Abstract
Background & Aims
Highly proliferative fetal liver stem/progenitor cells (FLSPC) repopulate livers of normal recipients by cell competition. We investigated the mechanisms by which FLSPC repopulate livers of older, compared with younger rats.
Methods
Fetal liver cells were transplanted from DPPIV+ F344 rats into DPPIV− rats of different ages (2, 6, 14, or 18 months); liver tissues were analyzed 6 months later. Cultured cells and liver tissues were analyzed by reverse transcription PCR, immunoblot, histochemistry, laser-capture microscopy, and TUNEL analyses.
Results
We observed 4–5-fold increases in liver repopulation when FLSPC were transplanted into older, compared with younger, rats. mRNA levels of cyclin-dependent kinase inhibitors increased progressively in livers of older rats; hepatocytes from 20-month old rats had 6.1-fold higher expression of p15INK4b and were less proliferative, in vitro, than hepatocytes from 2-month old rats. Expression of p15INK4b in cultured hepatocytes was upregulated by activin A, which increased in liver during aging. Activin A inhibited proliferation of adult hepatocytes, whereas FLSPC were unresponsive because they had reduced expression of activin receptors (e.g. ALK-4). In vivo, expanding cell clusters derived from transplanted FLSPC had lower levels of ALK-4 and p15INK4b and increased levels of Ki-67, compared with the host parenchema. Liver tissue of older rats had 3-fold more apoptotic cells than of younger rats.
Conclusions
FLSPC, resistant to activin A signaling, repopulate livers of older rats; hepatocytes in older rats have less proliferation, because of increased activin A and p15INK4b levels, and increased apoptosis than of younger rats. These factors and cell types might be manipulated to improve liver cell transplantation strategies in patients with liver diseases in which activin A levels are increased.
Keywords: p16INK4a, p19ARF and p21/CIP1, stem cell therapy, organ transplantation
INTRODUCTION
Since donor organ scarcity is the major limitation of liver transplantation (1), novel therapeutic approaches are urgently needed to treat chronic and genetic-based liver diseases. In this regard, considerable attention has been focused on the possibility to restore liver function through cell transplantation (2,3). This has been achieved in our laboratory in a rat model system, using ED14 FLSPC transplanted into young rats without requiring genetic or genotoxic manipulation of the host liver (4,5). Since transplanted FLSPC have higher proliferative activity than host hepatocytes, a proliferative differential exists between these cell types (5). This leads to cell competition, a process originally described in Drosophila wing development, during which more rapidly proliferating cells progressively replace less proliferative neighboring cells by inducing their apoptosis (6,7). Even though fetal liver cells are not likely to be used for human liver repopulation, the importance of using cell competition as a strategy to achieve effective therapeutic cell repopulation has been highlighted (8). Therefore, understanding the mechanism(s) that can drive liver repopulation through cell competition needs to be determined. Loss of liver regenerative capacity is the most dramatic alteration noted in aging liver (9,10). In normal liver, hepatocytes are in a quiescent state and turn over very slowly. However, following two-thirds partial hepatectomy (PH), remaining hepatocytes rapidly enter the cell cycle and total parenchymal cell number and mass are restored within 1-2 weeks (11-13). This proliferative response is significantly diminished in aging liver. Bucher et al. (14) demonstrated delayed proliferative activity in livers of 12-15 month old rats compared to 4 month old rats after PH. Stocker & Heine (15) determined the proliferating pool of epithelial cells after PH in rats and concluded that 70% of hepatocytes in 24-30 month old rats are non-dividing cells compared to 21% in 2-4 month old rats.
Cellular events leading to decreased regenerative capacity of hepatocytes in aging liver are complex and not fully understood. Cell cycle arrest is mediated by cyclin-dependent kinases (cdk) that are negatively regulated by two cyclin-dependent kinase inhibitor (CKI) families, the INK4 family (p15INK4b, p16INK4a, p18INK4c and p19INK4d) and the CIP/KIP family (p21/CIP1, p27/KIP1 and p57/KIP2) (16). INK family members inhibit the catalytic subunits of cdk4 and cdk6, whereas CIP/KIP proteins regulate cyclin D-, E- and A-dependent kinases (16). Furthermore, several CKIs from both families are senescence-associated (17) and are elevated during aging, including p16INK4a (18,19) and p21/CIP1 (20).
