Abstract
Zinc (Zn) is an essential micronutrient for humans, but Zn deficiency has become serious as equally as iron (Fe) and vitamin A deficiencies nowadays. Selection and breeding of high Zn-density crops is a suitable, cost-effective, and sustainable way to improve human health. However, the mechanism of high Zn density in rice grain is not fully understood, especially how Zn transports from soil to grains. Hydroponics experiments were carried out to compare Zn uptake and distribution in two different Zn-density rice genotypes using stable isotope technique. At seedling stage, IR68144 showed higher 68Zn uptake and transport rate to the shoot for the short-term, but no significant difference was observed in both genotypes for the long-term. Zn in xylem sap of IR68144 was consistently higher, and IR68144 exhibited higher Zn absorption ratio than IR64 at sufficient (2.0 µmol/L) or surplus (8.0 µmol/L) Zn supply level. IR64 and IR68144 showed similar patterns of 68Zn accumulation in new leaves at seedling stage and in developing grains at ripening stage, whereas 68Zn in new leaves and grains of IR68144 was consistently higher. These results suggested that a rapid root-to-shoot translocation and enhanced xylem loading capacity may be the crucial processes for high Zn density in rice grains.
Keywords: Zinc, Stable isotope, High Zn-density rice genotype, Translocation, Remobilization
1. Introduction
Rice (Oryza sativa L.) is a predominant staple food and a major source of dietary carbohydrate for more than half of the world’s population (Zimmermann and Hurrell, 2002). Unfortunately, it is a poor source of essential micronutrients such as iron (Fe), zinc (Zn), and vitamin A. In plants, Zn plays an significant role as integral co-factor of over 300 enzymes which are involved in biosyntheses and turnovers of proteins, nucleic acids, carbohydrates, and lipids. Furthermore, Zn has a critical structural role in many proteins (Marschner, 1995). Recent data showed that nearly 50% of the world’s population is at high risk of Zn deficiency (Welch and Graham, 2004), especially those are depend upon rice for their survival. Zn deficiency is serious as equally as iron (Fe) and vitamin A deficiencies. Zn deficiency causes a lot of health problems in humans, such as impairments of physical development, immune system, and brain function (Cakmak, 2008). Increasing the Zn content in rice grains through breeding, which provided sufficient genetic variation of high Zn-density rice, or through transgenic approaches, emerged as the times require and offered a suitable, cost-effective, and sustainable approach to solve this problem (Cakmak, 2008).
The root-soil interface is the first and most important barrier which affects Zn uptake (Welch and Graham, 2002). To increase Zn uptake by roots, the Zn availability in the rhizosphere must be increased (Welch, 1995), which can be performed by enhanced release rates of root-cell H+, metal chelating compounds and/or reductants, by increasing root absorptive surface area (fine roots and root hairs), and by association with mycorrhizal fungi (Liu et al., 2000; Ryan and Angus, 2003; Gao et al., 2007). However, knowledge on Zn transport in rice plant is scant (Grusak et al., 1999; Rengel, 1999). Zn transport in plants takes place through both the xylem and the phloem. Following absorption by the root, Zn is rapidly transported via the xylem to the shoot (Riceman and Jones, 1958). In rice plant, adequate Zn supply leads to a high proportion of Zn located in the shoots (especially stems), while with toxic level of Zn supply (150 µmol/L), a higher proportion of total Zn may accumulate in the roots (Jiang et al., 2007). Zn appears to be most mobile in all micronutrients and its remobilization is closely related to leaf senescence (Marschner, 1995; Uauy et al., 2006). It was shown that Zn can be transported in phloem of dicotyledonous plants, such as tobacco (Nicotiana glauca) (Hocking, 1980) and grape (Volschenk et al., 1999). In monocotyledonous wheat it also showed good transport of Zn from stem and leaves to developing grains (Pearson et al., 1995; 1996), as well as from older leaves to younger leaves (Page and Feller, 2005), indicating involvement of phloem transport. During grain filling stage, roots and stems are the largest Zn sources for translocation of Zn to the grains. However, grain could also accumulate Zn remobilized from leaves, as has been shown in soybean (Khan and Weaver, 1989) and wheat (Pearson and Rengel, 1995). Although the literature is limit for Zn transport from stems and leaves to rice grains, it is argued that it is different from wheat and barely (Stomph et al., 2009). Jiang et al. (2007) have investigated the uptake and distribution of Zn during rice grain development and suggested that most of the Zn accumulated in the grains originates from uptake by root after flowering and not from Zn remobilization from leaves, both under sufficient or surplus Zn supply. However, previous researches showed that foliar Zn fertilizer still could increase Zn content in rice (Broadley et al., 2007; Wissuwa et al., 2008), which suggested that Zn retranslocation in plant especially in late development stage may be also important for Zn density in rice. So far, manipulation of Zn transport in rice plant and its accumulation mechanism into grains are still unclear.
To resolve this unclearness, Zn stable isotope tracing method was employed to make a clear concept on Zn uptake and distribution in two different Zn-density rice genotypes, and xylem sap was also determined to compare xylem loading capacities of both genotypes. The aims of this present study were: (1) to compare 68Zn uptake capacities by roots of two genotypes differing in grain Zn density; (2) to compare xylem loading capacity of Zn from root to shoot; and, (3) to follow long-distance transport of root-applied 68Zn to new leaves at seedling stage and developing grains at ripening stage.
2. Materials and methods
2.1. Plant culture
Hydroponics experiments were carried out at Huajiachi campus, Zhejiang University, Hangzhou, China. Seeds of rice (Oryza sativa L.) cv. IR68144 (high Zn-density genotype) and IR64 (low Zn-density genotype) were obtained from the International Rice Research Institute, Manila, Philippines. It has been reported that Zn in the unpolished rice grains of IR68144 was about 37.0 mg/kg, whereas much lower Zn (25.5 mg/kg) was recorded in IR64 (Sellappan et al., 2009). Seeds were surface-sterilized by washing with 70% ethanol for 1 min and soaking in 0.01 g/ml sodium hypochlorite for 5 min, rinsed thoroughly in deionized water (resistivity ≥18.2 MΩ∙cm), and imbibed in deionized water for 48 h at 30 °C. Then seeds were germinated in quartz sand washed with 5% (v/v) HCl. For two weeks, only deionized water was supplied. After 14 d when seedlings grew onto two-leaf stage, they were transplanted into 2.5-L black plastic buckets which were covered with a polystyrol plate with seven evenly spaced holes (2 cm in diameter). The composition of nutrient solution was the same as described by Yang et al. (1994): 1.5 mmol/L NH4NO3, 1.0 mmol/L CaCl2, 1.6 mmol/L MgSO4, 1.0 mmol/L K2SO4, 0.3 mmol/L KH2PO4, 0.05 µmol/L H3BO3, 5.0 µmol/L MnSO4, 0.2 µmol/L CuSO4, and 0.05 µmol/L (NH4)6Mo7O24. Fe was supplied as ethylenediaminetetraacetic acid iron sodium (Na2FeEDTA) at 20 µmol/L. The nutrient solution was changed weekly until the plants were eight weeks of age and every 3 d thereafter. The solution pH was adjusted to 5.5±0.1 every other day with NaOH or HCl. Buckets were placed in a growth chamber with a controlled atmospheric temperature (35/25 °C) and photoperiod (16-h light/8-h dark), with 75% relative humidity. Lights were made of fluorescence and incandescence, and intensity was 2000 lx.
