Abstract
Breast tumour stem cells have been reported to differentiate in the epithelial lineage but a cross-lineage potential has not been investigated. We aimed to evaluate whether breast tumour stem cells were able to differentiate also into the endothelial lineage. We isolated and cloned a population of breast tumour stem cells, cultured as mammospheres that expressed the stem markers nestin and Oct-4 and not epithelial and endothelial differentiation markers, and formed serially transplantable tumours in SCID mice. When cultured in the presence of serum, mammosphere-derived clones differentiated in the epithelial lineage. When cultured in the presence of VEGF, the same clones were also able to differentiate in the endothelial lineage acquiring endothelial markers and properties, such as the ability to organize in Matrigel into capillary-like structures. In the transplanted tumours, originated from mammospheres, we demonstrate that some of the intratumour vessels were of human origin, suggesting an in vivo endothelial differentiation of mammosphere-derived cells. Finally, endothelial cell clones originated from mammospheres were able, when implanted in Matrigel in SCID mice, to form after 7 days a human vessel network and, after 3–4 weeks, an epithelial tumour suggesting that in the endothelial-differentiated cells a tumourigenic stem cell population is maintained. In conclusion, the results of the present study demonstrate that stem cells of breast cancer have the ability to differentiate not only in epithelial but also in endothelial lineage, further supporting the hypothesis that the tumour-initiating population possesses stem cell characteristics relevant for tumour growth and vascularization.
Keywords: mammospheres, tumour-derived stem cells, angiogenesis, breast carcinoma
Introduction
Emerging evidence indicates that the capacity of a tumour to grow and propagate resides in a small population of tumour cells, termed cancer stem cells or tumour-initiating cells [1]. Tumour cancer stem cells have been identified in several solid tumours, and although the specific markers may differ from one tumour to another, they share the ability to growth in non-differentiating conditions and to self-renew, to differentiate in tumour cell types and to generate serially transplantable tumours [2, 3]. In breast carcinoma, the tumour-initiating cells have been identified by Al-Hajj et al.[4] as a rare population of CD44+CD24−low/epithelial specific antigen+ cells. The ability of breast tumour progenitor/stem cells to grow in non-adherent structures called mammospheres led to the possibility to isolate, expand in culture and characterize this population [5]. Long-term human mammosphere cultures were shown to be composed by undifferentiated, self-renewing tumour cells, which could differentiate into both epithelial (luminal and ductal) and myoepithelial cell types, expressing markers of the mature mammary gland epithelium, in the presence of serum and of an adhesion substrate [6, 7]. Therefore, the known differentiation potential of breast cancer stem cells is toward the breast glandular epithelial lineages. However, it is at present unknown whether breast cancer stem cells may exhibit cross-lineage differentiation capabilities, and in particular whether they can also differentiate into endothelial cells.
In breast cancer, there are several reports showing the presence of dysfunctional and disorganized vessels with a defective endothelial monolayer [8]. In addition, the possibility that tumour cells themselves organize in microvascular channels, so-called ‘vasculogenic mimicry’[9], has been extensively described in inflammatory breast cancer [10]. In the present study, we aimed to evaluate whether breast tumour stem cells may also give rise to a progeny of endothelial differentiated cells, acquiring endothelial markers in vitro and whether they participate to tumour vascular-ization in vivo.
For this purpose, we generated tumour-initiating breast stem cell clones and we evaluated whether each individual clone was able to differentiate into both epithelial and endothelial cells. We therefore evaluated the endothelial differentiation of breast cancer stem cells in vivo and their involvement in tumour angiogenesis. Finally, we studied the ability of the endothelial clones to generate the vascular and the epithelial component of tumours in severe combined immunodeficiency (SCID) mice.
Material and methods
Isolation and in vitro expansion of progenitor cells from breast tumour specimens
Tumour specimens were obtained from a consenting patient according to the Ethics Commitee of the S. Giovanni Battista Hospital of Torino, Italy. The histologic assessment showed a lobular-infiltrating carcinoma of the pleomorphic type expressing oestrogen receptor in about 60% of cells. Tumour specimen was finely minced with scissors and then digested by incubation for 1 h at 37°C in DMEM containing collagenase II (Sigma Chemical Company, St. Louis, MO, USA). After washings in medium plus 10% FCS (GIBCO, Grand Island, NY, USA), the cell suspension was forced through a graded series of meshes to separate the cell components from stroma and aggregates. After filtration through a 40-μm pore filter (Becton Dickinson, San Jose, CA, USA), single cells were plated at 1000 cells/ml in serum-free DMEM-F12 (Cambrex BioScience, Venviers, Belgium), supplemented with 10 ng/ml basic fibroblast growth factor (bFGF), 20 ng/ml epidermal growth factor (EGF), 5 (μg/ml insulin and 0.4% bovine serum albumin (all from Sigma), as described in [6]. After 7 days, the appearance of non-adherent spherical clusters of cells, i.e. mammospheres, was observed. Mammospheres were then collected on the bottom of a conical tube by spontaneous precipitation (20 min. at room temperature), in order to remove non-living cells. Subsequently after 2–3 days, mammospheres were collected by gentle centrifugation (800 rpm) and disaggregated through enzymatic and mechanical dissociation using trypsin and pipetting, respectively. Recovered cells were expanded at 1000 cells/ml in the serum-free medium described above and the process was repeated every 7 days.
Clonal sphere formation assay
Primary mammospheres were dissociated as described above and 100 cells were plated in a 96-well culture plate to obtain a single cell/well in 200 (μl of growth medium; 25 μl of medium per well were added every 5 days. The number of clonal mammospheres for each 96-well culture plate was evaluated after 14 days of culture. This procedure was repeated for the tertiary spheres.
