Abstract
An efficient induction system and regeneration protocol based on mature barley embryos were developed. Embryos isolated from mature seeds, dehusked by hand and inoculated with longitudinally bisected sections, showed low contamination and high primary callus-forming capability. The influences of nine culture media on primary callus induction and germination from the mature embryos of barley cultivars Golden Promise and Zaoshu 3 were analyzed. The results showed that the two cultivars had much higher values of primary callus induction in the B16M6D medium as compared to the other eight medium formulations, with a frequency of 74.3% and 78.4% for Golden Promise and Zaoshu 3, respectively. Furthermore, Zaoshu 3 demonstrated particularly high stability in callus induction over the different media, indicating its potential utilization in callus induction and regeneration for its good agronomic traits and wide adaption. There were significant differences amongst 11 barley genotypes in terms of primary callus induction in the optimum medium, with percentages of callus induction and germination response ranging from 17.9% to 78.4% and 2.8% to 47.4%, respectively. Green plantlets of Dong 17, Golden Promise, and Zaoshu 3 were successfully developed from primary calli through embryogenesis, with green plant differentiation frequencies ranging from 9.7% to 21.0% across genotypes.
Keywords: Barley, Mature embryo, Genotypic difference, Primary callus, Plant regeneration
1. Introduction
The improvement of abiotic stress tolerance in barley depends on the understanding of genetic variation within this crop and the availability of precise methods for desirable genetic transformation (Ellis et al., 2000). Once the genotypes for desirable characters are identified by preliminary evaluation, the transferral of desired genes to an elite barley germplasm can be attempted (Özgen et al., 2005). However, molecular enhancement in barley remains hampered due to the lack of efficient and long-term regenerable culture systems. Despite the fact that barley presents as a promising model plant for the development methods of genetic engineering, its transformation is marked by some complications. For barley, the protocols for callus induction and plant regeneration are not efficiently and widely used because of high genotype-specific dependency (Taniguchi et al., 1991; Akula et al., 1999; Ganeshan et al., 2003) and low regeneration efficiency (Rengel, 1987; Bregitzer et al., 1998; Chen et al., 2006). Therefore, the application of genetic engineering has been restricted to only a few barley germplasms that possess high tissue culture capability; the so-called model cultivars mostly contain a low agronomic value. Most culture systems for callus formation and plant regeneration were developed for model barley varieties such as Golden Promise (Wan and Lemaux, 1994; Tingay et al., 1997; Holme et al., 2006; Kumlehn et al., 2006). It is imperative, therefore, to explore and establish regeneration systems for more elite barley genotypes that contain not only high tissue culture potential but also reasonable agronomic traits.
In cereals, green plantlets can be successfully developed from a variety of explants, including embryos (Chauhan et al., 2007), mature embryos (Cho et al., 2004), leaves (Chugh and Khurana, 2003) or seedling segments (Becher et al., 1992), apical meristems (Goldman et al., 2003), coleoptiles (Sahrawat and Chand, 2004), and microspores (Jähne et al., 1994). For barley, morphogenic calli can be induced more frequently from immature embryos (Caligari et al., 1987; Bregitzer et al., 1998). Moreover, highly efficient and reproducible plant regeneration protocols were primarily established through the use of immature embryotic materials (Repellin et al., 2001). However, immature embryotic materials require the continuous growth of donor barley plants in environmentally-controlled greenhouses, thereby placing high demands on labor, time, and space. Furthermore, it is difficult to control the developmental stage of tissue culture within immature embryos. The use of mature embryos has remarkable advantages, such as a simplified cultivation of tissues excised from mature dry seeds and the availability of large amounts of initial materials without growth season restrictions. The establishment of embryogenesis and plant regeneration system based on mature embryos has been reported in recent studies (Sharma et al., 2004; He and Jia, 2008), but optimal systems for the isolation and culture mode of explants either are underdeveloped or lack adequate recognition.
Described in this paper is an optimized primary callus induction medium, as well as an accessible and reproducible isolation and induction system for mature barley embryos. The differences in primary callus induction and plant regeneration among barley genotypes are evaluated. Also reported is a preliminary screening of several local Chinese barley cultivars with high primary callus induction and regenerability. The Chinese cultivars selected for the present study were planted locally, in wide distribution, and are deemed of specific value in agronomy. Finally, from the somatic embryogenesis of mature embryos, an efficient and reproducible regeneration protocol was established for Zaoshu 3.
