Abstract
Background
Amniotic membrane transplantation (AMT) has a long tradition in ophthalmic surgery and has become very popular recently because of newly developed methods of tissue preservation.
Methods
We selectively review the literature on recent developments, mechanisms of action, and established indications of AMT in the treatment of various diseases of the ocular surface. We searched the PubMed database for articles that appeared from 1994 to 2009 with the key words “amniotic membrane,” “cornea,” and/or “conjunctiva.”
Results
Amniotic membrane (AM) can function in the eye as a basement membrane substitute or as a temporary graft. It has anti-inflammatory and anti-scarring effects and contains growth factors that promote epithelial wound healing on the surface of the eye. AMT has been found to be a good alternative for corneal and conjunctival reconstruction in many clinical situations, including acute burns, persistent epithelial defects of the cornea, and diseases that cause conjunctival scarring. Nonetheless, there have been no more than a few randomized and controlled trials of AMT to date. Other studies have shown that AM can serve as a culture substrate to expand epithelial progenitor cells for use in ocular surface reconstruction.
Conclusion
AMT is an established technique in the treatment of various diseases of the external eye. In the last few years, AMT has brought about major advances in the reconstructive surgery of the ocular surface.
Transplantation of preserved human amniotic membrane (AM) can be considered one of the major new developments in surgery of the ocular surface. Although the first ophthalmological use of AM documented in the international literature took place almost 70 years ago, amniotic membrane transplantation (AMT) has only been performed in larger numbers of patients since 1995, with promising results (1– 3).
Various disorders of the ocular surface, including persistent epithelial defects of the cornea, acute chemical burns with long-term loss of integrity of the ocular surface epithelium or conjunctival scarring as a result of the healing of mucous membrane disorders still pose a clinical challenge in ophthalmic surgery. Since modern preservation methods were introduced, the innermost layer of the placenta, the AM, procured in sterile conditions following a Cesarean section, has experienced a renaissance as a basement membrane substitute. Today it is hard to imagine reconstructive surgery of the ocular surface without it (1– 3). In 2008, a total of 2308 AMTs were performed in Germany (seehttp://www.dog.org/?cat=121, in German). The many possible applications of AM established since then range from grafts via patches to a culture substrate/carrier for ex vivo cultivation of ocular surface epithelium. Each type of application yields histologically different integration patterns for AM in the host corneal tissue (4). The most important indications in reconstructive surgery of the ocular surface are persistent epithelial defects of the cornea with corneal ulceration of varying etiology, covering defects after surgical removal of large conjunctival lesions, acute chemical burns, symblepharon and fornix reconstruction in healing conjunctival disorders, and limbal stem cell deficiency of the cornea with simultaneous stem cell grafting (5– 7).
This review article presents information obtained from the authors’ scientific and clinical activities and a selective search of PubMed literature using the search terms “amniotic membrane,” “cornea,” and/or “conjunctiva” ranging from 1994 to 2009. It reports new developments, mechanisms of action, and established indications of AMT.
Procurement and histology
The AM is the innermost layer of the placenta, located next to the fetus. Histologically, it is a multilayer membrane approximately 0.02 to 0.5 mm thick. After serologically detectable pathogens (HIV-1/2; hepatitis B, C; HTLV-I/II; syphilis) have been ruled out, it is procured under sterile conditions during a Cesarean section, packed in a sterile environment, and swiftly prepared in a sterile working area. Appropriate standardized operating guidelines for procuring and manufacturing human AM from donor placenta are currently being developed with the support of the Tissue Transplantation and Biotechnology Section of the German Society of Ophthalmology (Deutsche Ophthalmologische Gesellschaft, DOG) (e1). When appropriately prepared it consists of a relatively thick basement membrane with devitalized amniotic epithelial cells and an avascular, almost acellular stroma. Cryopreservation involves a cryomedium (glycerin: DMEM medium 1:1), with a storage temperature of –75 °C to –85 °C and maximum storage time of approximately 12 to 24 months. If professionally procured and preserved, AM’s biological properties are retained, and as a result this natural matrix can be used to replace damaged stromal tissue of the surface of the eye (4, 5).