In vitro cell differentiation
To evaluate the differentiative ability of cells in the mammospheres, mammospheres clones (n= 13) obtained from primary mammospheres were expanded, dissociated and grown in the differentiative epithelial and endothelial media. Differentiation was also tested for secondary or tertiary single cell-derived mammospheres obtained as described above. Epithelial differentiation was obtained plating the single cell suspension from disaggregated mammospheres in the presence of RPMI plus 10% FCS, without the addition of growth factors. Endothelial differentiation was obtained culturing the cells in EBM medium (Cambrex Bio Science) with VEGF (10 ng/ml) (Sigma) and 10% FCS on Endothelial Cell Attachment Factor (Sigma) [11].
Endothelial cell clones were obtained placing single cells from dissociated mammospheres in 96-well plates in endothelial medium, and the generated clones expanded thereafter.
Capillary-like structure
In vitro formation of tubular structures was studied on growth factor reduced Matrigel (Becton Dickinson). Cells (4 × 104 cells/well) were seeded onto Matrigel-coated wells (let to gelify at 37°C for 1 hr) in RPMI containing 0.25% BSA. Cells were periodically observed with a Nikon inverted microscope and experimental results recorded. Image analysis was performed with the MicroImage analysis system (Cast Imaging srl, Venice, Italy), as described in [12].
In vivo angiogenic and tumourigenic potential of mammosphere-derived cells
Cells derived from CD24−/CD44+ mammosphere clones or from CD24+/CD44+ differentiated epithelial cells or from endothelial cells derived from mammosphere clones (n= 3 for each condition) were collected and were implanted subcutaneously into SCID mice (Charles River, Jackson Laboratories, Bar Harbor, ME, USA) within growth-factor depleted Matrigel basement membrane (from 100 to 1 × 105 cells). Cells were harvested using trypsin-EDTA, washed with PBS, counted in a microcytometer chamber and resuspended in 150 μl DMEM. Cells were chilled on ice, added to 150 μl of Matrigel at 4°C and injected subcutaneously into the left back of SCID mice via a 26-gauge needle using a 1-ml syringe. After 3–4 weeks, mice were sacrificed and tumours recovered and processed for histology. For serial transplant experiments, tumours were digested in Matrigel digesting solution (Becton Dickinson) and collagenase II and the recovered cells processed to culture in mammosphere conditions. Mammospheres were disaggregated and cells injected to evaluate second tumour generation. The process was repeated for tertiary tumour generation.
To evaluate angiogenesis, endothelial differentiated clones were implanted subcutaneously into SCID mice within growth-factor depleted Matrigel (1×104 cells) and Matrigel plugs recovered after 7–30 days.
Tumour microvessel density was assessed by counting intratumoural human and mouse CD31-positive vessels in ×20 magnification fields. Fifty microscopic fields were analysed for each experimental condition.
Immunofluorescence
Cytofluorimetric analysis was performed using the following antibodies, all FITC or PE conjugated: anti-CD44 mAb (Sigma), anti-CD24 mAb (PharMingen, BD Biosciences, Franklin Lakes, NJ, USA), anti-CD31, anti-CD146/Muc-18 and anti-CD105 mAbs (Dako, Copenhagen, Denmark), anti-VEGF receptor1, anti-KDR and anti-VEGF receptor 3 mAbs (R&D System, Minneapolis, MN, USA). Isotype-matched FITC or PE-conjugated control mouse IgG were from Dako. Cells were incubated for 30 min. at 4°C with the appropriate Ab or with the irrelevant control in PBS containing 2% heat-inactivated human serum. For staining of the cytoplasmic antigens, single cells were fixed in 3.5% paraformaldehyde contained 2% of sucrose at 4°C for 10 min. and then permeabilized with HEPES Triton X-100 Buffer 0.1% for 10 min. at 4°C and incubated with anti-cytokeratin (CK)14, anti-CK18 or anti-panCK Abs (Santa Cruz Biotecnology, Santa Cruz, CA, USA). Where needed a second step reagent, cells were stained by the addition of conjugated polyclonal rabbit anti-goat immunoglobulins\FITC (Dako) or polyclonal goat anti-rabbit immunoglobulins\FITC (Sigma) and incubated for further 30 min. at 4°C. Cells were analysed on a FACScan (Becton Dickinson). In total, 10,000 cells were analysed in each experimental point.
For confocal microscopy analysis, indirect immunofluorescence was performed on mammosphere-derived cells on cytospin preparation of a single cell suspension using the non-enzymatic cell solution (Sigma). For cells grown in adhesion, indirect immunofluorescence was performed on cells cultured on chamber slides (Nunc, Rochester, NY, USA). Cells were fixed in 3.5% paraformaldehyde containing 2% sucrose and, when needed, permeabilized with Hepes-Triton X-100 Buffer [13]. The following antibodies were used: rabbit anti-von Willebrand Factor (vWF) Ab and anti-α-smooth muscle actin (α-SMA) mAb (Dako), rabbit anti-pan-CK Ab, goat anti-CK14, rabbit anti-CK18 Abs (Santa Cruz), anti-vimentin mAb (Sigma, clone 13.2), goat anti-Oct-4 Ab (Abcam, Cambridge, UK) and rabbit anti-nestin Ab (Chemicon, Temecula, CA, USA). Sections from cryostatic or paraffin-embedded sample of tumours recovered from SCID mice were stained for rabbit anti-human Class I antigen Ab (BioLegend, San Diego, CA, USA), goat anti-mouse (β2-microglobulin Ab (Santa Cruz Biotechnology), and anti-human CD31 mAb (Dako, clone JC70A) or rat anti-mouse CD31 mAb (Abcam). For CD31 staining, 0.1 trypsin treatment (15 min. at 37°C) was performed for antigen retrieval. Alexa Fluor 488 or Texas Red-conjugated anti-rabbit, anti-goat, anti-rat and anti-mouse IgG (all from Molecular Probes, Leiden, The Netherlands) were used as secondary antibodies. Confocal microscopy analysis was performed using a Zeiss LSM 5 Pascal model confocal microscope (Carl Zeiss Int. Oberkochen, Germany). Hoechst 33258 dye (Sigma) was added for nuclear staining.