2. Materials and methods
2.1. Plant materials
Mature seeds of 12 barley (Hordeum vulgare L.) genotypes were used, consisting of 11 hulled barley genotypes (Dong 17, Fuxuan 48, Golden Promise, Humai 1, Igri, TL43, Triumph, Weisuobuzhi, Zaoshu 3, ZAU 3, and Zhepi 1) and one hulless landrace (Yuyaohuanghumimai). Due to their high yield potential, wide adaptability, and high tolerance to waterlogging, the representative cultivars Fuxuan 48, Humai 1, Zaoshu 3, ZAU 3, and Zhepi 1 were widely planted in the Yangtze River Delta region during the last two decades.
2.2. Effects of isolation and sterilization methods of mature embryos on primary callus induction
Fuxuan 48 was inoculated in four induction treatments to determine the callus induction response. Seeds were soaked in water for 2 h, and then dehusked by hand or by 50% (v/v) concentrated sulfuric acid. The sections containing whole mature embryos (3–5 mm in length) were, then, dissected from the dehusked seeds, surface-sterilized in 75% (v/v) ethanol for 30 s, and then immersed in 15% (v/v) sodium hypochlorite for 15 min. Meanwhile, the intact, dehusked seeds of Fuxuan 48 were surface-sterilized in 75% ethanol for 30 s, and then immersed in 50% sodium hypochlorite for 30 min. The isolated embryos or caryopses were rinsed three times with sterile distilled water and longitudinally bisected into two halves. Approximately 20–25 explants were placed, bisected section down, in Petri dishes containing medium B13S2D.
2.3. Effects of induction medium and genotype on primary callus induction
The basal media used in this study were MS (Murashige and Skoog, 1962), B5 (Gamborg et al., 1968), and N6 (Chu, 1978), and were supplemented with different concentrations of 2,4-dichlorophenoxy-acetic acid (2,4-D) and carbon sources (Table 1). Added to all media were 0.5 g/L of proline and 0.5 g/L of casein hydrolysate. Unless otherwise indicated, all medium components, except plant hormone, were stirred, adjusted to pH 5.8, solidified with 0.55% agar, and autoclaved at 115 kPa and 120 °C for 20 min. All growth factors were filter-sterilized before added to the autoclaved medium. To optimize the primary callus inductions of cvs. Zaoshu 3 and Golden Promise, explants were induced on each of the nine induction media. To detect the genotypic difference in primary callus induction, mature embryos of 11 genotypes were dehusked by hand, extracted of endosperm, and inoculated on B16M6D medium.
Table 1.
Nine different medium formulations for primary callus induction
| Culture medium | Macronutrient | Micronutrient | Vitamin | Carbon source | 2,4-D (mg/L) |
| B13S2D | N6 | MS | B5 | 30 g/L sucrose | 2 |
| B13S6D | N6 | MS | B5 | 30 g/L sucrose | 6 |
| B13M6D | N6 | MS | B5 | 30 g/L maltose | 6 |
| MS3M2D | MS | MS | MS | 30 g/L maltose | 2 |
| MS3M6D | MS | MS | MS | 30 g/L maltose | 6 |
| B16M6D | N6 | MS | B5 | 60 g/L maltose | 6 |
| B5MS6M6D | MS | MS | B5 | 60 g/L maltose | 6 |
| MS6M6D | MS | MS | MS | 60 g/L maltose | 6 |
| N6MS6M6D | N6 | MS | MS | 60 g/L maltose | 6 |
After two weeks incubation in darkness at 23–25 °C, the frequencies of primary callus induction/germination response were calculated by counting the explants that formed callus/germinated out of the total number of mature embryos. Since most explants exposed to this germination intensity would likely have ceased callusing, the germination response value represents the frequency of explants sharing a similar germination intensity and having an average shoot length of 3–5 cm and root length of 1–2 cm.
2.4. Embryogenic callus formation and plant regeneration
After three weeks of induction, primary callus was separated from explants, then transferred to a B13M medium supplemented with 3.0 mg/L 2,4-D and 0.1 mg/L 6-benzylaminopurine (BA) to commence embryogenesis at 23–25 °C in darkness for three weeks. The proliferated callus was then cultured in a regeneration medium B13M containing 0.4 mg/L BA under low light conditions [20–30 μE/(m2∙s)] at 24 °C and during a 14-h/10-h (light/dark) photoperiod. Embryogenic callus with green structures and leaf primordial could be obtained after five to six weeks of incubation. Eleven to twelve weeks after culture initiation, green shoots and plantlets were successfully developed from embryogenic callus through a biweekly subculture. To induce a strong root system, well-developed shoots or plantlets (2–3 cm in length) were separated from the regenerating embryoids and transferred to a rooting medium consisting of 1/2 MS medium, 20 g/L sucrose, 2.0 mg/L α-naphtalene acetic acid (NAA), and 0.4% agar. Conditions selected for plant regeneration and rooting of plantlets were based on those described by Sharma et al. (2005). The well-rooted plants were transferred to soil and placed in a growth camber (16-h light period, 15 °C).
