1998;10(1):99–103. (in Chinese) [Google Scholar]
- 32.Yang JS, Chen YW. Studies on the constituents of Aquilaria sinensis (Lour.) Gilg. I. Isolation and structure elucidation of two new sesquiterpenes, baimuxinic acid and baimuxinal. Acta Pharm Sin. 1983;18(3):191–198. (in Chinese) [PubMed] [Google Scholar]
- 33.Yang JS, Chen YW. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. II. Isolation and structure of baimuxinol and dehydrobaimuxinol. Acta Pharm Sin. 1986;21(7):516–520. (in Chinese) [PubMed] [Google Scholar]
- 34.Yang JS, Wang YL, Su YL. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. IV. Isolation and characterization of 2-(2-phenylethyl) chromone derivatives. Acta Pharm Sin. 1989;24(9):678–683. (in Chinese) [PubMed] [Google Scholar]
- 35.Yang JS, Wang YL, Su YL, He CH, Zheng QT, Yang J. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. III. Elucidation of the structure of isobaimuxinol and isolation and identification of the constituents of lower boiling fraction of the volatile oil. Acta Pharm Sin. 1989;24(4):264–268. (in Chinese) [PubMed] [Google Scholar]
- 36.Yang JS, Wang YL, Su YL. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. V. Isolation and characterization of three 2-(2-phenylethyl) chromone derivatives. Acta Pharm Sin. 1990;25(3):186–190. (in Chinese) [PubMed] [Google Scholar]
- 37.Zhou MH, Wang HG, Suolang JB, Kou JP, Yu BY. Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg. leaves extract. J Ethnophamacol. 2008;117(2):345–350. doi: 10.1016/j.jep.2008.02.005. [DOI] [PubMed] [Google Scholar]