Combined fluorescence in situ hybridization (FISH) and immunofluorescence analysis
A combined immunofluorescence and FISH staining was performed to detect CD31 expression on human chromosome 17 positive cells [13]. FISH was performed using the Vysion kit for the detection of human chromosome 17 (Vysis Inc., Downers Grove, IL, USA). SG CEP 17 DNA probe hybridizes to the centromere (band region 17p11.1-q11.1, locus D17Z1) of human chromosome 17. Previous experiments showed negative reaction of mouse tissue [14]. Hybridization was performed on 5-μm sections according to the manufacturer's guidelines. In brief, the sections were deparaffinized, dehydrated in 100% ethanol and dried at 45–50°C for 2–5 min. Slides were then subjected to protease digestion for 10–20 min. at 38°C, denatured (72°C for 5 min.) and hybridized (37°C) with prewarmed probes (CEP17 Spectrum Green; Vysis Inc.) overnight (16–18 hrs) in HYBrite hybridization system (Vysis Inc.). After hybridization, the rubber cement was removed and the slides were immersed in 2× SSC for 5–10 min. at room temperature. At this point, slides were processed for immunofluorescence detection of CD31 protein. Slides were incubated for 60 min. at room temperature with the anti-human CD31 mAb (Dako, clone JC70A). After a brief wash in Tris buffer, immunodetection was performed using a Texas Red anti-mouse IgG (Molecular Probes) for 60 min. at room temperature, washed three times in Tris buffer for 5 min. each, and rinsed briefly in Aqua bidest. Nuclei were counterstained with Hoechst 33258 dye (Sigma), and then slides were observed with a confocal microscope.
Immunohistochemistry
Sections from paraffin-embedded blocks of human tumours obtained in SCID mice were collected onto poly-Llysine-coated slides and stained using the following antibodies: rabbit anti-pan-CK, anti-mouse β2-microglobulin, or rabbit anti-HLA class I Ab (all from Santa Cruz Biotechnology); mouse anti-CK AE1/AE3 mAb (clone AE1-AE3-PCK26), and anti-oestrogen receptor mAb (clone 6F11) (both from Ventana Medical Systems S.A., Illkirch CEDEX, France), anti-epithelial membrane antigen (EMA) mAb (clone E29), anti-vimentin mAb (clone R9), polyclonal anti-cErb2 Ab and polyclonal anti-epithelial growth factor receptor (EGFR) (all from Dako). Endogenous peroxidase activity was blocked with 6% H2O2 for 8 min. at room temperature. Primary antibodies were applied to slides overnight or for 1 hr at 4°C. Horse radish peroxidase-labeled anti-rabbit or anti-mouse Envision polymers (Dako) were incubated for 1 hr 30 min. The reaction product was developed using 3,3-diaminobenzidine. Omission of the primary Ab or substitution with an unrelated rabbit serum or mouse IgG served as negative control.
Electron microscopy
Transmission electron microscopy was performed on Karnowsky's fixed osmium tetraoxide post-fixed tissues, embedded in epoxy resin according to standard procedures [15]. Ultra-thin sections were stained with uranyl acetate and lead citrate and were examined with a Jeol JEM 1010 electron microscope.
Results
Isolation and characterization of breast cancer stem cells
Breast cancer stem cells were generated using the protocol described by Ponti et al. [6] from a lobular infiltrating breast carcinoma. Mammospheres were obtained by culturing enzimatically dissociated single-cell suspension at 1000 cells/ml in serum-free medium supplemented with EGF, bFGF and insulin (Fig. 1A). Primary mammospheres, enzymatically digested after 10 days and re-plated as single cell suspension, generated second passage mammospheres. Cells were serially passaged using this procedure and propagated in culture for >50 passages. Moreover, the mammospheres were cloned by limiting dilution of dissociated cells, by plating one single cell per well into 96-well culture plates. Clonal, non-adherent secondary mammospheres formed, which in turn gave rise to tertiary mammosphere clones (Fig. 1B), indicating the presence of self-renewing cells. The mammosphere clones (n= 24) were maintained in culture for >3 months.
1.