2.5. Statistical analysis
Identical and independent experiments were performed for each cultivar. The data were analyzed using the statistical package statistical analysis system (SAS) Version 8 (SAS Institute Inc., Cary, NC, USA). A two-way factorial analysis was conducted to study the effects of medium component and genotype on induction response. Differences in primary callus induction, germination response, and plant regeneration amongst the 12 studied genotypes were evaluated by analysis of variance (ANOVA) testing. The significance of group differences was determined by a least significant difference (LSD) formulation.
3. Results
3.1. In vitro responses of mature embryos in four induction treatments
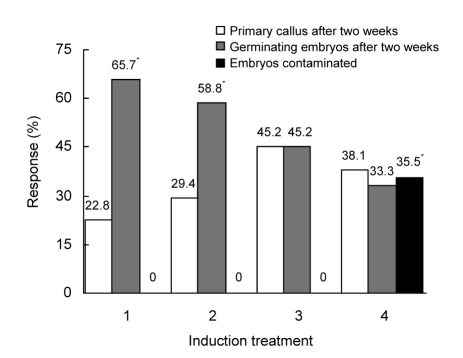
Explants of Fuxuan 48 had more stable induction responses but were more vulnerable to contamination as compared to Golden Promise; therefore, Fuxuan 48 was selected for this experiment. During initial experiments, the mature embryos showed differential induction responses in medium B13S2D (Fig. 1). Explants dehusked by hand, with endosperm removed, showed relatively great primary callus-forming capability. Moreover, isolating embryos from mature seeds could effectively eliminate contamination, even if sodium hypochlorite strength was reduced from 50% (for 30 min) to 15% (for 15 min) during surface-sterilization. Removing the hull with concentrated sulfuric acid is more convenient than when performed by hand, regardless of a statistically higher frequency of germinating embryos. Seven to nine days following culture initiation, the caryopsis dehusked by sulfuric acid germinated intensively and main shoots elongated rapidly, with callus forming 5–7 d later. The callusing response took 3–4 d later for the explants dehusked by sulfuric acid as compared to those dehusked by hand, probably due to the injury of sulfuric acid to the embryos.
Fig. 1.
Effects of four induction treatments on callus induction in cv. Fuxuan 48
Induction treatment: 1, dehusked by sulfuric acid/endosperm contained; 2, dehusked by sulfuric acid/endosperm removed; 3, dehusked by hand/endosperm removed; 4, dehusked by hand/endosperm contained. * indicates that values are significantly different at P=0.05 according to the ANOVA test. A total of 40 to 50 explants were used as starting materials
3.2. Optimization of culture medium for induction of primary callus
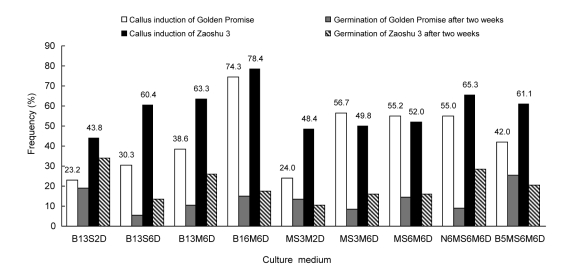
The purpose of this experiment was to determine the influences of nine different media on primary callus induction of two barley cultivars, Zaoshu 3 and Golden Promise (Fig. 2). The statistical analysis showed that primary callus induction of the mature embryos differed among medium types between the two cultivars, thereby indicating an interaction between cultivar and medium. On the whole, the value of primary callus induction in medium B16M6D was significantly higher than those within the other media. Regardless of genotype, explants induced in B13S2D resulted in a slightly higher (about 10%) frequency of germinating embryos as compared to the other media, indicating that germination may be reasonable at low concentrations of sucrose and 2,4-D. Moreover, the results suggest that a higher maltose level (60 g/L) combined with a higher 2,4-D concentration (6 mg/L) could efficiently promote callusing response.
Fig. 2.