For the different experiments described below, 1.0 µmol/L ZnSO4 in nutrient solution was replaced by 1.0 µmol/L 68ZnSO4 for stable isotope tracing during treatments. The nutrient solution containing 1.0 µmol/L 68ZnSO4 was also replaced every 4 d. 68Zn-enriched isotope was purchased as solid powder of 68ZnO from the Cambridge Isotope Laboratory. The isotope abundances were 98.60% 68Zn, 0.44% 64Zn, 0.39% 66Zn, 0.54% 67Zn, and 0.03% 70Zn. The solution of 68ZnSO4 was prepared as follows: 21.0 mg 68ZnO (powder) was dissolved in 5 ml 1.0 mol/L H2SO4, and then gently stirred for 48 h until it was completely dissolved. The solution was then diluted by using deionized water, and solution pH adjusted to 5.0 by adding 1.0 mol/L NaOH. The solution was then transferred to a 250-ml volumetric flask and made up to volume. The final concentration of 68ZnSO4 in the solution was 1.0 mmol/L. The abundances of Zn isotope in non-enriched ZnSO4 were 18.75% 68Zn, 48.63% 64Zn, 27.90% 66Zn, 4.10% 67Zn, and 0.62% 70Zn.
2.2. Time-course of 68Zn uptake by seedling stage rice plants
Rice plants were grown in normal nutrient solution up to 30 d (seedling stage) as described above. Before treatment, plants were cultured in normal nutrient solution except Zn supply for 7 d as a starvation treatment, and each pot had three individual plants as replication which were then transferred into a nutrient solution with 1.0 µmol/L 68Zn supply from 68ZnSO4 (isotopic abundance 98.60%). Different plant samples (new leaves, old leaves, stems, and roots) were collected for determinations of 68Zn abundance and total Zn concentration at various time intervals of 0, 2, 4, 6, and 8 d after transferred to 68ZnSO4 solution. At the onset of each sampling time, a 1.0 ml of nutrient solution was taken from each pot for 68Zn concentration determination. Treatments were replicated three times.
2.3. Xylem sap collection and analysis
Plants of both genotypes (IR68144 and IR64) were grown hydroponically for ten weeks, and used for xylem sap collection. Plants of both genotypes were placed in the uptake solutions (2 mmol/L MES-Tris, 0.5 mmol/L CaCl2; pH=5.8) with different Zn treatments including 0.5, 2.0, and 8.0 µmol/L Zn. Xylem sap collection procedure was according to Lu et al. (2009). Twelve plants from each treatment were de-topped using sharp blades at ~5.0 cm above the roots. Immediately after de-topping, each stem was rinsed with deionized water and blotted with absorbent paper to remove contaminants from cut cells. After discarding ~0.3 ml of sap, each cut surface was blotted again and 10-cm silicon tubing was fitted over the stem. Sap flowing from the tubing was collected in sterile vials at time-points of 4, 8, 12, and 24 h after treatment. At the onset of each xylem sap collection, a 1.0 ml aliquot of the uptake solution was taken from each pot for Zn determination. For xylem sap samples, a subsample of 0.5 ml was mixed with 4.5 ml of 2% (v/v) nitric acid. Zn concentrations in all samples were determined by inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500a, USA).
2.4. Kinetics of 68Zn transport to developing grains
Plants were pre-cultured in normal nutrient solution until anthesis stage, and thereafter the procedure was analogous as in the preceding experiments: plants were transferred to nutrient solution with 1.0 µmol/L 68Zn supply from 68ZnSO4 (isotopic abundance being 98.60%). Whole rice grains were collected for determinations of 68Zn abundances and total Zn concentrations at various time intervals of 0, 5, 10, 15, 20, and 25 d after transfer to the treated solution. Other elements (Fe, P, etc.) in rice grains were also determined by ICP-MS (Agilent 7500a, USA). At the onset of each sampling time, a 1.0 ml of nutrient solution was taken from each pot for 68Zn concentration determination. Each treatment was replicated three times.
2.5. Sample preparation and digestion
After harvest, plant parts were separated and washed in running deionized water to remove superficial nutrient solution. Roots were submerged in a 1-1 bath containing 1 mmol/L LaCl3+0.05 mmol/L CaCl2 for 10 min to remove Zn bounded in apoplast (Rengel, 1999).
Samples were oven-dried and milled by using MM301 (Retsch, Germany) with agate ball and internal wall. For digestion, 0.1 g (accuracy 0.1 mg) samples were mixed with 4 ml nitric acid (HNO3, reagent grade) and 1 ml hydrogen peroxide (H2O2, analytical reagent; Beijing Chemical Works, China). Digestion was performed by using a hot block system (LabTech ED36, Germany). All samples were digested and analyzed in triplicates.
2.6. Determination of 68Zn tracer concentration in rice plants by ICP-MS
Total Zn concentration (c Zn,t, mg/kd dry weight (DW)) and final ratio of 68Zn/66Zn (R fin) in rice plants were obtained directly by analysis of ICP-atomic emission spectroscopy (AES) (SHIMADZU ICPS-7510) and ICP-MS (Agilent 7500a), respectively. The ultimate goal of total Zn and Zn isotopic analyses was to determine the concentration of the newly-accumulated Zn into various parts of the rice plants during the tracing periods. Concentrations of the newly-accumulated Zn in different plant parts (c Zn,a, mg/kg DW) were calculated from total Zn concentrations post exposure in the respective plant parts, the final 68Zn/66Zn ratio in the respective parts, and the relative fractions of 68Zn and 66Zn in both the exposure media (f 68-enr and f 66-enr) and in the respective parts of control rice plants (f 68-nat and f 66-nat), according to Eq. (1) (Todd et al., 2009; Wolf et al., 2009):
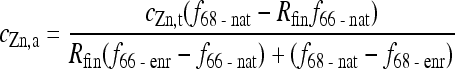 , ,
|
(1) |
where f 68-nat and f 66-nat are the natural abundances of 68Zn and 66Zn in normal nutrient solution (18.75% and 27.90%, respectively); f 68-enr and f 66-enr represent the abundance of 68Zn and 66Zn in 68Zn-enriched ZnSO4 bought from the Cambridge Isotope Laboratory (98.60% and 0.39%, respectively). Please note that all data of 68Zn in the tables and figures of this paper refer to c Zn,a only. They do not include the 68Zn accumulated with ‘normal Zn’ (c Zn,t−c Zn,a).