Clinical importance of biological properties
Clinical trials suggest that AM transplantation promotes epithelialization and differentiation of the epithelium of the ocular surface (5– 9). The most important growth factors that promote wound healing, which have been isolated mainly from the amniotic epithelium but also from the AM stroma, are epidermal growth factor and keratocyte growth factor (8, 9). Structural proteins such as laminin and type VII collagen in the AM basement membrane explain the observed epitheliotropic effects (9, 10). Intrinsic neurotropic substances make AM an ideal substrate for reconstruction of the epithelium of the ocular surface (11, 12).
The inhibition of TGF-ß signal transduction in corneal and conjunctival fibroblasts in vitro explains AM’s anti-scarring effect in the treatment of various disorders of the surface of the eye (13). In the initial phase after AMT, there is typically a significant reduction in inflammation. In vitro, AM reduces expression of various growth factors and pro-inflammatory cytokines (14). In addition, anti-inflammatory cytokines such as interleukin-10 and interleukin-1 receptor antagonist are released in the epithelium and stroma of the AM and may modulate inflammatory processes. They may play a role in the healing of acute chemical burns of the cornea covered by AM (15, 16). AM also has an immunomodulatory effect (17), and tissue rejection is therefore rarely observed in clinical use of AM.
The effects of cryopreservation
As the cells of cryopreserved AM are devitalized after thawing, no enzyme activity appears, and no intact RNA can be extracted, these substances are released from the damaged, devitalized cells. Because these factors are removed after application of cryopreserved AM, longer-term use is problematic. It is therefore advisable to replace an AM used as a patch in ocular surface reconstruction at regular weekly intervals until the desired epithelial wound closure occurs. Unlike the generally preferred cryopreservation of AM, other AM preparation procedures which are in use, such as acid pretreatment and subsequent air drying (18), lead to near-complete loss of its biological properties, and as a result their possibilities for clinical application and their efficacy seem limited (19).
Basic surgical techniques
AM has three main aims in clinical use:
To promote epithelialization
To reduce pain
To minimize inflammation of the ocular surface.
This allows procedures to be performed in a sequential manner, such as cornea transplantation with a reduced risk of rejection (5, 20). These aims are achieved using various different surgical procedures for AMT (5, 6).
Inlay or graft technique
In the inlay technique, the AM is applied as a permanent basement membrane substitute. The main indications for this are persistent epithelial defects, corneal ulceration or to cover defects following excision of conjunctival tumors (5, 6, 20– 23, e2) (Box). AM is sewn in with the epithelium/basement membrane side facing outwards, using 10-0 nylon sutures, following wound debridement, so that neighboring recipient epithelial cells can migrate onto the AM and the wound begins to close (5, 6). In deep defects, e.g. corneal ulceration, multiple layers of AM can be used (20, 21). Epithelialization of the AM integrates AM into the host tissue (4). It remains detectable for months, sometimes years, and in defects of the cornea is even colonized by local keratocytes (4).
Box. Indications of amniotic membrane transplantation*.
Reconstruction of the corneal surface
Persistent epithelial defect with corneal ulceration
Reconstruction of the surface of the conjunctiva
Acute chemical burns
-
Removal of epithelial or subepithelial lesions
(band keratopathy, scars, tumors)
Painful bullous keratopathy
-
Partial or complete limbal stem cell deficiency
(with stem cell grafting)
Reconstruction of the surface of the conjunctiva
Acute chemical burns and acute Stevens-Johnson syndrome
Covering defects after removal of large conjunctival lesions (tumors, conjunctival intraepithelial neoplasia, scars, conjunctival folds parallel to the edges of the eyelids)
Symblepharon, fornix reconstruction
Anophthalmia
Bleb revisions
Scleral thinning
Pterygium
*According to (5– 7, 16, 21– 25). The literature contains no evidence-based, randomized studies comparing AMT with the alternative options for these indications
Onlay or patch technique
In the onlay technique, a large AM is temporarily placed on the surface of the eye as a “natural” patch (5, 6). Unlike the inlay technique, where the AM remains permanently on the cornea, with the onlay technique the AM patch typically becomes detached from the surface of the cornea after one to two weeks. Classical indications range from acute burns to acute herpetic keratitis and the acute stage of Stevens-Johnson syndrome (5, 6, 16, 20, 24). With these indications AM’s biological properties, particularly its anti-inflammatory mechanisms of action, are used, although as mentioned above these last only a limited time (8, 9, 19). If delayed wound healing is demonstrated clinically, on the basis of the above-mentioned experimental data it seems to be advisable to change the AM patch weekly until the wound closes as desired. The orientation of the AM plays only a minor role with this surgical procedure: in most cases attaching the AM loosely in the episcleral space or to the bulbar conjunctiva using 8-0 monofilament vicryl sutures is considered sufficient (5, 6).
