Abstract
Scrophularia ningpoensis has long been used in the Chinese Materia Medica for inflammation. Like other herbal medicines, S. ningpoensis collected from different localities may considerably differ in their therapeutic efficacy, and the one grown in Zhejiang Province is recognized as geo-authentic. However, it is difficult to confirm the geographical authenticity by similar morphological characteristics. In the present study, inter-simple sequence repeat (ISSR) markers were conducted to detect S. ningpoensis from different origins. A 1 259-bp fragment amplified by primer UBC874 was found only in geo-authentic ones. By cloning and sequencing that specific band, sequence characterized amplified region (SCAR) markers were designed to distinguish geo-authentic S. ningpoensis from others. This is a rapid and easy method that can be used to identify the geographical authenticity of S. ningpoensis.
Keywords: Inter-simple sequence repeat (ISSR), Sequence characterized amplified region (SCAR), Scrophularia ningpoensis, Chinese Materia Medica, Traditional Chinese medicine
1. Introduction
Scrophularia ningpoensis Hemsley, known as “Zhexuanshen”, used in the Chinese Materia Medica (CMM), belonging to the family Scrophulariaceae, has a long history of widespread use in China (Kajimoto et al., 1989; Fernández et al., 1996; Miyase and Mimatsu, 1999; Giner et al., 2000). S. ningpoensis named by Forbes and Hemsley (1890) based on the specimens collected in Tiantong County, Ningbo City of Zhejiang Province, is endemic to China and now is widely cultivated in China as well. It is used to treat inflammation, laryngitis, tonsillitis, abscesses of carbuncles (Reid, 1996), and constipation (Yen, 1992). Recent research revealed that this medicinal species, which has high antiangiogenic activity, also can be used as an anticancer agent (Sagar et al., 2006).
The major bioactive components of S. ningpoensis have been reported to be harpagoside, angoroside C, acteoside, and cinnamic acid (Liu et al., 1995; Miyazawa et al., 1998; de Santos Galíndez et al., 2002; Díaz et al., 2004). However, determined by bioactive components, the quality and efficacy of CMM depend significantly on its geographical origin (Woo et al., 1999). The chemical differences of Radix Scrophulariae among various production regions were demonstrated to different extents. That grown in Zhejiang Province has better medicinal effect and is recognized as geo-authentic (Wang and Wang, 2007). Several methods based on high-performance liquid chromatography (HPLC) or combined with liquid chromatography-electrospray ionisation-mass spectrometry (LC-ESI-MS) were developed to quality and quantify the bioactive compounds in S. ningpoensis (Liu et al., 2007; Zhu et al., 2008). Our previous studies on HPLC fingerprints of S. ningpoensis have revealed that three of the four major bioactive compounds, harpagoside, angoroside C, and cinnamic acid, were largely variable among samples collected from different regions (Yang et al., 2010). The materials from Zhejiang Province produced the highest contents of the bioactive compounds, which have the most anti-inflammatory effect (Yang et al., 2010). Medicinal parts (roots) of S. ningpoensis originating from different geographical areas share similar morphological characters. Therefore, it is very difficult to distinguish S. ningpoensis of Zhejiang from others by using morphological methods.
The quality control of CMM is important for safe and effective use (Chung et al., 2006). Medicinal plants collected from different localities are considerably different in their therapeutic efficacy (Woo et al., 1999). Recent developments in molecular biology techniques make DNA markers be useful for the identification and standardization of CMM (Yang et al., 2001). Our group has established species-specific polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) methods for identifications of Actinidia macrosperma and Sinopodophyllum hexandrum (Gong et al., 2006; Zhao et al., 2007), and sequence characterized amplified region (SCAR) markers for Sinocalycanthus chinensis (Ye et al., 2006).