Influences of nine different culture media on primary callus induction and germination from mature embryos of barley cvs. Golden Promise and Zaoshu 3
Frequencies of primary callus and germinating embryos with 3–5 cm long shoot were recorded in each combination two weeks after culture initiation. Average data were from two independent experiments each with 60 to 70 mature embryos as materials
Golden Promise showed a frequency of callus induction ranging from 23% to 74% in the nine media, indicating that the cultivar has specificity in its response to culture media during callus induction. By contrast, the local cultivar Zaoshu 3 had a smaller variation in the frequency of callus induction over the different media, ranging from 50% to 60%, suggesting that the cultivar is relatively insensitive to the media. For Golden Promise, however, increasing the 2,4-D level from 2 mg/L to 6 mg/L resulted in a significant increase of callus induction frequency and decrease of germination. Interestingly, Zaoshu 3 showed higher primary callus induction when macronutrients in the MS medium were substituted by those in the N6 medium. Moreover, the same pattern took place for Zaoshu 3 when vitamins in the MS medium were replaced by those in the B5 medium. This provides conclusive evidence that the medium B16M6D was optimal for the primary callus induction of the mature embryos. It was, therefore, used as the initial culture medium for callus induction in the subsequent experiments.
3.3. Genotypic difference in primary callus induction in B16M6D medium
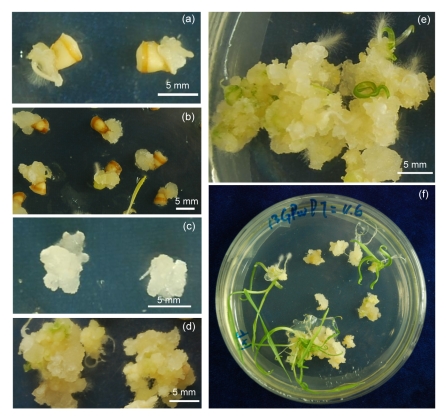
Due to the low concentration of sodium hypochlorite in surface-sterilization, as well as the direct contact of longitudinally bisected embryos with the induction media, the developments of plumule and radicle were initiated within 24 h, and became visible after 3 d of incubation. Meanwhile, the callusing response was observed as showing the non-embryogenic callus, termed “primary callus,” maintaining proliferation coupled with elongation of the main shoot (Fig. 3a). The primary callus could be obtained after two weeks of induction culture (Fig. 3b). At this time, the plumule and radicle lengths of most germinated explants were 3–5 cm and 0.5–1.0 cm, respectively. A few explants germinated significantly to a plumule length of 3–5 cm after 7-d induction and moreover, the plumule would continue growing for one week further, reaching a length of up to 15 cm and lifting the explants out of the medium. Callus proliferation was, therefore, inhibited. Consequently, in order to maintain the embryogenic potential, it was deemed reasonable to discard the primary calli from the callusing explants after two to three weeks.
Fig. 3.
Primary callus induction, embryogenic callus formation, and plant regeneration from mature embryos of cv. Zaoshu 3
(a) Developing primary callus along with plumule and radicle from mature embryos after 3 d of incubation; (b) Induction response of isolated embryos two weeks after culture initiation; (c) The soft, translucent, and watery primary callus discarded from mature embryos; (d) Embryogenic callus with nodular and compact structures in the Petri dish; (e) Regenerating embryos with small shoots, plantlets, and clusters of embryoids; (f) Green plantlets developed from embryoids 13 weeks after culture initiation
Frequencies of primary callus induction and germination responses of mature embryos in 11 barley genotypes are shown in Table 2. The nutrient-modified medium B1, containing 60 g/L maltose and 6 mg/L 2,4-D, bore a high frequency of primary callus induction higher than 50% for Zaoshu 3, Golden Promise, Dong 17, and Zhepi 1. These four genotypes, in addition to ZAU 3, were, therefore, selected for the plant regeneration experiment. The results indicated that Zaoshu 3 had the highest percentage of callus induction, followed by Golden Promise and Dong 17; whereas other genotypes had significantly lower percentages. Humai 1, Triumph, Yuyaohuanghumimai and Igri showed particularly low percentages of primary callus induction, with Igri being the lowest (17.9%), indicating a remarkable genotypic difference in the frequency of primary callus induction. The germination response of Humai 1 was 47.4%; the highest amongst the 11 genotypes. This high germination response could be attributed to low callus induction. The primary callus derived from embryos of hulless barley was generally loose, petal-like, and low in embryogenesis potential. The soft, translucent, and watery calli, dissected from the explants of the five barley cultivars with high callus induction (Zaoshu 3, Golden Promise, Dong 17, Zhepi 1, and ZAU 3), were transferred to the subculture medium for proliferation and embryogenesis (Fig. 3c).
Table 2.