Inlay and onlay technique
This technique, also called the sandwich technique, is a combination of the two described above and is used mainly in serious disorders of the ocular surface such as deep and extensive corneal ulceration, or in surgical revisions (5, 6). The main purpose of the onlay is to protect the inlay and promote its epithelialization (4, 5). This method is favored due to its high success rate (65% to 80%), and low rate of recurrence (approx. 20% to 35%) (5, 20).
Amniotic membrane as culture substrate and carrier
The AM has only recently been described as a culture substrate for ex vivo expansion of the epithelium of the ocular surface (7, 25, e3– e5). Here the AM functions as a biomatrix and is placed on the surface of the eye together with the cultivated cells (e3, e4). This technique is mainly used to treat limbal stem cell deficiency, but also in reconstruction of major surface defects of the outer eye (7, 25, e5, e6).
Indications of transplantation
So many indications of AMT have already been described in the literature that they would go well beyond the boundaries of this review article (5– 7, 16, 21– 25, e2, e5, e6). The main indications of AMT are therefore summarized in the Box. Unfortunately, reliable clinical data have only been published in small to medium numbers of cases, with a relatively short follow-up period, and overwhelmingly in retrospectively analyzed case series (e6), probably due to the low incidence of individual disorders of the ocular surface. There are no Cochrane Reviews or meta-analyses available from either the Cochrane Library or PubMed on the subjects discussed in this review article. Controlled, randomized, multicenter, long-term trials involving large numbers of patients must be conducted in the future in order to substantiate AMT’s clinical potential in reconstructive surgery of the surface of the eye.
Reconstruction of the corneal surface
Persistent epithelial defects and ulceration of the cornea
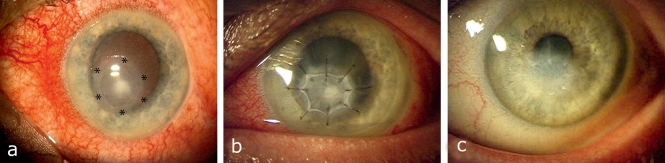
Disorders of the cornea are one of the most common indications of AMT in the USA, accounting for 41% of cases (9). The treatment of corneal ulcers still poses a major challenge in ophthalmology, despite various conservative and surgical options (5, 20, 21). An intact corneal epithelium is a decisive factor in ocular surface stability. Simple epithelial defects generally heal with no complications. If they are untreated or inappropriately treated, corneal ulceration, descemetocele, and even perforation can quickly develop. AM is used clinically as a basement membrane substitute in patients with persistent epithelial defects both with and without corneal ulceration (5, 20). After a median follow-up time of 18 months, Seitz et al. achieved epithelial closure with no recurrence in 65% of cases (5) (Figure 1). The success rate of AMT can be significantly improved using subsequent measures such as inducing upper eyelid ptosis via Botox injection or tarsorrhaphy (5, 20). The reoperation rate then falls from approx. 44% to 30% (20).
Figure 1.