As to the identification of geo-authentic CMM, chemical fingerprints are the most used method, but the whole genome patterns are proven to be useful, accurate, and convenient as well. For instance, different arbitrarily primed (AP)-PCR fingerprints are used to distinguish samples of Astragalus membranaceus originated from different localities (Yip and Kwan, 2006). For Codonopsis pilosula, AP-PCR and random amplification of polymorphic DNA (RAPD) fingerprints revealed different patterns according to different geographic origins (Zhang et al., 1999). Similarly, Vitex rotundifolia samples from 14 different regions were divided by inter-simple sequence repeat (ISSR) markers (Hu et al., 2007). In this study, we used ISSR method to detect the whole genome of S. ningpoensis from different geographical origins and found a specific fragment only in populations originated from Zhejiang Province. Then based on that specific fragment, we designed a pair of diagnostic primers to identify S. ningpoensis of Zhejiang Province.
2. Materials and methods
2.1. Plant materials and DNA extraction
A total of 189 samples of S. ningpoensis originated from seven different Provinces were used in this study, wherein 85 individuals from three geographical origins were surveyed for ISSR and all samples were tested by designed SCAR primers (Table 1). The voucher specimens were deposited in the Herbarium of the Zhejiang University (HZU). DNA was isolated from silica-gel dried leaf by a modified hexadecyl trimethyl ammonium bromide (CTAB) method (Doyle, 1991).
Table 1.
Sampling localities and codes of S. ningpoensis
| Originated location | Locality code | Sample size | ISSR |
| Yaochuan, Pan’an County, Zhejiang | YC | 15 | √ |
| Renchuan, Pan’an County, Zhejiang | RC | 15 | √ |
| Guangmingcun, Pan’an County, Zhejiang | GM | 15 | √ |
| Shanghu, Pan’an County, Zhejiang | PA | 10 | √ |
| Xianju County, Zhejiang | XJ | 10 | √ |
| Hubei | HB | 10 | √ |
| Shanxi | SX | 10 | √ |
| Jinfo Mountain, Chongqing | JF | 19 | |
| Jingang Mountain, Jiangxi | JX | 15 | |
| Pingjiang County, Hunan | HN | 15 | |
| Jiuhua Mountain, Anhui | AH | 15 | |
| Tianmu Mountain, Zhejiang | TM | 15 | |
| Dapan Mountain, Zhejiang | DP | 10 | |
| Matou County, Jiangxi | MT | 15 |
2.2. ISSR-PCR amplification
Out of 100 ISSR markers (UBC primer set No. 9, Biotechnology Laboratory, University of British Columbia, Vancouver, Canada; http://www.ubc.ca/), twelve primers (Table 2) that produced the strongest, clearest, and most reproducible bands were selected for further study. A 25 μl PCR amplification run contained 25 ng of genomic DNA, 2.5 μl 10× buffer, 2 mmol/L MgCl2, 0.2 mmol/L dNTPs, 0.4 μmol/L of primers, and 2.0 U Taq DNA polymerase (Shanghai Sangon Biotechnology Co. Ltd., Shanghai, China). ISSR-PCR amplifications were performed in a GeneAmp® PCR System 9700 thermal cycler (Applied Biosystems, Foster City, USA) with programme: 94 °C for 4 min; 45 cycles of 94 °C for 30 s, 49.4–65.0 °C for 45 s, and 72 °C for 1.5 min; 72 °C for 10 min (the specific annealing temperature for every ISSR primer is in Table 2). For every PCR run, a negative control without template DNA was also included. And every PCR amplification was repeated at least twice. PCR products were electrophoresed on 1.5% (v/v) agarose gels along with DNA Marker DL2000 (TaKaRa Biotechnology Co. Ltd., Dalian, China), then stained with ethidium bromide (EB), visualized with ultraviolet, and photographed.
Table 2.