2.7. Statistical analysis
All data were statistically analyzed using SPSS Version 12.0. Differences between treatments were determined by the least significant difference (P<0.05) from the analysis of variance (ANOVA). Differences between two genotypes were tested by a paired t-test (P<0.05 or P<0.01). The figures were generated using the software SigmaPlot 10.0.
3. Results
3.1. 68Zn absorption and transportation by root at seedling stage
Under adequate 68Zn supply level, the high Zn-density genotype (IR68144) had a higher shoot dry matter yield, but a lower root dry matter yield, as compared with those in the low Zn-density genotype (IR64), and therefore, the root/shoot ratio of the former was significantly (P<0.01) lower regardless of the sampling time (Table 1). In the advanced growth stage, root/shoot ratios of both genotypes decreased due to higher shoot growth rate (Table 1).
Table 1.
Dry weights (DWs) of roots and shoots of high (IR68144) and low (IR64) Zn-density rice genotypes during supplying 68ZnSO4 for 8 d at seedling stage
| Time (d) | Shoot DW (g/plant) |
Root DW (g/plant) |
Root/shoot ratio |
|||
| IR68144 | IR64 | IR68144 | IR64 | IR68144 | IR64 | |
| 2 | 1.10* | 0.84 | 0.28 | 0.33* | 0.25 | 0.39* |
| 4 | 1.33* | 1.14 | 0.29 | 0.34* | 0.21 | 0.30* |
| 6 | 1.62* | 1.34 | 0.33 | 0.39* | 0.20 | 0.29* |
| 8 | 1.78* | 1.50 | 0.35 | 0.43* | 0.19 | 0.29* |
Plants were grown in normal nutrient solution up to 30 d (seedling stage), and then transferred into nutrient solution with 1.0 µmol/L 68Zn. Data represent a mean of three plants
Statistical significance at P<0.01 between the two genotypes
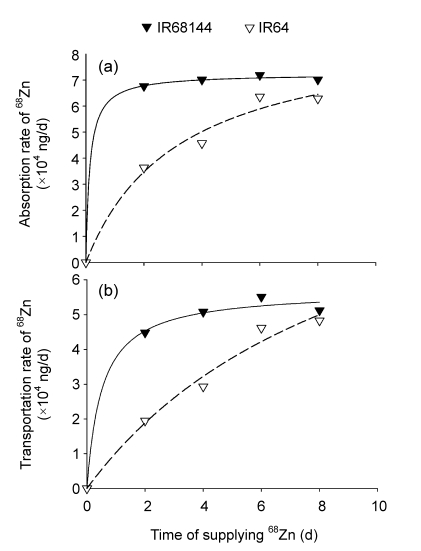
After treatments with 68ZnSO4, the concentrations of 68Zn in shoot parts (new leaves, older leaves, and stems) of the high Zn-density genotype (IR68144) were higher than those of the low Zn-density genotype (IR64), but lower in root parts (Table 2). The concentrations of 68Zn in new leaves and stems of both genotypes increased by the time of supplying 68Zn, while in older leaves it was continuously decreased (Table 2). In root, the concentration of 68Zn was almost stable, with exception of slight increase in IR64 and decrease in IR68144 after 2 d. The total amount of 68Zn taken up by each plant increased with time, whereas higher uptake rate was observed in IR68144 (Fig. 1a). In a short time (first 2 d), more 68Zn was absorbed in rice plant and translocated to the shoot in the high Zn-density genotype (IR68144), and became stable at 8 d (Fig. 1).
Table 2.
Concentration of 68Zn in the dried tissues of two rice genotypes during supplying 68ZnSO4 for 8 d at seedling stage
| Sampling time (d) | Concentration of 68Zn (mg/kg) |
|||||||
| IR64 |
IR68144 |
|||||||
| Old leaves | New leaves | Stem | Root | Old leaves | New leaves | Stem | Root | |
| 2 | 0.63a | 0.32a | 13.00a | 16.48a | 2.25a | 0.98a | 18.44a | 15.33a |
| 4 | 0.42b | 2.35b | 15.18b | 16.74a | 1.75b | 2.71b | 19.81a | 15.24a |
| 6 | 0.25c | 5.58c | 22.17c | 18.54b | 1.54b | 7.00c | 19.55a | 14.68b |
| 8 | 0.13d | 9.85d | 31.03d | 19.10b | 1.33c | 11.69d | 15.26b | 14.41b |
Plants were grown in normal nutrient solution up to 30 d (seedling stage), then transferred into nutrient solution with 1.0 µmol/L 68ZnSO4. Data represent a mean of three plants. Different superscript letters at the same column indicate statistical significance at P<0.05
Fig. 1.
Time-dependent kinetics of 68Zn absorption and transportation of two rice genotypes after supplying 68ZnSO4
(a) Total amount of 68Zn uptake by whole plant; (b) Total amount of 68Zn translocated to shoot. Rice plants were grown in normal nutrient solution up to 30 d (seedling stage), and then transferred into nutrient solution with 1.0 µmol/L 68Zn supply from 68ZnSO4. Samples were collected for determination of 68Zn abundance and total Zn concentration at various time intervals of 0, 2, 4, 6, and 8d after transfer
3.2. Kinetics of 68Zn accumulation in new leaves and grains
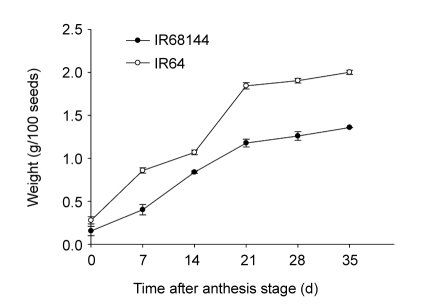
The concentrations of 68Zn in new leaves and grains of both rice genotypes increased rapidly by the time 68ZnSO4 was supplied (Fig. 2), indicating that 68Zn in the nutrient solution was absorbed and accumulated into the new leaves and grains. The characteristics of 68Zn accumulation in both genotypes were quite similar (Fig. 2). By comparison, 68Zn concentration in IR68144 was higher than in IR64 (Fig. 2). In the developing grains, 68Zn concentration reached the highest level at 15d and then decreased; this is different from the new leaves and may be due to the growing complexion of rice grains. The 15th day after anthesis stage showed the greatest rate of grain filling, and large amounts of carbohydrate were transported into grains, increasing the biomass quite quickly (Fig. 3). Afterwards, 68Zn concentration decreased due to dilution effects (Fig. 2).