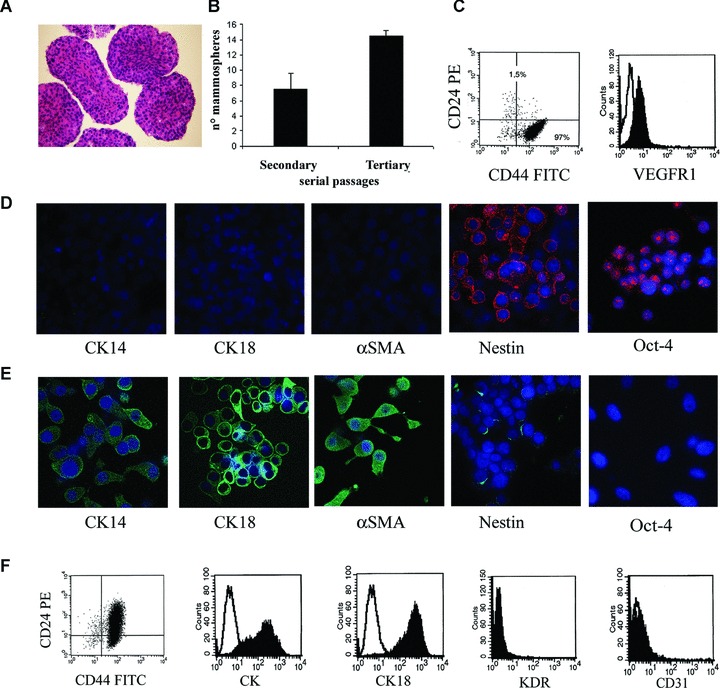
Mammosphere generation, characterization and epithelial differentiation. Mammospheres were obtained by culture of dissociated cells from a human breast tumour in mammosphere medium containing EGF and bFGF. (A) Representative micrograph showing the ematoxilin and eosin staining of paraffin-embedded, formalin-fixed mammospheres. (B) Mammosphere clone formation analysis in secondary and tertiary passages. The number of mammosphere clones/100 cells generated from single cells increased in the third passage. (C) Representative FACS analysis of mammosphere cell clones showing the expression of CD44 and not CD24 and of VEGF receptor 1 (VEGFR1; the dark area indicates the specific Ab, the white area the isotypic control). (D) Representative immunofluorescence expression by mammosphere cell clones of the stem cell markers Oct-4 and nestin, but not of the differentiation markers CK14, CK18 and α-SMA. (E) Serum supplementation and withdrawal of growth factors induced the expression of lineage specific markers of the mature mammary epithelium (CK14, CK18 and α-SMA) in cultured cells from mammosphere clones, with loss of the stem cell markers Oct-4 and nestin. (F) Representative FACS analysis of epithelial differentiated cells showing the acquirement of CD24, the expression of pan-CK and CK18 and the absence of the endothelial cell markers KDR and CD31. The dark area indicates the specific Ab, the white area the isotypic control. Original magnification: panel A: ×100; panel D and E: ×650. Nuclei were counterstained with Hoechst dye.
The cells of the mammospheres were CD44+/CD24− and showed absence of differentiation markers of the cell types of the glandular epithelium as they did not express CK14, CK18 or α-SMA. Moreover, breast tumour progenitor cells expressed the stem cell markers nestin and Oct-4, indicating that they are comparable to other breast tumour progenitor cells described in the literature [4–6] (Fig. 1C and D). Moreover, the cells expressed the VEGF receptor 1 (Fig. 1C). This phenotype was maintained in all clones.
In vitro epithelial and endothelial differentiation of mammosphere-derived CD44+/CD24− clones
Thirteen mammosphere-derived CD44+/CD24− clones were studied for epithelial and endothelial differentiation. When cultured in the presence of serum without growth factor supplementation, cells from dissociated mammosphere clones grew in adherent conditions to the plastic and acquired markers associated to myoepithelial cells (CK14 and α-SMA) and luminal/ductal cells (CK18) as well as the epithelial differentiation marker CD24, lacking in undifferentiated cells. The observed epithelial differentiation (96 ± 5.4% of cells acquiring pan-CK) was associated with a reduction of the stem cell markers nestin and Oct-4 expression (Fig. 1E and F). The cells were negative for the endothelial markers VEGF receptor 2 (KDR) and CD31 (Fig. 1F).
The same mammosphere clones, used for the epithelial differentiation, were plated in parallel in endothelial differentiating medium (EBM with 10 ng/ml VEGF and 10% FCS) to evaluate their possible differentiation toward an endothelial phenotype. Cells from mammosphere clones acquired an endothelial phenotype after 2 weeks of culture (85 ± 3.0% of cells acquiring CD31). Cells acquired expression of endothelial markers such as vascular endothelial-cadherin, vWF, KDR, CD146, VEGF receptor 3, CD31 and CD105, but did not acquire CK18 (Fig. 2A) and CK14 (not shown). All these endothelial markers were negative in undifferentiated cells of mammospheres. No such a differentiative ability was obtained using cells cultured in serum and previously differentiated in epithelial-myoepithelial cells after 2 weeks of culture. Endothelial differentiated cells also acquired the ability to organize into capillary-like structures when plated onto Matrigel (Fig. 2B). This ability was not shared by undifferentiated breast cancer progenitors (Fig. 2B) or by cells differentiated into an epithelial phenotype (not shown).
2.
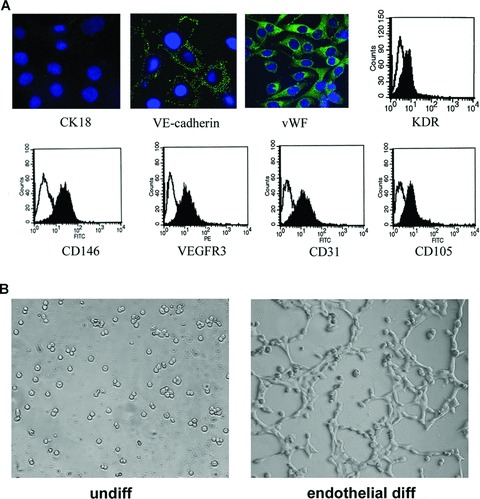
Endothelial differentiation of mammosphere clones cultured in endothelial differentiating medium containing VEGF. (A) Representative micrographs showing the expression of endothelial markers after 2-week culture by immunofluorescence staining of vascular endothelial (VE)-cadherin and vWF, and representative FACS analysis (KDR, CD105, CD31, CD146, VEGF receptor 3 (VEGFR3): black area; the white area is the isotypic control) showing the endothelial differentiation. CK18 was negative. (B) Representative micrographs showing the formation of capillary-like structures on Matrigel by endothelial-differentiated breast tumour stem cells, but not by undifferentiated breast tumour progenitor cells. Capillary-like formation assay was evaluated after 6 hrs. (Original magnification: panel A ×650; panel B ×200).