Frequencies of primary callus induction and germination responses of mature embryos in 11 barley genotypes1
| Genotype | nei | Primary callus induction (%)2 | Germination after two weeks (%)2 |
| Dong 17 | 161 | 64.8±4.4b | 32.7±2.6ab |
| Golden Promise | 154 | 74.3±3.7ab | 15.2±2.7efg |
| Igri | 148 | 17.9±5.7f | 7.0±3.2fg |
| Zhepi 1 | 362 | 53.7±3.3c | 20.2±3.5ce |
| Weisuobuzhi | 145 | 42.8±7.8cd | 42.3±9.7a |
| ZAU 3 | 511 | 49.3±1.9cd | 30.2±3.1bd |
| Zaoshu 3 | 171 | 78.4±2.6a | 17.3±2.0ef |
| Humai 1 | 126 | 24.5±5.0ef | 47.4±5.1a |
| Yuyaohuanghumimai | 166 | 27.1±3.0ef | 2.8±1.4g |
| TL43 | 175 | 38.8±6.4de | 18.2±3.2def |
| Triumph | 149 | 27.3±6.6ef | 19.9±6.3def |
n ei: number of explants inoculated
Three independent experiments with three replicates each were carried out for each cultivar; for Zhepi 1 and ZAU 3, six and eight replicates were done, respectively
Values (mean±SE) followed by the same superscript letters are not significantly different at P=0.05 according to the ANOVA test
3.4. Embryogenesis and plant regeneration from five barley cultivars
The basic medium B1 containing 30 g/L maltose and 0.4 mg/L BA was found to be efficient for the induction of embryogenic callus with biweekly subculture when incubated for 3 d in the dark, followed by two to four weeks under light. The proliferated calli with shiny, nodular, and compact structure were of great embryogenic potential (Fig. 3d). Embryogenic callus with excellent regenerability was obtained after nine to ten weeks of culture. The successive subcultures promoted the formation of numerous clusters of embryoids at different developmental stages with leaf primordial and/or small plumule (Fig. 3e). During embryogenesis, the cultivars Zhepi 1 and ZAU 3 presented great callus proliferative ability and the embryogenic calli were also capable of turning green under light conditions. Nevertheless, the calli were not effective for differentiation due to genotype dependence and they eventually turned brown. Plumules and plantlets spontaneously derived from embryoids were observed in the next three weeks for Zaoshu 3 (Fig. 3f). In addition, the media free of plant hormone or supplemented with 3 mg/L 2,4-D and 0.1 mg/L BA were also efficient for plant regeneration, whereas embryogenesis took place on the hormone-free medium two to three weeks later than on the hormone-containing medium (data not shown). Regenerated plantlets of 3 cm in length were transferred into the rooting medium, and a strong root system developed within two to three weeks. The well-rooted plantlets were transferred to soil and placed in a growth cabinet. Generally, no phenotypic difference was found between the regenerants and seed-initiated seedlings of Zaoshu 3.
Table 3 shows percentages of embryogenic callus induction, plant regeneration efficiency, and average number of plants per regenerated callus from the five barley cultivars. The percentage of green plants was 21.0%, 14.4%, and 9.7% for Zaoshu 3, Golden Promise, and Dong 17, respectively, being much higher than those for Zhepi 1 and ZAU 3. The cultivar Golden Promise had strong regenerability in terms of average number of plants per regenerated callus. Interestingly, Dong 17, which had the highest frequency of embryogenic callus, showed a relatively weak regenerability when compared with Zaoshu 3 and Golden Promise.
Table 3.