Twelve ISSR primers used in the present study to develop SCAR markers
| Primer code | Sequence (5′–3′) | Annealing temp. (°C) |
| UBC809 | AGAGAGAGAGAGAGAGG | 60.5 |
| UBC810 | GAGAGAGAGAGAGAT | 53.0 |
| UBC811 | GAGAGAGAGAGAGAC | 52.7 |
| UBC812 | GAGAGAGAGAGAGAA | 50.8 |
| UBC827 | ACACACACACACACACG | 60.5 |
| UBC834 | AGAGAGAGAGAGAGAGYT | 49.4 |
| UBC855 | ACACACACACACACACYT | 62.0 |
| UBC859 | TGTGTGTGTGTGTGTGRC | 57.0 |
| UBC874 | CCCTCCCTCCCTCCCT | 65.0 |
| UBC881 | GGGTGGGGTGGGGTG | 60.5 |
| UBC887 | DVDTCTCTCTCTCTCTC | 50.8 |
| UBC889 | DBDACACACACACACAC | 50.8 |
2.3. Cloning and sequencing of ISSR marker fragment
The specific band only amplified in S. ningpoensis of Zhejiang Province was excised from 2% (v/v) agarose gels and purified DNA fragment was cloned using pUCm-T vector (Sangon, Shanghai, China). The GENECLEAN II kit (BIO 101 Inc., Carlsbad, USA) is used to purify DNA fragment. Sequencing was run on an ABI 3700 Sequencer (ABU, Italy) by Shanghai Sangon Biotechnology Co., Ltd. Sequences were edited by SEQUENCHER (Version 4.0.5 Gene Codes Corporation, Ann Arbor, MI, USA).
2.4. Primer design and SCAR-PCR
Based on the sequencing results, a pair of primers (Table 3) was designed using the software of primer-primer 5.0 (Premier Biosoft International; Palo Alto, CA, USA) and synthesized by Shanghai Sangon Biotechnology Co., Ltd. The diagnostic PCR by primers CC874u and CC874d was carried out by programme: 94 °C for 5 min; 35 cycles of 94 °C for 30 s; 59 °C for 45 s; 72 °C for 1.5 min; 72 °C for 10 min. The reaction mixture is the same as ISSR-PCR, containing 0.2 μmol/L of the upper primer and 0.2 μmol/L of the lower primer. PCR products were run in 1.5% (v/v) agarose/EB gels.
Table 3.
SCAR primers derived from cloned ISSR band of S. ningpoensis from Zhejiang Province*
| SCAR primer | Sequence (5′–3′) |
| CC874u | CTATCATCGTCTTTGTCCATCC |
| CC874d | TGCTTTGAAACATTTGAACTTG |
ISSR primer: UBC874; Annealing temperature: 59.0 °C
3. Results
3.1. Screening the specific ISSR marker
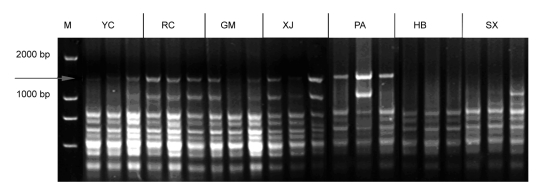
In this study, 85 individuals originated from three different provinces (Table 1) were surveyed by ISSR-PCR to develop SCAR markers. ISSR-PCR results show that primer UBC874 provided an approximately 1 300-bp band unique to populations originated from Zhejiang Province (Fig. 1).
Fig. 1.
PCR profiles of UBC874 in S. ningpoensis
Arrow indicates the specific band, which is only in samples from Zhejiang Province. M: DNA marker. YC, RC, GM, XJ, PA, HB, and SX are locality codes as shown in Table 1
3.2. Conversion of ISSR marker to SCAR marker
This specific band amplified by ISSR primer UBC874 was only found in the genome of S. ningpoensis from Zhejiang Province, but not in the genome of others. After gel-purified, cloned and sequenced, this DNA fragment turned out to be 1 306 bp (GenBank accession No. EU082804.1; GI: 156185939). Based on analysis of that sequence, a pair of 22-bp SCAR primers (Table 3), CC874u and CC874d, was designed for the amplification of this DNA fragment. The upper primer was 70 bp from 5′ and the lower primer being 110 bp from 3′ amplified a 1 126 bp fragment from samples of Zhejiang Province.