Fig. 2.
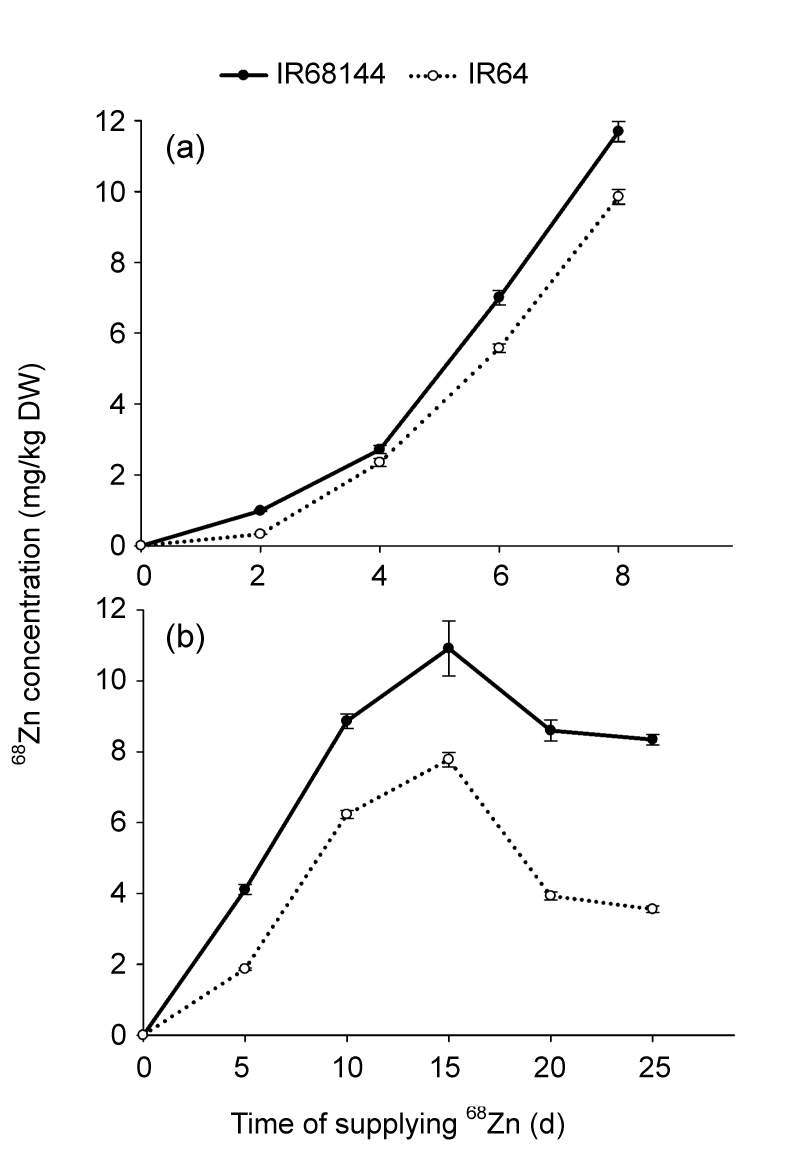
Effect of 68ZnSO4 treatment on the concentration of isotope tracer 68Zn in new leaves and developing grains of two rice genotypes after supplying 68ZnSO4
(a) New leaves were collected at various time intervals of 0, 2, 4, 6, and 8 d; (b) Grains were collected at various time intervals of 0, 5, 10, 15, 20, and 25d after transfer. Rice plants were grown in normal nutrient solution for 30 d (seedling stage) and 100d (anthesis stage), respectively, and then transferred into nutrient solution with 1.0 µmol/L 68Zn supply from 68ZnSO4. Data represent mean±SE of three replicates
Fig. 3.
Growth weight of grains in two rice genotypes under normal hydroponic condition
Rice plants were grown in normal nutrient solution for 100 d (anthesis stage), and then rice grains were collected at various time intervals of 0, 7, 14, 21, 28, and 35d after that. Weight of 100 seeds per individual plants was determined. Data represent mean±SE of five replicates
Besides, Table 3 shows that transportation of 68Zn into rice grains continues up to maturity. The accumulation of 68Zn in grains of both genotypes increased rapidly with time of supplying 68Zn; however, before 15d the accumulation of 68Zn in IR64 was higher than that of IR68144 because of the earlier grain filling stage of IR64 (Table 3). After 15d, 68Zn accumulation of IR68144 was higher than that of IR64. The amount of Zn remobilized from vegetative tissues was increased even at late grain filling stage, with a great portion of total Zn allocated in grains, which was much higher than the level of Zn directly absorbed by the root after the anthesis stage.
Table 3.