In the present study, we compared the repopulation potential of FLSPC after their transplantation into young vs. older rats and discovered that liver repopulation is much higher in older recipients. In aging liver, we observed a progressive increase in p15INK4b expression, which could be induced in isolated hepatocytes by activin A, a potent growth suppressor that is also progressively increased in vivo during rat liver aging. These findings suggest a potential mechanism whereby FLSPC, which have a growth advantage through their resistance to activin A-induced growth inhibition, are able to repopulate the recipient liver and more effectively repopulate the aging liver, in which both activin A tissue levels and cell competition between transplanted FLSPC and host hepatocytes are increased.
MATERIALS AND METHODS
Animals
Pregnant, ED14 DPPIV+ F344 rats were purchased from Taconic Farms. Male DPPIV− F344 rats were provided by the Liver Research Center, Albert Einstein College of Medicine (AECOM). All animal studies were conducted under protocols approved by the Animal Care Use Committee of ACOM in accordance with NIH guidelines.
Isolation of fetal liver cells, cell transplantation and liver repopulation
Unfractionated fetal liver cells were isolated from ED14 fetal livers of DPPIV+ pregnant F344 rats, as described previously (4,5). Fetal liver cells (~1.5 × 107 cells) were transplanted into rats of different ages (2, 6, 14 months; n = 4/4/5) in conjunction with 2/3 PH. At 6 months after cell transplantation, rats were sacrificed. After enzymehistochemistry for DPPIV, 2 liver cryosections from each rat were scanned, liver replacement by DPPIV+ cells was determined by a computerized procedure and the average % liver repopulation was calculated (4,5).
Hepatocyte isolation
Detailed information concerning cell isolation procedures can be found in Supplementary Material & Methods.
Cell culture
Unfractionated fetal liver cells (1.0-1.5 × 106 cells, of which 5.0-7.5 × 104 cells were epithelial FLSPC) and 1.0 × 105 hepatocytes from 2 and 20 month old rats were plated on collagen-coated 12-well plates and incubated in DMEM containing 10% FBS overnight, after which the medium was switched to hormonally defined hepatocyte growth medium. After 24 hours, cells were incubated w/o or with activin A (PeproTech) at various concentrations for 24 hours.
RT-PCR, Western Blot analysis and qRT-PCR array analysis
Detailed information about RT-PCR and Western blot protocols can be found in Supplementary Material & Methods. Custom-made RT2Profiler™ PCR arrays (SA Biosciences) containing 370 genes from seven signaling pathways and 14 controls (Supplementary Material & Methods) were used to measure mRNA expression levels in cultured hepatic cells after activin A treatment.
Immunohistochemistry
Cytospins were stained with mouse anti-Ki-67 (BD Biosciences), followed by alkaline phosphatase-conjugated horse anti-mouse IgG, developed with Vector® Black Substrate Kit (Vector) and counterstained with hematoxylin. For activin receptor detection, cytospins were stained with rabbit anti-ActR-IIB or anti-ALK-4 (Santa Cruz) and Cy™2-conjugated donkey anti-rabbit IgG (Jackson). Detection of apoptosis in transplanted cell clusters vs. surrounding host parenchyma (TUNEL assay) was performed as described previously (5).
Laser capture microscopy
Six months after cell transplantation into a 14 month old recipient, a liver cryosection was stained for DPPIV, cell areas from DPPIV+ clusters and DPPIV− surrounding liver were laser-captured and used for qRT-PCR analysis (see Supplementary Material & Methods).
Statistics
Data are reported as mean ± SEM. Significance was analyzed by Student’s t-test or Mann-Whitney rank sum test with SigmaStat 2.01 software (SPSS Scientific).
RESULTS
Comparison of liver repopulation by transplanted FLSPC into rats of different ages
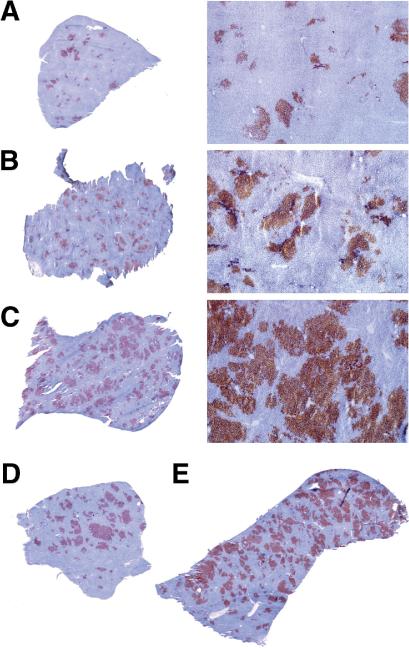
Previously, we transplanted unfractionated ED14 fetal liver cells into rats at age 2-3 months (4,5). Interestingly, after transplanting ED14 fetal liver cells into 12 month old rats, we discovered that the cluster size of transplanted cells is much larger and liver repopulation is much higher in older compared to younger rats. To determine whether there is a correlation between the % liver replacement by FLSPC and the age of the host recipient at the time of cell transplantation, we performed additional cell transplantation studies in older recipients and compared the liver repopulation with FLSPC transplanted into young rats. Six months after FLSPC transplantation into 2 month old rats, we obtained 4.5 ± 1.4% replacement of liver mass (Fig. 1A). In rats of 6 months age at the time of cell transplantation, we observed a 2.6-fold increase in liver repopulation (11.5 ± 2.8%, Fig. 1B). The average repopulation was 5-fold higher in 14 month old rats (22.6 ± 6.3%, Fig. 1C) compared to 2 month old recipients and there was a dramatic increase in cluster size with many cell clusters becoming confluent and encompassing multiple lobules (Fig. 1C).