3.3. Testing designed SCAR primers
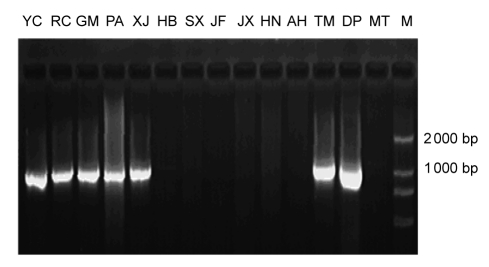
All samples listed in Table 1 were amplified by SCAR primers CC874u and CC874d to test their specificities. PCR products showed that a single band about 1 000 bp was only in accessions (Table 1) originated from Zhejiang Province (Fig. 2). Therefore, primers, CC874u and CC874d, designed in this study were proven to be diagnostic probe markers for identifying geo-authentic S. ningpoensis originated from Zhejiang Province.
Fig. 2.
Banding pattern of S. ningpoensis originated from Zhejiang Province (showing a distinct and reproducible band) and other provinces (showing no positive marker) with the designed primers CC874u/CC874d
M: DNA marker. YC, RC, GM, PA, XJ, HB, SX, JF, JX, HN, AH, TM, DP, and MT are locality codes as shown in Table 1
4. Discussion
Nowadays, with the booming of the herbal medicine market, standardization of traditional Chinese medicine has become more and more important. CMM is different from western and chemical medicines. The multiple sources and geo-authenticity of CMM generate unique confusion, e.g., different herbs species sharing one name, one herb using different names, and even one species collected from different localities having different medical effects (Zhao et al., 2006). S. ningpoensis has been widely used in CMM, but the geographical origin has always been a problem as mentioned above. Thus, to deal with the increasing dissatisfaction among consumers with the quality of herbal products and to obtain safe and effective application of CMM, an effective method to distinguish S. ningpoensis according to its geographical origins is critical (Moraes et al., 2005).
In general, morphological analysis, chemical chromatography, and DNA markers are used for authentication. Morphological method is conducted by observing, touching, smelling, and tasting (Zhao et al., 2006). Although, it is fast and easy, it also largely depends on personal experience. In many cases, morphological characteristics are often variable, and may disappear when crude drugs only contain the medical part like roots of a whole plant. And some herbs from the same genus or family are difficult to identify because of the similar morphological characteristics. In this study, all samples are the same species, S. ningpoensis, collected from different localities. Thus, it is almost impossible to distinguish geo-authentic S. ningpoensis from others by morphological method.
In recent years, chromatography has been widely used in the authentication of CMM (Hua et al., 2003). In particular, the HPLC chromatography fingerprinting technique, which can provide more precise information, is used for the identification of geographical origins within the same species (Lu et al., 2005). HPLC is not perfect, however, and needs large amounts of samples. And the result is always influenced by harvest time, storage period, and processing method of CMM, since the chemical gradients of plants are easily affected by those factors (Lum et al., 2005). As to the process of HPLC experiments, standard chromatograms are first collected for a number of authentic species of samples. Then, four to nine characteristic peaks in a fingerprint chromatogram are chosen for authentication and identification purpose (Hu et al., 2005). Thus, this method is considered to be complicated and time-consuming.