Accumulation of 68Zn into rice grains of each individual plant at various time when 68ZnSO4 supply was initiated at anthesis stage
| Genotype | Time (d) | 68Zna (µg/plant) | Znb (µg/plant) | Total Znc (µg/plant) | 68Zn content (%) |
| IR64 | 0 | 0 | 10.95±0.32 | 10.95 | 0 |
| 5 | 0.77±0.02 | 31.05±0.59 | 31.82 | 2.41 | |
| 10 | 10.81±0.19 | 46.32±0.73 | 57.13 | 18.92 | |
| 15 | 14.86±0.41 | 59.29±0.91 | 74.15 | 20.01 | |
| 20 | 15.62±0.29 | 74.81±1.03 | 90.43 | 17.27 | |
| 25 | 17.74±0.48 | 75.39±0.89 | 93.13 | 19.05 | |
| IR68144 | 0 | 0 | 10.68±0.47 | 10.68 | 0 |
| 5 | 0.55±0.02 | 32.67±0.53 | 33.22 | 1.66 | |
| 10 | 5.74±0.14 | 54.09±0.87 | 59.83 | 9.60 | |
| 15 | 19.88±1.42 | 66.21±0.71 | 86.09 | 23.09 | |
| 20 | 22.73±0.77 | 76.50±1.32 | 99.23 | 22.90 | |
| 25 | 27.07±0.48 | 79.43±1.03 | 106.50 | 25.42 | |
Plants were grown in normal nutrient solution for 100 d (anthesis stage), and then transferred into nutrient solution with 1.0 µmol/L 68Zn. Data represent mean±SE for three replications
68Zn refers to Zn absorbed from nutrient solution after anthesis stage
Zn refers to Zn remobilized from vegetative tissues
Total Zn means the sum of both
3.3. Zn transport in xylem
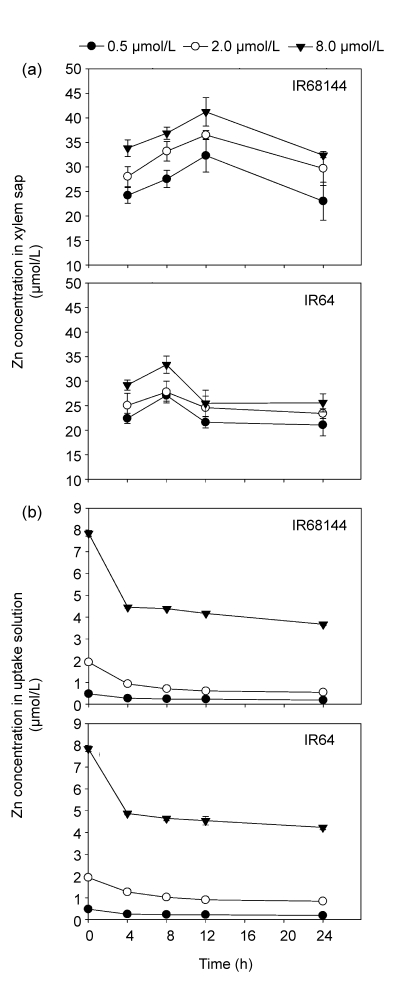
Time-dependent variation of Zn concentration in xylem sap of the plants and the uptake solution is shown in Fig. 4 for both IR68144 and IR64. Zn concentrations in the xylem sap of both genotypes increased with increasing Zn level in the uptake solution at each time point (Fig. 4a), but showed different rate patterns. For high Zn-density genotype (IR68144), Zn concentration increased rapidly up to 12 h and then decreased, whereas for IR64, it increased to 8 h and then decreased. Regardless of treatments, Zn concentration in the xylem sap of IR68144 was consistently higher than that of IR64, especially with 8.0 µmol/L Zn supply level (Fig. 4a). In contrast to Zn concentration in xylem sap, Zn concentration in the root of IR64 was much higher than that of IR68144 (data not shown). Zinc concentration in the uptake solution decreased gradually, especially during first 4 h (Fig. 4b). Zn concentration was consistently higher in the xylem sap of both rice genotypes than in the uptake solution (Fig. 4). At the Zn-deficient level (0.5 µmol/L Zn supply), Zn in the external solution deceased by 50% within 4 h, and no significant difference was observed for the two genotypes (Fig. 4b), but the decreasing ratio of Zn in solution was approximately 50% for IR68144, but less than 40% for IR64, at sufficient (2.0 µmol/L) or surplus (8.0 µmol/L) Zn supply. After 24 h, IR68144 also had higher Zn absorption ratio than IR64 at sufficient (2.0 µmol/L) or surplus (8.0 µmol/L) Zn supply, showing lower Zn concentration in uptake solution (Fig. 4b).
Fig. 4.
Time-course Zn concentrations in the xylem sap (a) and uptake solution (b) of IR68144 and IR64 with treatments of 0.5, 2.0 and 8.0 µmol/L Zn
Twelve plants in the same pot were treated as one replicate. Data represent mean±SE of three replicates
4. Discussion
The physiological basis of Zn transport in rice plants and the controlling process of Zn accumulation in edible portion of seeds are not understood with any certainty (Welch and Graham, 1999; Graham et al., 2001). The first and most important barrier of rice plants to accumulate more Zn in edible tissues resides at the root-soil interface (i.e., the rhizosphere) for Zn absorption (Welch, 1995). In this study, 68ZnSO4 was used as the source of Zn in hydroponics experiments. The results show that more 68Zn was absorbed in rice plants and translocated to the shoot by roots of high Zn-density rice genotype (IR68144) in early 2 d (Fig. 1), with higher 68Zn concentration in new leaves (Table 2), when compared with those of IR64. Fibrous root system of IR68144 had more root tips and total surface area than that of IR64 (Table 4), so 68Zn could be absorbed more quickly from nutrient solution in the short-term.
Table 4.
Root morphologies of two rice genotypes under normal hydroponics condition
| Genotype | Total length (cm/plant) | Total surface area (cm2/plant) | Total volume (cm3/plant) | Length of root with diameter <0.4 mm (cm) | Number of root tips (tip/plant) |
| IR64 | 478.39±51.71Aa | 56.11±12.18Bb | 0.53±0.17Aa | 313.88±10.56Aa | 2 046.11±356.36Bb |
| IR68144 | 559.32±62.46Aa | 65.71±18.23Aa | 0.58±0.22Aa | 355.91±25.41Aa | 3 707.67±339.64Aa |
Plants were grown in normal nutrient solution for 30 d (seedling stage). Data represent mean±SE of six independent replicates. Different upper and lower superscript letters indicate statistical significance at P<0.01 and P<0.05, respectively, between the two rice genotypes
Similar results were also obtained in barley and rice (Genc et al., 2007; Chen et al., 2009). Genc et al. (2007) showed that the greater Zn uptake and better growth in Pallas (wild-type barley) compared with brb (root-hairless mutant) in Zn-deficient soil, which could be attributed primarily to greater root surface area due to root hairs in Pallas, rather than other root morphological differences. Interestingly, 68Zn concentration in stems of IR68144 decreased after 6 d when 68Zn was deficient in the solution; however, it was not seen in IR64 (Table 2). This suggested that 68Zn deposited in stems of IR68144 might be retranslocated into new growing tissues under Zn-deficient condition.
The efficiency of root-to-shoot translocation is theoretically dependent on four processes (Lasat et al., 1996; Palmgren et al., 2008): (1) Zn sequestration in the root; (2) efficiency of the radial symplastic passage; (3) xylem loading capacity; and, (4) Zn movement efficiency in the xylem vessels. It has been suggested that decreased root cell sequestration may facilitate enhancing Zn root-to-shoot translocation in the hyperaccumulators (Yang et al., 2006); however, very limited literature was available on Zn sequestration in the root of rice. Root uptake of divalent cations typically exhibits two phases: apoplastic binding and symplastic uptake (Hart et al., 1998; Zhao et al., 2002). Passive (apoplastic) uptake involves diffusion of ions in the soil solution into the root endodermis along a chemical potential (concentration) gradient, while active ion uptake occurs against the concentration gradient with high selectivity of ions and energy-consuming mechanism (Marschner, 1995). In this study, a higher Zn concentration in xylem sap was observed compared to the uptake solution, and still increased within 4–8 h while Zn concentration in the uptake solution was decreasing (Fig. 4). We believe that root to shoot translocation of Zn in both rice genotypes is through symplastic passage. Additionally, Zn concentration in the xylem sap of IR68144 was consistently higher than that of IR64, especially with 8.0 µmol/L Zn supply level (Fig. 4a), but lower Zn concentration in root in contrast to IR64 (Table 2), showing enhanced transport capacity of Zn to shoot. Thus, we suggest that efficient transport of Zn into root symplasm and efflux into xylem vessels may play an important role in Zn accumulation into grains.