Moreover, the percentage of differentiation into epithelial and endothelial cells did not significantly vary from primary to single cell-derived secondary and tertiary mammospheres (Fig. 3).
3.
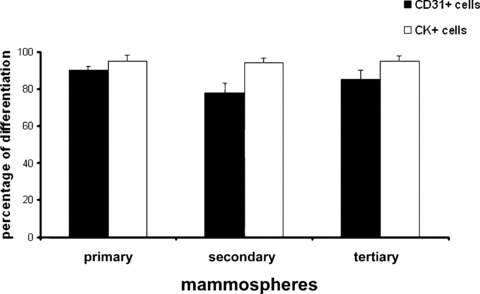
Endothelial and epithelial differentiation of mammospheres and derived secondary and tertiary clones. The percentage of cells differentiated into endothelial or epithelial phenotype was evaluated by the cytofluorimetric expression of CD31 (black columns) or pan-CK (white columns), respectively. Data are the mean +/−S.D. of 4 experiments.
In addition, to avoid the possible presence of epithelial differentiated/committed cells residing into mammosphere clones contaminating the endothelial lineages, we generated endothelial clones from mammosphere-derived single cells cultured in endothelial differentiating medium. The clonogenic ability was 6 ± 1.2%. Nine clones were characterized and were shown to express all the endothelial markers studied. CD31 was expressed in 87 ± 5.1% of the cells whereas about 10% of the cells remained undifferentiated. When endothelial clones were cultured in epithelial medium (RPMI + 10%FCS), reversal of the vascular phenotype into an epithelial phenotype was obtained after culture for more than 2 months (pan-CK positive cells: 96 ± 3.4%, CD31 positive cells: 0%), suggesting that this reversal is not dependent on a switch between epithelial and vascular phenotype but rather on the expansion of remaining undifferentiated stem cells.
In vivo tumourigenesis and vasculogenesis of breast stem cells
In vivo, undifferentiated CD44+/CD24− and epithelial differentiated CD44+/CD24+ cells derived from mammospheres were implanted subcutaneously in the left and right abdominal side of the same SCID mouse, to comparatively evaluate their tumouri-genic potential. As shown in Table 1, as low as 100 CD44+/CD24− breast cancer progenitor cells were able to generate in 4 weeks an aggressive epithelial tumour, resembling the tumour of origin (Table 1, Fig. 4). In contrast, 100 epithelial differentiated CD44+/CD24+ cells were unable to form tumours. For serial tumour generation, breast cancer SC were obtained by dissociation of tumours developed in SCID mice and by culturing the cells in mammosphere conditions. Mammospheres were then dissociated and re-injected for three serial tumour passages (n= 6 tumours each passage with 100% incidence). By immunohisto-chemistry, the tumours expressed low molecular weight CKs (AE1/AE3), EMA and vimentin (Fig. 5A). In contrast, tumours were negative for CK5/6, EGFR, c-Erb2 (Fig. 5A) and oestrogen receptor. The human origin of the tumours was shown by the expression of the HLA class I antigen, and not the mouse beta2 microglobulin (Fig. 6A and B). We analysed whether vessels developed within the tumour may derive from the transplanted breast tumour stem cells. By FISH analysis, we found that a small percentage of cells lining the tumour capillaries (hCD31 positive) expressed the human chromosome 17 (Fig. 6C). The surrounding murine tissue was negative for the human chromosome 17. Moreover, we found that the majority of vessels detected around the tumours were of murine origin as they expressed the mouse β2 microglobulin, whereas several of the intratumour vessels were of human origin, as detected by HLA class I and human CD31 expression (vessel density: hCD31/HLA+: 1.32 ± 0.26; mCD31/β2+: 4.6 ± 0.5) (Fig. 5B). These results suggest that at least some of the intratumour vessels derived from the implanted clones of breast tumour stem cells.
1.
Tumour-initiating ability of breast stem cells
| 102 cells | 104 cells | 105 cells | ||||
|---|---|---|---|---|---|---|
| 1st pass | 2nd pass | 3rd pass | ||||
| CD24−/CD44+ stem cells | 5/5 | 6/6 | 6/6 | 6/6 | 6/6 | |
| CD24+/CD44+ epithelial differentiated cells | 0/5 | ND | ND | 4/6 | 6/6 | |
| Endothelial differentiated cells | 0/5 | ND | ND | 4/5 | 6/6 | |
| Endothelial differentiated cell clones | 0/8 | ND | ND | 8/8 | ND | |
Tumours were generated in SCID mice by subcutaneous injection of cells derived from CD24−/CD44+ mammosphere clones or from CD24+/CD44+ differentiated epithelial cells or from endothelial cells derived from mammosphere clones (n= 3 different lines for each condition), at different cell numbers. After 4 weeks, mice were sacrificed and tumours recovered and processed for histology. For serial transplant experiments, 102 cells from mammospheres obtained from primary tumours were re-injected to evaluate second tumour generation and the same procedure was applied for third tumour generation. Moreover, endothelial differentiated clones (n= 3 different clones) were implanted subcutaneously into SCID mice within growth-factor depleted Matrigel (1 × 104 cells) and tumours recovered after 4 weeks. ND = not done.
4.
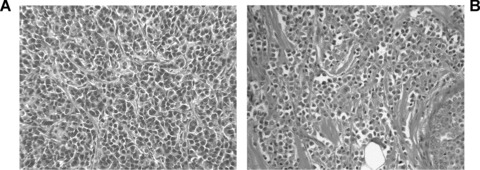
Morphological appearance of tumours derived from mammospheres. Representative micrographs showing the morphological appearance of a tumour originated from 102 cells from mammospheres (A), resembling the original lobular breast carcinoma (B). Original magnification: ×100.