Frequencies of embryogenic callus induction and plant regeneration from mature embryos in five barley cultivars1
| Cultivar | Number of callus incubated | Embryogenic callus (%)2, 4 | Green plantlets (%)3, 4 | Albinos (%)3 | Average number of plants per regenerated callus4 |
| Zaoshu 3 | 319 | 67.7±3.5b | 21.0±3.7a | 2.2±1.1 | 3.1±0.3ab |
| Golden Promise | 143 | 63.2±7.2b | 14.4±6.9ab | 1.2±1.1 | 4.2±0.9a |
| Dong 17 | 71 | 93.7±3.6a | 9.7±3.2ab | 9.7±4.4 | 1.7±0.2b |
| Zhepi 1 | 81 | 71.0±5.2b | 0b | 0 | 0b |
| ZAU 3 | 48 | 51.6±8.9b | 0b | 0 | 0b |
Values (mean±SE) are calculated after three subcultures on regeneration medium
Frequency was estimated as the ratio of the number of embryogenic calli or greenish calli to the total number of calli used
Regeneration capacity of calli was calculated as the ratio of the number of calli with green or albino shoots to the number of calli plated on regeneration medium
Values followed by the same superscript letters are not significantly different at P=0.05 according to the ANOVA test
4. Discussion
Recently, mature embryos of barley were evaluated as starting materials in different culture modes, including endosperm-supported culture (He and Jia, 2008) and meristematic shoot segment culture, in which explants are excised from germinated mature embryos (Sharma et al., 2004). However, the isolation and obtaining of explants were not at that time readily available; therefore, the reliability needed further confirmation. We have optimized the conditions of callus induction and plant regeneration. The frequency of somatic embryogenesis was improved with segmentation from mature embryos into pieces in wheat (Delporte et al., 2001). In the present study, when the 2/3 endosperm of soaked seeds was removed, the isolated embryos were easily dehusked by hand, and the embryos were cut into two halves. The endosperm-excised explants resulted in a higher callus induction frequency than complete seeds. A similar observation was reported by Zapata et al. (2004), who found that the percentage of callus induction from isolated embryos was generally higher than those from roots, shoots, and complete seeds. The success of in vitro regeneration via embryogenesis is probably attributed to excised explants containing embryonic axis, as the suitability of the embryonic axis explants was demonstrated in previous studies (Krishnamurthy et al., 2000; Sharma et al., 2005).
Furthermore, barley regeneration systems via embryogenesis, based on different combinations of growth regulators, have been reported. Regenerated barley plantlets were successfully obtained on the medium containing 1.0 mg/L indole-3-butyric acid (IBA) and 0.1 mg/L kinetin (Zapata et al., 2004), 4 mg/L 4-amino-3,5,6-trichloropicolinic acid (picloram) (Akula et al., 1999), or 2 mg/L BA (He and Jia, 2008). In contrast, our results showed that the plant regeneration is successful on the medium B13M containing 0.4 mg/L BA. Meanwhile, the current results prove that embryogenic callus formation and plant regenerability are also genotype-dependent. Similar results were found by Bi et al. (2007). The hormone composition suitable for embryogenic callus formation and regeneration varies with genotypes, suggesting that the abilities of embryogenesis and regeneration for the callus derived from mature barley embryo may be dependent on not only hormone composition in the medium, but also on the hormone level in the callus.
Specific modifications of the culture medium strongly influence the frequencies of primary callus induction and the germinating embryo. It has been reported that the formation of embryogenic callus in various barley cultivars was depended on 2,4-D concentration, and 2–3 mg/L was adequate in most cases (Bregitzer et al., 1998; Zapata et al., 2004). However, it has been further demonstrated that for cv. Golden Promise, the germination and callusing response of mature embryos are affected by 2,4-D concentration, with the optimum level being 6 mg/L, combined with an increase in maltose level (Sharma et al., 2005). The inconsistence in the optimum 2,4-D concentration among the available reports may be attributed to the properties of embryos, which differ in developmental stages. In the current study, a marked increase in callus induction and decrease in germinating embryos were found when the media B1 and MS were supplemented with 6 mg/L 2,4-D and 60 g/L maltose for cv. Golden Promise, which was consistent with the results found by Sharma et al. (2005). However, Golden Promise had a relatively high percentage of callus formation in this study, as compared to Sharma et al. (2005)’s protocol. Interestingly, the findings were not observed in cv. Zaoshu 3. Moreover, the macronutrients in the N6 medium, as well as vitamins in B5 medium, appeared favorable for callusing response for the cultivar, indicating that there are remarkable interactions between genotypes and medium components. In other words, the levels of auxins and nutrients for callus induction are cultivar specific.
To date, Golden Promise has been well-known for its high tissue culture ability, and hence this genotype has been widely used in barley tissue transformation (Wan and Lemaux, 1994; Tingay et al., 1997; Holme et al., 2006; Kumlehn et al., 2006). In the current study, several genotypes were explored to establish callus induction and plantlet regeneration system. The results showed that genotype-specific dependency is universal and inevitable, and callus induction and plantlet regeneration vary with genotype. Therefore, the medium formulations have to be optimized according to the given genotype. However, Zaoshu 3 showed particularly high stability in callus induction over the different media and higher regenerability compared to Golden Promise, indicating its potential utilization in transgenic systems because of its reasonable agronomic traits and wide adaptation.
In conclusion, we developed a reproducible and highly efficient protocol, based on mature dry barley seeds, and found a new genotype Zaoshu 3 with higher tissue culture ability. The developed callus induction and regeneration system may provide an effective method for molecular breeding and genetic transformation of barley.