Results of the present study show similar patterns of 68Zn accumulation into new leaves at seedling stage and into developing grains at ripening stage by root supply (Fig. 2). The concentrations of 68Zn in new leaves and grains of IR68144 were higher than those of IR64 at the same developing stage. It is hypothesis that rice plants with high Zn concentration in new leaves at seedling stage will have high Zn density in grains, but it still needs further investigation. If so, it will become more convenient for the plant breeder to choose high Zn-density rice genotypes. After anthesis stage, both of the rice genotypes continued to uptake the root-supplied Zn with high accumulation occurring in the rice grains (Table 3). This was consistent with the findings of cotton (Constable et al., 1988), red spring wheat (Miller et al., 1994), and aerobic rice (Jiang et al., 2007). Of the total plant Zn, 50% (cotton), 10% (wheat), or 20% (rice) was taken up between anthesis and maturity. Here, in the high Zn-density genotype (IR68144) it was 25%, higher than that in the low Zn-density genotype IR64 (20%), indicating that IR68144 had a more efficient transport system for Zn bypass from root to grain. It was reported that Zn was a mobile micronutrient and could remobilize from vegetative tissues (such as stems, roots, and senescence leaves) into grains, which was confirmed in wheat (Pearson et al., 1995; 1996). However, in rice, there is little evidence to show the capacity of phloem remobilization of Zn from vegetative tissues to grains. A large proportion of Zn in the grains was remobilized from vegetative tissues during grain developing stage (Table 3), which amounts to three to four times greater than that directly absorbed by roots after anthesis stage in IR68144 and IR64. Therefore, we suggest that the phloem remobilization capacity could be an important factor which is responsible for high Zn density in rice grains.
In the cereal grains, Zn is preferentially stored together with phytate, which is a strong chelator of divalent cations and significantly reduces mineral bioavailability (Bohn et al., 2008). Phytate is the primary storage form of phosphate and inositol in cereal seeds (Bouis, 2000). It accumulates rapidly during seed development and can account for up to several percent of the seed DW (Lott, 1984). In this study, P accumulation in the grains of IR64 was higher than that of IR68144 during the grain filling stage, which was consistent with the result of Liu et al. (2004). The content of Fe in developing grains was also analyzed (Fig. 5b) and had the same tendency as Zn accumulation in grains. Hao et al. (2005) found that Zn and Fe were distributed through the whole transverse section of IR68144 grains, whereas the elements were not detected in the center of IR64 grains. Although there was no significant difference between the protein contents of polished rice (approximately 10%) with the two genotypes (data not shown), the grains of IR68144 had significantly higher methionine content and lower tyrosine content than those of IR64 (P<0.01). Those results suggest that high Zn density in rice grains is also related with the chemical compositions of grains, such as P and other elements.
Fig. 5.
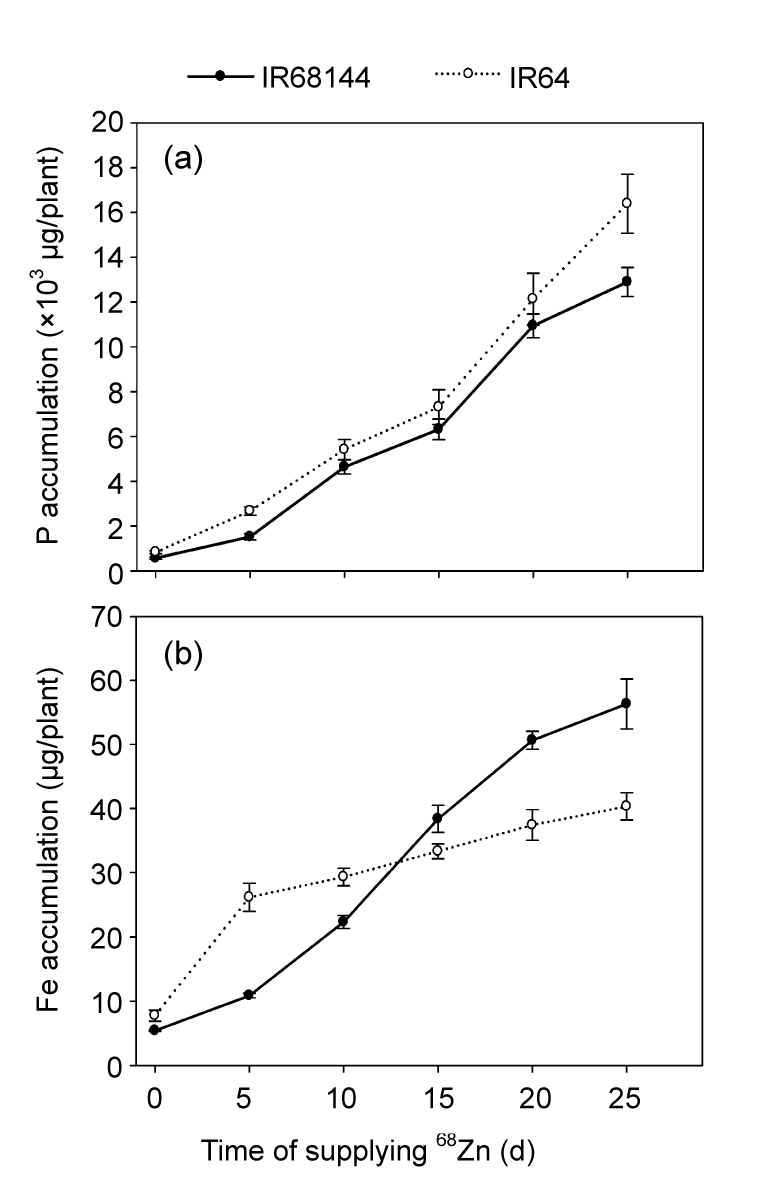
Accumulations of P (a) and Fe (b) during the rice grain developments of two rice genotypes
Rice plants were grown in normal nutrient solution for 100 d (anthesis stage), and then rice grains were collected at various time intervals of 0, 5, 10, 15, 20, and 25 d after that. Accumulations of P and Fe were determined by ICP-MS. Data represent mean±SE of three replicates
Our findings suggest that for Zn density in rice grains, the mobility of Zn within the plants seems to be more important than the root uptake ability. In the low Zn-density genotype, a great amount of Zn absorbed by the root was deposited in the roots and stems, and less was remobilized into developing grains compared to high Zn-density genotype. Efforts on promoting this portion of Zn to grains could be the key point for enhancing Zn density in seeds. Root-shoot distribution of Zn has been suggested to be controlled mainly by heavy metal transporting ATPases (Hussain et al., 2004), which were thought to transport Zn across the plasma membrane of root vascular cells into the xylem for transport to the shoot. Increasing the expression of HMA4 or a closely related gene is therefore likely to enhance the rates of Zn translocation from root to shoot for biofortification purposes (Hanikenne et al., 2008). The yellow stripe-like transporter (YSL) proteins transport metal-nicotianamine complexes and have mainly been studied with respect to Fe homeostasis (Koike et al., 2004; Schaaf et al., 2005). However, YSL transporters could also have roles in Zn mobilization. Waters and Grusak (2008) found that these proteins were required for efficient mobilization of Zn from sentence leaves and fruit hulls into seeds. However, little literature was available on the effect of these transporters in rice, and further investigation is necessary to understand the mechanisms of manipulating Zn transport in rice plants.