5.
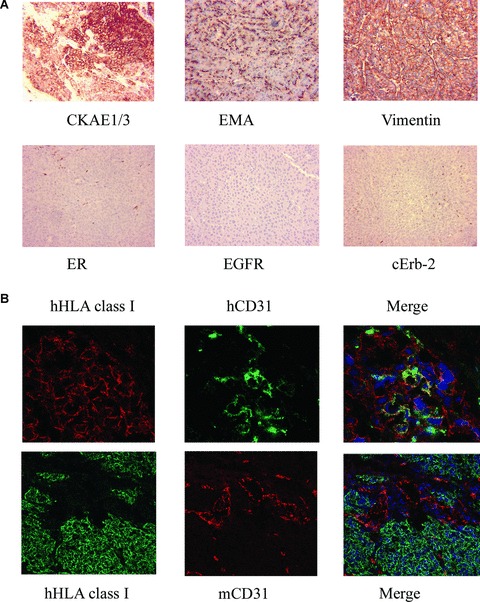
Breast tumour stem cells formed tumours in SCID mice and contributed to tumour vessel formation. (A) Representative micrographs of tumour sections showing positivity for low-weight cytokeratins AE1/AE3 (CKAE1/3), EMA and vimentin, but not for CK5/6ER, EGF-R and c-Erb2. (Original magnifications: ×100). (B) Representative immunofluorescence micrographs showing co-localization of human HLA class I and human CD31 (hCD31) in vessels within the mammosphere-generated tumours, as seen by confocal microscopy (Original magnifications: ×400). In contrast, the co-localization of human HLA and mouse CD31 (mCD31) in several vessels around and within the implanted tumour was absent (Original magnifications: ×250). Data are representative of six experiments with similar results.
6.
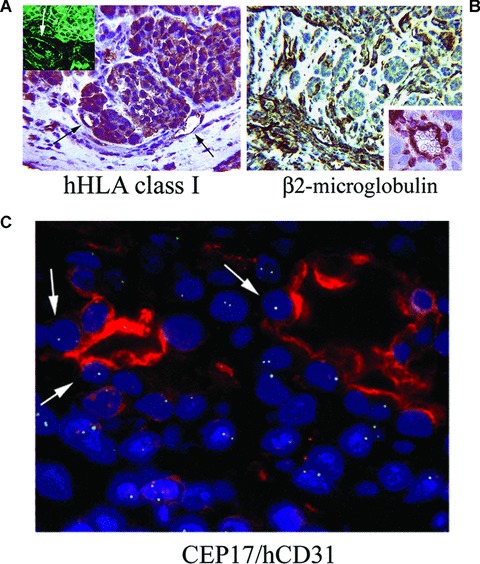
Origin of vessels present in breast tumour stem cells formed tumours. (A) Immunohistochemistry for human HLA class I antigen showed the positivity of human endothelial cells within tumour (arrows) and of tumour cells. In the inset, human HLA class I antigen is detected by immunofluorescence (the arrow indicates a positive vessel). (B) Immunohistochemistry for mouse β2 microglobulin showed the presence of murine vessels and isolated cells at the periphery of the tumour (inset). (Original magnifications: ×250). (C) FISH analysis for chromosome 17 followed by immunofluorescence analysis for human CD31 Ab showed the co-expression by endothelial cells of the human chromosome 17 (arrows). (Original magnification: ×650). Data are representative of six experiments with similar results.
In vivo tumourigenesis and vasculogenesis of endothelial clones
We tested whether endothelial differentiated cells derived from mammospheres (n= 3) maintained the ability to generate tumours when injected subcutaneously in Matrigel in SCID mice, and whether they also participated to tumour vasculogenesis. In addition, to dispel the presence of contaminating differentiated cells, we also generated and tested clones from single endothelial differentiated cells (n= 3 clones). As shown in Table 1, the number of cells required for tumour generation was 104–105. After 7 days, endothelial differentiated cells from mammosphere clones gave rise to angiogenesis in Matrigel characterized by development of a network of HLA class I positive human vessels connected with the mouse vasculature (Fig. 7A and B). At this time, only few tumour clusters were seen. After 3 weeks, the epithelial component expanded, generating tumours (Fig. 7C), expressing CK, vimentin and EMA (not shown), thus resembling the tumour originated by mammospheres.
7.
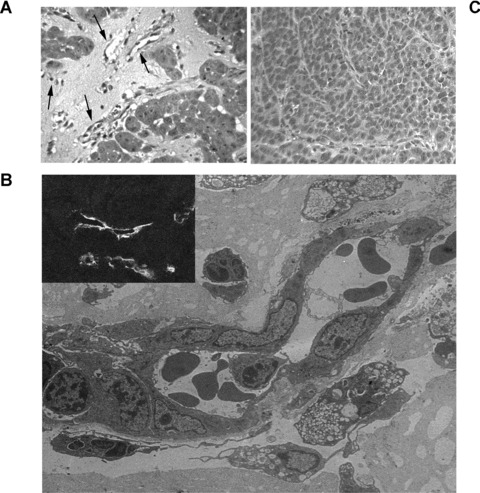
Vessels and tumour formation by endothelial differentiated tumour stem cell clones subcutaneously injected in SCID mice. (A) Representative micrograph showing the organization of endothelial differentiated breast tumour stem cells injected subcutaneously in Matrigel after 7 days. Several vessels connected with the mouse vasculature and containing erythrocytes were observed (arrows). (B) Representative micrograph of electron microscopy of vessels formed within Matrigel by endothelial differentiated breast tumour progenitor cells injected subcutaneously in Matrigel after 7 days. The inset shows the immunofluorescence staining of vessels for human HLA Class I. (C) Representative micrograph showing the formation of an epithelial tumour by the endothelial differentiated breast tumour progenitor cells injected subcutaneously in Matrigel after 3 weeks. (Original magnifications: Panel A and C ×250; panel B ×6000; inset, ×400). Data are representative of eight different tumors.