Footnotes
Project supported by the Ministry of Science and Technology of China (No. 2006BAD02B04-3), the Ministry of Agriculture of China (Nos. N20070331-01 and 2008zx08010-003), and the National Natural Science Foundation of China (No. 30630047)
References
- 1.Akula C, Akula A, Henry R. Improved regeneration efficiency from mature embryos of barley cultivars. Biol Plant. 1999;42(4):505–513. doi: 10.1023/A:1002694410575. [DOI] [Google Scholar]
- 2.Becher T, Haberland G, Koop HU. Callus formation and plant regeneration in standard and microexplants from seedlings of barley (Hordeum vulgare L.) Plant Cell Rep. 1992;11(1):39–43. doi: 10.1007/BF00231837. [DOI] [PubMed] [Google Scholar]
- 3.Bi RM, Kou M, Chen LG, Mao SR, Wang HG. Plant regeneration through callus initiation from mature embryo of Triticum . Plant Breeding. 2007;126(1):9–12. doi: 10.1111/j.1439-0523.2007.01327.x. [DOI] [Google Scholar]
- 4.Bregitzer P, Dahleen LS, Campbell RD. Enhancement of plant regeneration from callus of commercial barley cultivars. Plant Cell Rep. 1998;17(12):941–945. doi: 10.1007/s002990050514. [DOI] [PubMed] [Google Scholar]
- 5.Caligari PDS, Powell W, Goodall V. The in vitro genetics of barley (Hordeum vulgare L.): genetical analysis of immature embryo response to 2,4-dichlorophenoxyacetic acid. Heredity. 1987;59(2):285–292. doi: 10.1038/hdy.1987.125. [DOI] [Google Scholar]
- 6.Chauhan H, Desai SA, Khurana P. Comparative analysis of the differential regeneration response of various genotypes of Triticum aestivum, Triticum durum and Triticum dicoccum . Plant Cell Tiss Org Cult. 2007;91(3):191–199. doi: 10.1007/s11240-007-9285-5. [DOI] [Google Scholar]
- 7.Chen JY, Yue RQ, Xu HX, Chen XJ. Study on plant regeneration of wheat mature embryos under endosperm-supported culture. Agric Sci China. 2006;5(8):572–578. doi: 10.1016/S1671-2927(06)60094-1. [DOI] [Google Scholar]
- 8.Cho MJ, Yano H, Okamoto D, Kim HK, Jung HR, Newcomb K, Le VK, Yoo HS, Langham R, Buchanan BB, et al. Stable transformation of rice (Oryza sativa L.) via microprojectile bombardment of highly regenerative, green tissues derived from mature seed. Plant Cell Rep. 2004;22(7):483–489. doi: 10.1007/s00299-003-0713-7. [DOI] [PubMed] [Google Scholar]
- 9.Chu CC. The N6 Medium and Its Applications to Anther Culture of Cereal Crops; Proceedings of the Symposium on Plant Tissue Culture; Beijing, China: Science Press; 1978. pp. 43–50. [Google Scholar]
- 10.Chugh A, Khurana P. Regeneration via somatic embryogenesis from leaf basal segments and genetic transformation of bread and emmer wheat by particle bombardment. Plant Cell Tiss Org Cult. 2003;74(2):151–161. doi: 10.1023/A:1023945610740. [DOI] [Google Scholar]
- 11.Delporte F, Mostade O, Jacquemin JM. Plant regeneration through callus initiation from thin mature embryo fragments of wheat. Plant Cell Tiss Org Cult. 2001;67(1):73–80. doi: 10.1023/A1011697316212. [DOI] [Google Scholar]
- 12.Ellis RP, Forster BP, Robinson D, Handley LL, Gordon DC, Russell JR, Powell W. Wild barley: a source of genes for crop improvement in the 21st century? J Exp Bot. 2000;51(342):9–17. doi: 10.1093/jexbot/51.342.9. [DOI] [PubMed] [Google Scholar]
- 13.Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 14.Ganeshan S, Baga M, Harvey BL, Rossnagel BG, Scoles GJ, Chibbar RN. Production of multiple shoots from thidiazuron-treated mature embryos and leaf-base/apical meristems of barley (Hordeum vulgare L.) Plant Cell Tiss Org Cult. 2003;73(1):57–64. doi: 10.1023/A:1022631807797. [DOI] [Google Scholar]
- 15.Goldman JJ, Hanna WW, Fleming G, Ozias-Akins P. Fertile transgenic pearl millet [Pennisetum glaucum (L.) R. Br.] plants recovered through microprojectile bombardment and phosphinothricin selection of apical meristem-, inflorescence-, and immature embryo-derived embryogenic tissue. Plant Cell Rep. 2003;21(10):999–1009. doi: 10.1007/s00299-003-0615-8. [DOI] [PubMed] [Google Scholar]