In comparison to traditional and other existing methods, DNA markers have more advantages: sensitive, reliable, accurate, stable, convenient, and only a tiny amount of sample is sufficient. Among many types of DNA markers, for ISSR markers, no prior sequence reference is required, and they are more convenient than SSR and other markers (Zietkiewicz et al., 1994). Because of the longer primers and higher annealing temperature, ISSR can provide more reproducibility and stability than RAPD markers (Camacho and Liston, 2001). In addition, experiments of ISSR are much easier and less expensive than amplified fragment length polymorphism (AFLP) (Passinho-Soares et al., 2006). Considering the high reproducibility and polymorphic nature, the simple process of experiments, the stability, and low cost, using ISSR markers for CMM authentication is more practical and reasonable. A pair of primers developed in this study is an extension of ISSR markers. Although ISSR markers have the potential to provide a rapid, reliable, and simple authentication, as a universal primer, the specificity of ISSR is not as good as species-specific molecular markers. Also, in many cases, it can be an advantage to amplify a single fragment (Techen et al., 2006). This study provides a diagnostic PCR method to identify S. ningpoensis according to geographical origin by using species-specific molecular markers, and proves that DNA markers are much more useful for the identification of geographical origins than morphological and phytochemical methods.
Acknowledgments
The authors thank Mr. Shu-qing SUN (Zhejiang University, China) and Mr. Ming-shui ZHAO (Tianmu Natural Reserve Institute, Zhejiang, China) for collecting samples during fieldwork.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2007CB411600), the National Natural Science Foundation of China (No. 31070205), and the Key Agricultural Program of Pan’an County of Zhejiang Province, China (No. 2005ZB01)
References
- 1.Camacho FJ, Liston A. Population structure and genetics diversity of Botrychium pumicola (Ophioglossaceae) based on ISSR. Am J Bot. 2001;88(6):1065–1070. doi: 10.2307/2657089. [DOI] [PubMed] [Google Scholar]
- 2.Chung SY, Cheng FC, Lee MS, Lin JY, Lin MC, Wang MF. Ginkgo biloba leaf extract (EGb761) combined with neuroprotective agents reduces the infarct volumes of gerbil ischemic brain. Am J Chin Med. 2006;34(5):803–817. doi: 10.1142/S0192415X06004302. [DOI] [PubMed] [Google Scholar]
- 3.de Santos Galíndez J, Lanza AMD, Matellano LF. Biologically active substances from the genus Scrophularia . Pharm Biol. 2002;40(1):45–59. doi: 10.1076/phbi.40.1.45.5864. [DOI] [Google Scholar]
- 4.Díaz AM, Abad MJ, Fernández L, Silván AM, de Santos J, Bermejo P. Phenylpropanoid glycosides from Scrophularia scorodonia: in vitro anti-inflammatory activity. Life Sci. 2004;74(20):2515–2526. doi: 10.1016/j.lfs.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Doyle JJ. DNA Protocols for Plants—CTAB Total DNA Isolation. In: Hewitt G, Johnston A, editors. Molecular Techniques in Taxonomy. Berlin, Germany: Springer Verlag; 1991. pp. 283–294. [Google Scholar]
- 6.Fernández MA, García MD, Sáenz MT. Antibacterial activity of the phenolic acids fraction of Scrophularia frutescens and Scrophularia sambucifolia . J Ethnopharmacol. 1996;53(1):11–14. doi: 10.1016/0378-8741(96)01419-5. [DOI] [PubMed] [Google Scholar]