The ability of endothelial differentiated clones to generate epithelial tumours in vivo may suggest that in the endothelial differentiated breast cancer stem cells a non-differentiated population is maintained and it is able to differentiate into tumour epithelial cells in vivo.
Discussion
In the present study, we demonstrate that stem/progenitor cells of breast carcinomas cultured as mammospheres could differentiate not only in epithelial cells but also into endothelial cells both in vitro and in vivo.
A tumour-initiating population able to sustain and maintain the tumour has been identified in several organs [1]. The ability to growth in suspension, as floating spheres has been described as a culture system that allows the propagation of normal and tumour mammary stem cells in an undifferentiated state [16].
In the present paper, we used a population of breast tumour stem cells, cultured as mammospheres. Breast tumour stem cells expressed the stem markers nestin and Oct-4 and were negative for epithelial and endothelial differentiation markers. Mammospheres could be cultured in vitro for more then 50 passages and showed enhanced tumourigenesis in respect to differentiated cells. Clonal mammospheres were obtained by plating one single cell raising secondary mammosphere clones. Secondary mammospheres were submitted to subsequent cloning, generating long-term tertiary mammosphere clones. These results show the self-renewal and the tumour-initiating ability of this cell population comparable to that described by Ponti et al.[6].
Tumour-initiating cells or ‘cancer stem cells’ are characterized by their ability to display stem/progenitor cell properties: competence for self-renewal and capacity to differentiate in a heterogeneous tumour cell population [1, 2]. In addition, if the notion that tumour stem cells originate from mutated stem cells of the tissue is true, it is conceivable that tumour stem cells may differentiate in different lineages. This has been shown for melanoma-derived stem cells that are able to differentiate in multiple mesenchymal lineages such as adypocitic, osteocytic and chondrocytic lineages [17]. Breast tumour stem cells have been reported to differentiate in the epithelial linage but a cross-lineage potential has not been investigated.
In the present study, we demonstrated that breast tumour stem cells are able to differentiate also into endothelial cells. Undifferentiated breast tumour stem cells expressed the VEGF receptor 1, but not VEGF receptor 2 or 3, suggesting that VEGF-induced endothelial differentiation may depend, at least in the first phase, on the engagement of this receptor known to trigger endothelial differentiation of other stem cell types [18]. This was shown by the acquirement of endothelial markers and properties, such as the ability to organize in Matrigel into capillary-like structures, by differentiated cells. Several studies have suggested the possibility that tumour cells express endothelial markers. In human tissue specimens of ductal in situ carcinomas, the expression of CD31 has been shown in the epithelial cells [19]. Other studies supported the idea that melanoma cells may acquire functional behaviours that are similar to those of endothelial cells [20]. Aggressive melanoma cells that form blood-carrying channels in tumours also exhibited capillary-like structure formation and co-expressed epithelial and some endothelial-like markers [9, 21, 22].
In the present study, the endothelial differentiation from stem cells was not associated with the co-expression of epithelial markers. Moreover, stem cells once differentiated into epithelial cells did not acquire endothelial markers when cultured in the endothe-lial differentiation medium. These results suggest a differentiation rather then a process of ‘vasculogenic mimicry’. Evidence of an in vivo differentiation of stem cells into endothelial cells is provided by the observation that at least a fraction of the vessels present in the transplanted tumours, originated from mammospheres, were of human origin.
The concept that at least some of the vessels present in tumours may derive from the tumour stem cells indicate the contribution of an intratumour vasculogenesis to the tumour vascularization. Several reports indicate that endothelial cells within tumours are different from normal endothelial cells [12, 23], are cytogenetically abnormal [24] and express tumour suppressor genes and oncogenes [23, 25]. In addition, in the case of tumours of hematopoietic origin, endothelial cells were shown to share the tumour-specific genetic alteration, such as chromosomal translocations in B-cell lymphomas, the BCR/ABL fusion gene in leukaemias and the myeloma-specific 13q14 chromosomal deletion [26–28]. These data may suggest the hypothesis that a common progenitor targeted by neoplastic transformation can differentiate in tumour cells or in endothelial cells sharing the same genetic abnormalities [27]. Regarding solid tumours, it was recently shown the endothelial cells present in neuroblastomas presented the same genetic amplification of neuroblastoma cells indicating that tumour endothelial cells may derive from tumour cell [29]. Among the different explications, our data support the hypothesis that a stem cell fraction of the tumour may be able to differentiate both into tumour cells and into endothelial cells. In this context, the tumour microenvironment may orchestrate their differentiation.
In vivo, mammosphere-derived single cells cloned in endothelial differentiating medium were able to induce both vessels and tumour formation. This suggests that in the endothelial-differenti-ated cells, a stem cell population is maintained and it is able in vivo to originate the tumour. The maintenance of stem cells in a differentiated cell population, achieved by asymmetrical division of stem cells, has been reported in a number of cell lines in culture [30, 31], including the mammary cell line MCF7 [6, 32]. In the present experiments, a single stem cell cloned in endothelial differentiating medium gave raise to about 90% cells expressing an endothelial phenotype and a small percentage of undifferentiated cells. It is therefore possible that the tumourigenic potential retained by the endothelial-differentiated clones may depend on these cells with stem/progenitor characteristics. However, we cannot definitely rule out the possibility that endothelial differentiated cells may revert to an epithelial phenotype. The rapid formation of tumour cluster within Matrigel in vivo may suggest that from a single stem cell of a single clone, a population of progenitors with bipotent tumourigenic and vasculogenic differentiating property is generated.