Amniotic membrane transplantation (AMT) in infectious corneal ulceration (see area marked with stars): a) Before surgery; b) Three days after AMT; c) Three months after AMT a smooth corneal surface with no irritation has formed, with a paracentral corneal scar. Signs of inflammation have significantly decreased. Modified with the permission of Dr. R. Kaden-Verlag (publishers), Heidelberg
A multilayer technique is used to treat deep corneal ulceration, descemetocele, and small corneal perforations (21, e2). Solomon et al. report stable wound closure in 28 of 34 eyes (82%) with these corneal disorders in a retrospective analysis (21). Kruse et al. also achieved stabilization of the corneal surface in 9 of 11 patients with deep ulceration of the cornea, within a follow-up time of 12 months (e2). After AMT, corneal transplantation (keratoplasty) is required for further restoration of vision. Prognosis is substantially improved if an intact surface epithelium has formed on the surface of the eye and signs of inflammation have decreased (5, 20, 21). Turning to complications, calcification is observed in 12% of cases if eye drops containing phosphates are used, and infections in less than 1% of cases (5).
Acute chemical burns
Chemical burns to the ocular surface are a common problem in acute ophthalmological care. These can lead to complete corneal erosion and blood vessel rupture at the limbus and in the conjunctiva. The overriding aims of treatment are to prevent necrosis and scarring and to achieve swift epithelialization. Early AMT improves functional outcomes after chemical burns (2, 16, 24). Symblepharon occurred in only one case of slight to moderate chemical burns treated with prompt AMT (2, 16, 24). In a prospective randomized controlled trial Tamhane et al. documented that the epithelial closure rate was significantly higher in patients treated with an AM in the first week. For severe chemical burns, however, stem cell deficiency of the cornea with long-term, serious loss of visual acuity is often impossible to prevent (16, e7).
Tissue engineering of limbal epithelium using amniotic membrane
The corneal surface is very important to eyesight. Maintaining a functional corneal epithelium is therefore essential to vision. Epithelial stem cells of the corneal epithelium are located in the limbus, which forms an anatomical bridge between the conjunctiva and the cornea. Various disorders of the surface of the eye can lead to limbal stem cell deficiency as a result of destruction of limbal stem cells. Limbal stem cell deficiency is characterized by formation of fibrovascular pannus tissue on the corneal surface, increased glare sensitivity, and loss of vision. Long-term success of surface reconstruction can only be achieved by restoring the limbal stem cell population (e6).
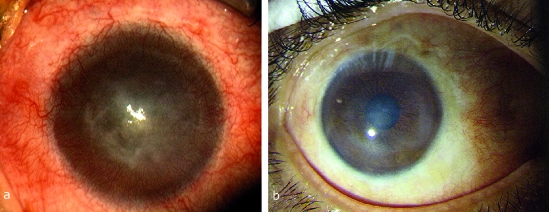
For many years the results of conventional limbus transplantation, which are not yet satisfactory, have given rise to a demand for stem cells from cell cultivation (e6). Further development of methods reliant on cell biology, particularly tissue engineering, now also make it possible to cultivate limbus stem cells. Autologous ex vivo expansion of limbal epithelial cells on AM has only recently been developed as a new procedure (7, 25, e3– e5) (Figure 2): in unilateral stem cell deficiency, in clinical and experimental conditions, a limbal biopsy measuring 1 to 2 mm was taken from the other, healthy eye, and epithelial cells were cultivated and expanded on the AM in vitro. This ex vivo expansion of limbal epithelial cells is less traumatic than conventional autologous limbal transplantation, in which up to 50% of the limbus from the healthy eye is transplanted onto the diseased eye. Tsai et al. were the first to describe their clinical experience in treating limbal stem cell deficiency using AM as a carrier and culture substrate (25). Long-term wound healing and increased visual acuity were achieved in 83% of treated patients. Vascularization as a sign of recurring stem cell deficiency did not occur within the described follow-up period. Many subsequent publications, some of which concern different cell cultivation procedures, have also described notable treatment success (7, e5). Unfortunately, there is currently no standardized procedure available, which makes the published studies harder to compare. Other disadvantages are the small, sometimes heterogeneous patient groups and usually short follow-up times, as yet preventing conclusive evaluation of this new form of treatment (7, e5, e6).
Figure 2.