- 7.Forbes FB, Hemsley WB. An enumeration of all the plants known from China proper, Formosa, Hainan, Corea, the Luchu archipelago, and the island of Hongkong together with their distribution and synonymy. J Linn Soc Bot. 1890;26(74):178–179. [Google Scholar]
- 8.Giner RM, Villalba ML, Recio MC, Máñez S, Cerdá-Nicolás M, Ríos JL. Anti-inflammatory glycoterpenoids from Scrophularia auriculata . Eur J Pharmacol. 2000;389(2-3):243–252. doi: 10.1016/S0014-2999(99)00846-8. [DOI] [PubMed] [Google Scholar]
- 9.Gong W, Fu CX, Luo YP, Qiu YX. Molecular identification of Sinopodophyllum hexandrum and Dysosma species using cpDNA sequences and PCR-RFLP markers. Planta Med. 2006;72(7):650–652. doi: 10.1055/s-2006-931535. [DOI] [PubMed] [Google Scholar]
- 10.Hu P, Luo GA, Zhao ZZ, Jiang ZH. Multi-component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chem Pharm Bull (Tokyo) 2005;53(6):677–683. doi: 10.1248/cpb.53.677. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Zhang Q, Xin H, Qin LP, Lu BR, Rahman K. Association between chemical and genetic variation of Vitex rotundifolia populations from different locations in China: its implication for quality control of medicinal plants. Biomed Chromatogr. 2007;21(9):967–975. doi: 10.1002/bmc.841. [DOI] [PubMed] [Google Scholar]
- 12.Hua R, Sun SQ, Zhou Q, Noda I, Wang BQ. Discrimination of fritillary according to geographical origin with Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. J Pharmaceut Biomed. 2003;33(2):199–209. doi: 10.1016/S0731-7085(03)00253-X. [DOI] [PubMed] [Google Scholar]
- 13.Kajimoto T, Hidaka M, Shoyama K, Nohara T. Iridoids from Scrophularia ninpoensis . Phytochemistry. 1989;28(10):2701–2704. doi: 10.1016/S0031-9422(00)98071-3. [DOI] [Google Scholar]
- 14.Liu CW, Bi ZM, Zhu YF, Li P. Simultaneous determination of four kinds of bioactive components in Radix Scrophulariae by HPLC. China Pharm J. 2007;42(21):1614–1616. (in Chinese) [Google Scholar]
- 15.Liu L, Hudgins WR, Shack S, Yin MQ, Samid D. Cinnamic acid: a natural product with potential use in cancer intervention. Int J Cancer. 1995;62(3):345–350. doi: 10.1002/ijc.2910620319. [DOI] [PubMed] [Google Scholar]
- 16.Lu GH, Chan K, Liang YZ, Leung KSY, Chan CL, Jiang ZH. Development of high performance liquid chromatographic fingerprints for distinguishing of Chinese Angelica from related umbelliferae herbs. J Chromatogr A. 2005;1073(1-2):383–392. doi: 10.1016/j.chroma.2004.11.080. [DOI] [PubMed] [Google Scholar]
- 17.Lum RM, Potter E, Dang T, Heber D, Hardy M, Hirsch MA. Identification of botanicals and potential contaminants through RFLP and sequencing. Planta Med. 2005;71(9):841–846. doi: 10.1055/s-2005-871230. [DOI] [PubMed] [Google Scholar]
- 18.Miyase T, Mimatsu A. Acylated iridoid andphenylethanoid glysocides from the aerial parts of Scrophularia nodosa . J Nat Prod. 1999;62(8):1079–1084. doi: 10.1021/np9805746. [DOI] [PubMed] [Google Scholar]
- 19.Miyazawa M, Okuno Y, Nakamura S, Kameoka H. Suppression of SOS-inducing activity of chemical mutagens by cinnamic acid derivatives from Scrophulia ningpoensis in the Salmonella typhimurium TA1535/pSK1002 umu test. J Agric Food Chem. 1998;46(3):904–910. doi: 10.1021/jf9704221. [DOI] [Google Scholar]
- 20.Moraes RM, Momm HG, Silva B, Maddox V, Easson GL, Lata H, Ferreira D. Geographic information system method for assessing chemo-diversity in Medicinal plants. Planta Med. 2005;71(12):1157–1164. doi: 10.1055/s-2005-873166. [DOI] [PubMed] [Google Scholar]