The ability of stem/progenitor cells to generate multiple lineages is considered a hallmark of stemness. The results of the present study demonstrate that tumour-initiating stem cells of breast cancer have the ability to differentiate not only in epithelial lineages but also in endothelial cells, further supporting the hypothesis that the tumour-initiating population possesses stem cell characteristics relevant for tumour growth and vascularization.
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), by the Italian Ministry of University and Research (MIUR) COFIN06 and ex60%, by Regione Piemonte and by Progetto S. Paolo Oncologia. We thank Carla Pecchioni and Patrizia Gugliotta for technical help.
References
- 1.Reya T, Morrison SJ, Clarcke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–82. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and trascriptional profiling of human mammary stem\progenitors cells. Genes Dev. 2003;17:1253. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponti D, Costa A, Zaffarono N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 7.Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:75–86. doi: 10.1007/s10911-005-2542-5. [DOI] [PubMed] [Google Scholar]
- 8.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–11. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angio-genesis. Am J Pathol. 2000;156:361–81. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirakawa K, Kobayashi H, Heike Y, Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, Konishi F, Terada M, Wakasugi H. Hemodynamics in vasculo-genic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62:560–6. [PubMed] [Google Scholar]
- 11.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 12.Grange C, Bussolati B, Bruno S, Fonsato V, Sapino A, Camussi G. Isolation and characterization of human breast tumor-derived endothelial cells. Oncol Rep. 2006;15:381–6. [PubMed] [Google Scholar]
- 13.Bruno S, Bussolati B, Grange C, Collino F, Graziano ME, Ferrando U, Camussi G. CD133+ renal progenitor cells contribute to tumor angiogenesis. Am J Pathol. 2006;169:2223–35. doi: 10.2353/ajpath.2006.060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lottner C, Schwarz S, Diermeier S, Hartmann A, Knuechel R, Hofstaedter F, Brockhoff G. Simultaneous detection of HER2/neu gene amplification and protein overexpression in paraffin-embedded breast cancer. J Pathol. 2005;205:577–84. doi: 10.1002/path.1742. [DOI] [PubMed] [Google Scholar]
- 15.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–55. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dontu G, Liu S, Wicha MS. Stem cells in mammary development and carcinogene-sis: implications for prevention and treatment. Stem Cell Rev. 2005;1:207–13. doi: 10.1385/SCR:1:3:207. [DOI] [PubMed] [Google Scholar]
- 17.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 18.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, Young PP. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovasculariza-tion. FASEB J. 2006;20:1495–7. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 19.Sapino A, Bongiovanni M, Cassoni P, Righi L, Arisio R, Deaglio S, Malavasi F. Expression of CD31 by cells of extensive ductal in situ and invasive carcinoma of the breast. J Pathol. 2001;194:254–61. doi: 10.1002/1096-9896(200106)194:2<254::AID-PATH880>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Rybak SM, Sanovich E, Hollingshead MG, Borgel SD, Newton DL, Melillo G, Kong D, Kaur G, Sausville EA. “Vasocrine” formation of tumor cell-lined vascular spaces: implications for rational design of antiangiogenic therapies. Cancer Res. 2003;63:2812–9. [PubMed] [Google Scholar]
- 21.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess AR, Seftor EA, Gardner LM, Carles-Kinch K, Schneider GB, Seftor RE, Kinch MS, Hendrix MJ. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2) Cancer Res. 2001;61:3250–5. [PubMed] [Google Scholar]
- 23.Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–61. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- 24.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–55. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 25.Fonsato V, Buttiglieri S, Deregibus MC, Puntorieri V, Bussolati B, Camussi G. Expression of Pax2 in human renal tumor-derived endothelial cells sustains apopto-sis resistance and angiogenesis. Am J Pathol. 2006;168:706–13. doi: 10.2353/ajpath.2006.050776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streubel B, Chott A, Huber D, Exner M, Jäger U, Wagner O, Schwarzinger I. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lym-phomas. N Engl J Med. 2004;351:250–9. doi: 10.1056/NEJMoa033153. [DOI] [PubMed] [Google Scholar]
- 27.Gunsilius E, Duba HC, Petzer AL, Kähler CM, Griinewald K, Stockhammer G, Gabl C, Dirnhofer S, Clausen J, Gastl G. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355:1688–91. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- 28.Rigolin GM, Fraulini C, Ciccone M, Mauro E, Bugli AM, De Angeli C, Negrini M, Cuneo A, Castoldi G. Neoplastic circulating endothelial cells in multiple myeloma with 13q14 deletion. Blood. 2006;107:2531–35. doi: 10.1182/blood-2005-04-1768. [DOI] [PubMed] [Google Scholar]
- 29.Pezzolo A, Parodi F, Corrias MV, Cinti R, Gambini C, Pistoia V. Tumor origin of endothelial cells in human neuroblastoma. J Clin Oncol. 2007;25:376–83. doi: 10.1200/JCO.2006.09.0696. [DOI] [PubMed] [Google Scholar]
- 30.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 31.Harper LJ, Piper K, Common J, Fortune F, Mackenzie IC. Stem cell patterns in cell lines derived from head and neck squa-mous cell carcinoma. J Oral Pathol Med. 2007;36:594–603. doi: 10.1111/j.1600-0714.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 32.Cariati M, Naderi A, Brown JP, Smalley MJ, Pinder SE, Caldas C, Purushotham AD. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int J Cancer. 2008;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]