Footnotes
Project supported by the HarvestPlus-China Program (No. HPC-8234), the Key International Cooperative Project (No. 2006DFA31030), and the Department of Education of Zhejiang Province (No. N20100339)
References
- 1.Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ-Sci B. 2008;9(3):165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouis HE. Special issue on improving human nutrition through agriculture. Food Nutr Bull. 2000;21(4):351–576. [Google Scholar]
- 3.Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173(4):677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 4.Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil. 2008;302(1-2):1–17. doi: 10.1007/s11104-007-9466-3. [DOI] [Google Scholar]
- 5.Chen WR, He ZL, Yang XE, Feng Y. Zinc efficiency is correlated with root morphology, ultrastructure, and antioxidative enzymes in rice. J Plant Nutr. 2009;32(2):287–305. doi: 10.1080/01904160802608627. [DOI] [Google Scholar]
- 6.Constable GA, Rochester IJ, Cook JB. Zinc, copper, iron, manganese and boron uptake by cotton on cracking clay soils of high pH. Aust J Exp Agric. 1988;28(3):351–356. doi: 10.1071/EA9880351. [DOI] [Google Scholar]
- 7.Gao XP, Thomas W, Kuyper E, Zou CQ, Zhang FS, Hoffland E. Mycorrhizal responsiveness of aerobic rice genotypes is negatively correlated with their zinc uptake when nonmycorrhizal. Plant Soil. 2007;290(1-2):283–291. doi: 10.1007/s11104-006-9160-x. [DOI] [Google Scholar]
- 8.Genc Y, Huang CY, Langridge P. A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J Exp Bot. 2007;58(11):2775–2784. doi: 10.1093/jxb/erm142. [DOI] [PubMed] [Google Scholar]
- 9.Graham RD, Welch RM, Bouis HE. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv Agron. 2001;70:77–142. doi: 10.1016/S0065-2113(01)70004-1. [DOI] [Google Scholar]
- 10.Grusak MA, Pearson JN, Marentes E. The physiology of micronutrient homeostasis in field crops. Field Crops Res. 1999;60(1-2):41–56. doi: 10.1016/S0378-4290(98)00132-4. [DOI] [Google Scholar]
- 11.Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Kramer U. Evolution of metal hyperaccumulation required cis-regulatory changes and copy number expansion of HMA4 . Nature. 2008;453(7193):391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- 12.Hao HL, Feng Y, Huang YY, Tian SK, Lu LL, Yang XE, Wei YZ. Situ analysis of cellular distribution of iron and zinc in rice grains using SRXRF method. High Energy Phys Nucl. 2005;29:56–60. (in Chinese) [Google Scholar]
- 13.Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV. Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol. 1998;116(4):1413–1420. doi: 10.1104/pp.116.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hocking PJ. The composition of phloem exudate and xylem sap from tree tobacco (Nicotiana glauca Grah.) Ann Bot. 1980;45(6):633–643. [Google Scholar]
- 15.Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, Young J, Camakaris J, Harper JF, Cobbett CS. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis . Plant Cell. 2004;16(5):1327–1339. doi: 10.1105/tpc.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Struik PC, Lingna J, van Keulen H, Zhao M, Stomph TJ. Uptake and distribution of root-applied or foliar applied 65Zn after flowering in aerobic rice. Ann Appl Biol. 2007;150(3):383–391. doi: 10.1111/j.1744-7348.2007.00138.x. [DOI] [Google Scholar]
- 17.Khan A, Weaver CM. Pattern of zinc-65 incorporation into soybean seeds by root absorption, stem injection, and foliar application. J Agric Food Chem. 1989;37(4):855–860. doi: 10.1021/jf00088a005. [DOI] [Google Scholar]
- 18.Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004;39(3):415–424. doi: 10.1111/j.1365-313X.2004.02146.x. [DOI] [PubMed] [Google Scholar]
- 19.Lasat MM, Baker AJM, Kochian LV. Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and non-accumulator species of Thlaspi . Plant Physiol. 1996;112(4):1715–1722. doi: 10.1104/pp.112.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu A, Hamel C, Hamilton RI, Ma BL. Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza. 2000;9(6):331–336. doi: 10.1007/s005720050277. [DOI] [Google Scholar]
- 21.Liu JC, Ockenden I, Truax M, Lott JNA. Phytic acid-phosphorus and other nutritionally important mineral nutrient elements in grains of wild-type and low phytic acid (lpa1–1) rice. Seed Sci Res. 2004;14(2):109–116. doi: 10.1079/SSR2004160. [DOI] [Google Scholar]
- 22.Lott JNA. Accumulation of Seed Reserves of Phosphorus and Other Minerals. In: Murray DR, editor. Seed Physiology. I. New York: Academic Press; 1984. pp. 139–166. [Google Scholar]
- 23.Lu LL, Tian SK, Yang XE, Li TQ, He ZL. Cadmium uptake and xylem loading are active processes in the hyper-accumulator Sedum alfredii . J Plant Physiol. 2009;166(6):579–587. doi: 10.1016/j.jplph.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Marschner H. Mineral Nutrition of Higher Plants. San Diego, California, USA: Academic Press; 1995. pp. 325–329. [Google Scholar]
- 25.Miller RO, Jacobsen JS, Skogley EO. Aerial accumulation and partitioning of nutrients by hard red spring wheat. Commun Soil Sci Plant Anal. 1994;25(11):1891–1911. doi: 10.1080/00103629409369162. [DOI] [Google Scholar]