- 21.Passinho-Soares H, Felix D, Maria Auxiliadora Kaplan MA, Margis-Pinheiro M, Margis R. Authentication of medicinal plant botanical identity by amplified fragmented length polymorphism dominant DNA marker: inferences from the Plectranthus genus. Planta Med. 2006;72(10):929–931. doi: 10.1055/s-2006-946673. [DOI] [PubMed] [Google Scholar]
- 22.Reid DP. Chinese Herbal Medicine. Boston, US: Shambhala Publications Inc. Press; 1996. p. 98. [Google Scholar]
- 23.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer—Part 1. Curr Oncol. 2006;13(1):14–26. doi: 10.3747/co.v13i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Techen N, Khan IA, Pan ZQ, Scheffler BE. The use of polymerase chain reaction (PCR) for the identification of Ephedra DNA in dietary supplements. Planta Med. 2006;72(3):241–247. doi: 10.1055/s-2005-916173. [DOI] [PubMed] [Google Scholar]
- 25.Wang JK, Wang JL. History and Genuine Character of Traditional Chinese Medicine. Beijing, China: Science and Technology of China Medicine Press; 2007. p. 94. (in Chinese) [Google Scholar]
- 26.Woo YA, Kim HJ, Cho J, Chung H. Discrimination of herbal medicines according to geographical origin with near infrared reflectance spectroscopy and pattern recognition techniques. J Pharmaceut Biomed Anal. 1999;21(2):407–413. doi: 10.1016/S0731-7085(99)00145-4. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Zhang DM, Liu JQ, Zheng JH. A molecular marker that is specific to medicinal rhubarb based on chloroplast trnL/trnF sequences. Planta Med. 2001;67(8):784–786. doi: 10.1055/s-2001-18341. [DOI] [PubMed] [Google Scholar]
- 28.Yang S, Chen C, Zhao Y, Xi W, Zhou X, Chen B, Fu C. Association between chemical and genetic variation of wild and cultivated populations of Scrophularia ningpoensis Hemsl. Planta Med. 2010 doi: 10.1055/s-0030-1250601. in press. [DOI] [PubMed] [Google Scholar]
- 29.Ye Q, Qiu YX, Quo YQ, Chen JX, Yang SZ, Zhao MS, Fu CX. Species-specific SCAR markers for authentication of Sinocalycanthus chinensis . J Zhejiang Univ-Sci B. 2006;7(11):868–872. doi: 10.1631/jzus.2006.B0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen KY. The Illustrated Chinese Materia Medica Crude and Prepared. Taiwan, China: SMC Publishing Inc. Press; 1992. p. 64. (in Chinese) [Google Scholar]
- 31.Yip PY, Kwan HS. Molecular identification of Astragalus membranaceus at the species and locality levels. J Ethnopharmacol. 2006;106(2):222–229. doi: 10.1016/j.jep.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Zhang YB, Ngan FN, Wang ZT, Ng TB, But PP, Shaw PC. Random primed polymerase chain reaction differentiates Codonopsis pilosula from different localities. Planta Med. 1999;65(2):157–160. doi: 10.1055/s-1999-14058. [DOI] [PubMed] [Google Scholar]
- 33.Zhao YP, Qiu YX, Gong W, Fu CX, Li JH. Authentication of Actinidia macrosperma using PCR-RFLP based on trnK sequences. Botanical Studies. 2007;48(3):239–242. [Google Scholar]
- 34.Zhao ZZ, Hu Y, Liang ZT, Yuen JP, Jiang ZH, Leung KS. Authentication is fundamental for standardization of Chinese Medicines. Planta Med. 2006;72(10):865–874. doi: 10.1055/s-2006-947209. [DOI] [PubMed] [Google Scholar]
- 35.Zhu YF, Bi ZM, Liu CW, Ren MT, Wu FH, Li P. Endothelial cell extraction and HPLC-ESI/TOF MS analysis for predicting potential bioactive components of Radix Scrophulariae. J China Pharm Univ. 2008;39:228–231. (in Chinese) [Google Scholar]
- 36.Zietkiewicz E, Rafalski A, Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20(2):176–183. doi: 10.1006/geno.1994.1151. [DOI] [PubMed] [Google Scholar]