- 16.He T, Jia JF. High frequency plant regeneration from mature embryo explants of highland barley (Hordeum vulgare L. var. nudum Hk. f.) under endosperm-supported culture. Plant Cell Tiss Org Cult. 2008;95(2):251–254. doi: 10.1007/s11240-008-9437-2. [DOI] [Google Scholar]
- 17.Holme BI, Brinch-Pedersen H, Lange M, Holm PB. Transformation of barley (Hordeum vulgare L.) by Agrobacterium tumefaciens infection of in vitro cultured ovules. Plant Cell Rep. 2006;25(12):1325–1335. doi: 10.1007/s00299-006-0188-4. [DOI] [PubMed] [Google Scholar]
- 18.Jähne A, Becker D, Brettschneider R, Lörz H. Regeneration of transgenic, microspore-derived, fertile barley. Theor Appl Genet. 1994;89(4):525–533. doi: 10.1007/BF00225390. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy KV, Suhasini K, Sagare AP, Meixner M, de Kathen A, Pickardt T, Schieder O. Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) embryo axes. Plant Cell Rep. 2000;19(3):235–240. doi: 10.1007/s002990050005. [DOI] [PubMed] [Google Scholar]
- 20.Kumlehn J, Serazetdinova L, Hensel G, Becker D, Loerz H. Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefaciens . Plant Biotechnol J. 2006;4(2):251–261. doi: 10.1111/j.1467-7652.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 21.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 22.Özgen M, Birsin MA, Önde S. The effect of hybrid vigor on callus induction and plant regeneration from mature embryo culture of barley (Hordeum vulgare) Plant Cell Tiss Org Cult. 2005;82(1):67–74. doi: 10.1007/s11240-004-6818-z. [DOI] [Google Scholar]
- 23.Rengel Z. Embryogenic callus induction and plant regeneration from cultured Hordeum vulgare mature embryos. Plant Physiol Bilchem. 1987;25:43–48. [Google Scholar]
- 24.Repellin A, Baga M, Jauhar PP, Chibbar RN. Genetic enrichment of cereal crops via alien gene transfer: new challenges. Plant Cell Tiss Org Cult. 2001;64(2/3):159–183. doi: 10.1023/A:1010633510352. [DOI] [Google Scholar]
- 25.Sahrawat AK, Chand S. High frequency plant regeneration from coleoptile tissue of barley (Hordeum vulgare L.) Plant Sci. 2004;167(1):27–34. doi: 10.1016/j.plantsci.2004.02.019. [DOI] [Google Scholar]
- 26.Sharma VK, Hänsch R, Mendel RR, Schulze J. A highly efficient plant regeneration system through multiple shoot differentiation from commercial cultivars of barley (Hordeum vulgare L.) using meristematic shoot segments excised from germinated mature embryos. Plant Cell Rep. 2004;23(1-2):9–16. doi: 10.1007/s00299-004-0800-4. [DOI] [PubMed] [Google Scholar]
- 27.Sharma VK, Hänsch R, Mendel RR, Schulze J. Mature embryo axis-based high frequency somatic embryogenesis and plant regeneration from multiple cultivars of barley (Hordeum vulgare L.) J Exp Bot. 2005;56(417):1913–1922. doi: 10.1093/jxb/eri186. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi M, Enomoto S, Komatsuda T, Nakajima K, Ohyama K. Varietal differences in the ability of callus formation and plant regeneration from mature embryo in barley (Hordeum vulgare L.) Jpn J Breed. 1991;41(4):571–579. [Google Scholar]
- 29.Tingay S, McElroy D, Kalla R, Fieg S, Wang M, Thornton S, Brettell R. Agrobacterium tumefaciens-mediated barley transformation. Plant J. 1997;11(6):1369–1376. doi: 10.1046/j.1365-313X.1997.11061369.x. [DOI] [Google Scholar]
- 30.Wan Y, Lemaux PG. Generation of large numbers of independently transformed fertile barley plants. Plant Physiol. 1994;104:37–48. doi: 10.1104/pp.104.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zapata JM, Sabater B, Martin M. Callus induction and in vitro regeneration from barley mature embryos. Biol Plant. 2004;48(3):473–476. doi: 10.1023/B:BIOP.0000041108.89399.85. [DOI] [Google Scholar]