Autologous transplantation of limbal epithelium cultivated ex vivo: a) Before surgery, with complete stem cell deficiency following a chemical burn. Visual acuity sufficient to perceive hand movements. b) Stable, clear corneal surface 4.5 years after reconstruction. Visual acuity has increased to 0.4 long-term. Modified according to Dev Ophthalmol 2010; 45: 57–70, with the permission of Karger-Verlag (publishers), Basel
Reconstruction of the conjunctival surface
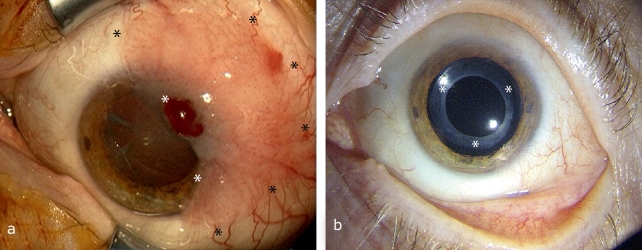
Excision of a squamous cell carcinoma or a melanoma from the area of the conjunctiva, for example, often causes major surface defects. After complete tumor removal, defects can be covered with an AM (22, 23). Further adjuvant measures to eradicate the tumor, such as radiation or local chemotherapy, can then follow. In cases such as these the AM functions as a basement membrane substitute and aids fast, usually scar-free wound healing by promoting the migration of neighboring conjunctival epithelial cells of the recipient (23) (Figure 3). Thanks to this, AM is more cosmetically acceptable than other methods such as transplantation of oral mucous membranes, and their intrinsic transparency also allows effective post-operative tumor monitoring (22, 23).
Figure 3.
Covering a defect following excision of conjunctival intraepithelial neoplasia: a) Before surgery, with a large conjunctival tumor (see area marked with stars); b) 4 years after tumor excision and AMT there are no signs of recurrence. Intraocular pseudophakia with no irritation (intraocular lens, see area marked with stars) following a cataract operation
Conclusion
AMT is used in acute ophthalmological care, to treat chronic diseases of the surface of the eye, and as the newest development, using tissue engineering, as a biomatrix to treat severe stem cell deficiency of the ocular surface. It provides practicing ophthalmologists with a particularly multifaceted instrument to tackle the challenges posed by disorders of the surface of the eye successfully. Controlled, randomized, multicenter, long-term trials involving large numbers of patients are needed in order to substantiate the clinically relevant potential of AMT in reconstructive surgery of the surface of the eye, which has been documented in many case studies.
Key Messages.
The amniotic membrane, the innermost layer of the placenta, plays a significant role in reconstructive surgery of the outer eye.
The amniotic membrane possesses biological properties which promote wound healing in various disorders of the outer eye.
Cryopreservation preserves the amniotic membrane’s biological properties for longer than other methods.
The main indications of amniotic membrane transplantation are corneal ulceration, covering defects in large conjunctival lesions, and acute chemical burns to the surface of the eye.
The amniotic membrane is used as a culture substrate and carrier in ex vivo expansion of corneal stem cells, to treat limbal stem cell deficiency.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
Supported by external grants from the Deutsche Ophthalmologische Gesellschaft (the medical scientific association of ophthalmology in Germany), Munich; the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Bonn, ME 1623/3–1; the Estnische Forschungsgemeinschaft (Estonian Research Foundation), Grant 5832; and the Forschungsförderung AG Trockenes Auge (the German research fund for dry eyes), Berlin, Germany.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.de Rotth A. Plastic repair of conjunctival defects with fetal membrane. Arch Ophthalmol. 1940;23:522–525. [Google Scholar]
- 2.Sorsby A, Symons HM. Amniotic membrane grafts in caustic burns of the eye: (Burns of the second degree) Br J Ophthalmol. 1946;30:337–345. [PubMed] [Google Scholar]
- 3.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–484. [PubMed] [Google Scholar]
- 4.Seitz B, Resch MD, Schlotzer-Schrehardt U, Hofmann-Rummelt C, Sauer R, Kruse FE. Histopathology and ultrastructure of human corneas after amniotic membrane transplantation. Arch Ophthalmol. 2006;124:1487–1490. doi: 10.1001/archopht.124.10.1487. [DOI] [PubMed] [Google Scholar]