- 26.Page V, Feller U. Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann Bot. 2005;96(3):425–434. doi: 10.1093/aob/mci189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmgren MG, Clemens S, Williams LE, Kraemer U, Borg S, Schjorring JK, Sanders D. Zinc biofortification of cereals: problems and solutions. Trends Plant Sci. 2008;13(9):464–473. doi: 10.1016/j.tplants.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Pearson JN, Rengel Z. Uptake and distribution of 65Zn and 54Mn in wheat grown at sufficient and deficient levels of Zn and Mn II. During grains development. J Exp Bot. 1995;46(7):841–845. doi: 10.1093/jxb/46.7.841. [DOI] [Google Scholar]
- 29.Pearson JN, Rengel Z, Jenner CF, Graham RD. Transport of zinc and manganese to developing wheat grains. Physiol Plant. 1995;95(3):449–455. doi: 10.1111/j.1399-3054.1995.tb00862.x. [DOI] [Google Scholar]
- 30.Pearson JN, Rengel Z, Jenner CF, Graham RD. Manipulation of xylem transport affects Zn and Mn transport into developing wheat grains of cultured ears. Physiol Plant. 1996;98(2):229–234. doi: 10.1034/j.1399-3054.1996.980202.x. [DOI] [Google Scholar]
- 31.Rengel Z. Physiological responses of wheat genotypes grown in chelator-buffered nutrient solutions with increasing concentrations of excess HEDTA. Plant Soil. 1999;215(2):193–202. doi: 10.1023/A:1004595112969. [DOI] [Google Scholar]
- 32.Riceman DS, Jones GB. Distribution of zinc in subterranean clover (Trifolium subterraneum L.) grown to maturity in a culture solution containing zinc labeled with the radioactive isotope 65Zn. Aust J Agric Res. 1958;9(6):730–744. doi: 10.1071/AR9580730. [DOI] [Google Scholar]
- 33.Ryan MH, Angus JF. Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: increased Zn-uptake but no increase in P-uptake or yield. Plant Soil. 2003;250(2):225–239. doi: 10.1023/A:1022839930134. [DOI] [Google Scholar]
- 34.Schaaf G, Schikora A, Haberle J, Vert G, Ludewig U, Briat JF, Curie C, von Wiren N. A putative function for the Arabidopsis Fe phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol. 2005;46(5):762–774. doi: 10.1093/pcp/pci081. [DOI] [PubMed] [Google Scholar]
- 35.Sellappan K, Datta K, Parkhi V, Datta SK. Rice caryopsis structure in relation to distribution of micronutrients (iron, zinc, β-carotene) of rice cultivars including transgenic indica rice. Plant Sci. 2009;177(6):557–562. doi: 10.1016/j.plantsci.2009.07.004. [DOI] [Google Scholar]
- 36.Stomph TJ, Jiang W, Struik PC. Zinc biofortification of cereals: rice differs from wheat and barley. Trends Plant Sci. 2009;14(3):123–124. doi: 10.1016/j.tplants.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Todd AS, Brinkman S, Wolf RE, Lamothe PJ, Smith KS, Ranville JF. An enriched stable-isotope approach to determine the gill-zinc binding properties of juvenile rainbow trout (oncorhynchus mykiss) during acute zinc exposures in hard and soft waters. Environ Toxicol Chem. 2009;28(6):1233–1243. doi: 10.1897/08-252.1. [DOI] [PubMed] [Google Scholar]
- 38.Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. A NAC gene regulating senescence improves grains protein, zinc and iron content in wheat. Science. 2006;314(5803):1298–1301. doi: 10.1126/science.1133649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volschenk CG, Hunter JJ, le Roux DJ, Watts JE. Effect of graft combination and position of application on assimilation and translocation of zinc in grapevines. J Plant Nutr. 1999;22(1):115–119. doi: 10.1080/01904169909365611. [DOI] [Google Scholar]
- 40.Waters BM, Grusak MA. Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3 . New Phytol. 2008;177(2):389–405. doi: 10.1111/j.1469-8137.2007.02288.x. [DOI] [PubMed] [Google Scholar]
- 41.Welch RM. Micronutrient nutrition of plants. Crit Rev Plant Sci. 1995;14(1):49–82. doi: 10.1080/713608066. [DOI] [Google Scholar]
- 42.Welch RM, Graham RD. A new paradigm for world agriculture: meeting human needs—productive, sustainable, nutritious. Field Crops Res. 1999;60(1-2):1–10. doi: 10.1016/S0378-4290(98)00129-4. [DOI] [Google Scholar]
- 43.Welch RM, Graham RD. Breeding crops for enhanced micronutrient content. Plant Soil. 2002;245(1):205–214. doi: 10.1023/A:1020668100330. [DOI] [Google Scholar]
- 44.Welch RM, Graham RD. Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot. 2004;55(396):353–364. doi: 10.1093/jxb/erh064. [DOI] [PubMed] [Google Scholar]
- 45.Wissuwa M, Ismail AM, Graham RD. Rice grains zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization. Plant Soil. 2008;306(1-2):37–48. doi: 10.1007/s11104-007-9368-4. [DOI] [Google Scholar]
- 46.Wolf RE, Todd AS, Brinkman S, Lamothe PJ, Smith KS, Ranville JF. Measurement of total Zn and Zn isotope ratios by quadrupole ICP-MS for evaluation of Zn uptake in gills of brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) Talanta. 2009;80(2):676–684. doi: 10.1016/j.talanta.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 47.Yang XE, Römheld V, Marschner H, Chaney RL. Application of chelator-buffered nutrient solution technique on zinc nutrition study in rice. Plant Soil. 1994;163(1):85–94. doi: 10.1007/BF00033944. [DOI] [Google Scholar]
- 48.Yang XE, Li TQ, Yang JC, He ZL, Lu LL, Meng FH. Zinc compartmentation in root, transport into xylem, and adsorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta. 2006;224(1):185–195. doi: 10.1007/s00425-005-0194-8. [DOI] [PubMed] [Google Scholar]
- 49.Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP. Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens . J Exp Bot. 2002;53(368):535–543. doi: 10.1093/jexbot/53.368.535. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann MB, Hurrell RF. Improving iron, zinc and vitamin A nutrition through plant biotechnology. Curr Opin Biotech. 2002;13(2):142–145. doi: 10.1016/S0958-1669(02)00304-X. [DOI] [PubMed] [Google Scholar]