- 5.Seitz B. Amniotic membrane transplantation. An indispensable therapy option for persistent corneal epithelial defects. Ophthalmologe. 2007;104:1075–1079. doi: 10.1007/s00347-007-1661-3. [DOI] [PubMed] [Google Scholar]
- 6.Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12:269–281. doi: 10.1097/00055735-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52:483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 9.Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–187. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- 11.Schroeder A, Theiss C, Steuhl KP, Meller K, Meller D. Effects of the human amniotic membrane on axonal outgrowth of dorsal root ganglia neurons in culture. Curr Eye Res. 2007;32:731–738. doi: 10.1080/02713680701530605. [DOI] [PubMed] [Google Scholar]
- 12.Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987–994. [PubMed] [Google Scholar]
- 13.Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–334. [PubMed] [Google Scholar]
- 14.Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–449. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Experimental Eye Research. 2000;70:329–337. doi: 10.1006/exer.1999.0794. [DOI] [PubMed] [Google Scholar]
- 16.Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 17.Ueta M, Kweon MN, Sano Y, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clinical and Experimental Immunology. 2002;129:464–470. doi: 10.1046/j.1365-2249.2002.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Versen-Hoynck F, Syring C, Bachmann S, Moller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts–light and scanning electron microscopic studies. Cell and Tissue banking. 2004;5:45–56. doi: 10.1023/b:catb.0000022276.47180.96. [DOI] [PubMed] [Google Scholar]
- 19.Thomasen H, Pauklin M, Steuhl KP, Meller D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefes Arch Clin Exp Ophthalmol. 2009;247:1691–1700. doi: 10.1007/s00417-009-1162-y. [DOI] [PubMed] [Google Scholar]
- 20.Fuchsluger T, Tuerkeli E, Westekemper H, Esser J, Steuhl KP, Meller D. Rate of epithelialisation and re-operations in corneal ulcers treated with amniotic membrane transplantation combined with botulinum toxin-induced ptosis. Graefes Arch Clin Exp Ophthalmol. 2007;245:955–964. doi: 10.1007/s00417-006-0493-1. [DOI] [PubMed] [Google Scholar]
- 21.Solomon A, Meller D, Prabhasawat P, et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694–703. doi: 10.1016/s0161-6420(01)01032-6. [DOI] [PubMed] [Google Scholar]
- 22.Paridaens D, Beekhuis H, van Den Bosch W, Remeyer L, Melles G. Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol. 2001;85:658–661. doi: 10.1136/bjo.85.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espana EM, Prabhasawat P, Grueterich M, Solomon A, Tseng SC. Amniotic membrane transplantation for reconstruction after excision of large ocular surface neoplasias. Br J Ophthalmol. 2002;86:640–645. doi: 10.1136/bjo.86.6.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheirkhah A, Johnson DA, Paranjpe DR, Raju VK, Casas V, Tseng SC. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008;126:1059–1066. doi: 10.1001/archopht.126.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- e1.Hahn A, Thanos M, Reinhard T, Seitz B, Steuhl KP, Meller D. Procedural guidelines : Arbeitsrichtlinien - gute fachliche Praxis zur Gewinnung und Herstellung von kryokonservierter humaner Amnionmembran aus Spenderplacenta. Ophthalmologe. 2010;107:1020–1031. doi: 10.1007/s00347-010-2269-6. [DOI] [PubMed] [Google Scholar]
- e2.Kruse FE, Rohrschneider K, Volcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106:1504–1510. doi: 10.1016/S0161-6420(99)90444-X. [DOI] [PubMed] [Google Scholar]
- e3.Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- e4.Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Shortt AJ, Secker GA, Rajan MS, et al. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008;115:1989–1997. doi: 10.1016/j.ophtha.2008.04.039. [DOI] [PubMed] [Google Scholar]
- e6.Cauchi PA, Ang GS, Azuara-Blanco A, Burr JM. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146:251–259. doi: 10.1016/j.ajo.2008.03.018. [DOI] [PubMed] [Google Scholar]
- e7.Tamhane A, Vajpayee RB, Biswas NR, et al. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005;112:1963–1969. doi: 10.1016/j.ophtha.2005.05.022. [DOI] [PubMed] [Google Scholar]