Figure 1.
Liver repopulation by ED14 fetal liver cells derived from wt DPPIV+ F344 rats transplanted into mutant DPPIV− F344 rats of different ages in conjunction with two-thirds PH. (A-C) Examples of liver repopulation showing whole liver sections and selected regions at higher magnification (×40). Rats were sacrificed 6 months after cell transplantation (~1.5 × 107 cells) into 2 (A), 6 (B), and 14 month old rats (C). (D,E) To test whether we could infuse high numbers of cells into older rats, we transplanted ~1.5 vs. ~5.0 × 107 fetal liver cells into two 18 month old recipients. Six months after cell transplantation, ~25% liver repopulation (D) and ~50% liver repopulation (E) was achieved, respectively.
To test whether we can infuse high numbers of cells into older rats, we transplanted ~1.5 vs. ~5.0 × 107 fetal liver cells into two 18 month old recipients. Six months after cell transplantation, we obtained ~25% and ~50% liver repopulation, respectively (Fig. 1D & E).
Expression of cdks, cyclins, and CKIs in fetal and aging liver
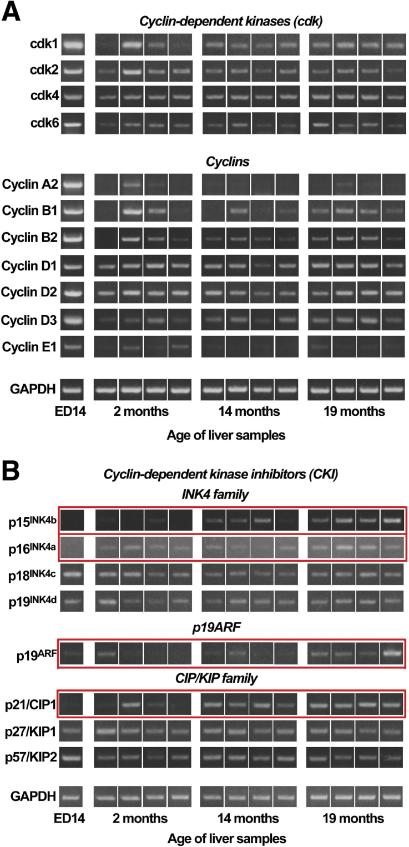
Impaired proliferative capability of the aging recipient liver (14,15) could be responsible for increased hepatic replacement by transplanted FLSPC in older compared to younger animals. Since cdks are key-molecules in cell proliferation and are regulated by cyclins (positive-regulators) and CKIs (negative-regulators), we determined their mRNA expression levels in liver of DPPIV− F344 rats of ages 2 and 14 months. Several members from both CKI-families have also been used to identify senescent cells (17), which accumulate in aging tissue. Therefore, we have included liver samples from older rats (age 19 months).
RT-PCR showed that expression of the majority of cdk and cyclin mRNAs was substantially higher in fetal liver compared to adult liver; however, no changes were found in cdk and cyclin mRNA expression in rat liver between ages 2, 14, and 19 months (Fig. 2A). No obvious differences were observed in mRNA expression of cdk4, cyclin D1 and D2 in fetal and aging liver (Fig. 2A). These data were confirmed by qRT-PCR for selected genes (data not shown).
Figure 2.
Gene expression of cdks and their regulators in fetal and aging liver. RNA extracts from pooled ED14 fetal livers (ED14) and liver samples from rats of different ages were analyzed for mRNA expression. (A) Expression of cdks and cyclins. (B) mRNA expression of CKIs, consisting of INK4 and CIP/KIP family members. Since several CKIs from both families are senescence biomarkers, p19ARF (an element of the p16INK4a/ARF locus) was included as an additional senescence marker. These experiments were performed at least two times.
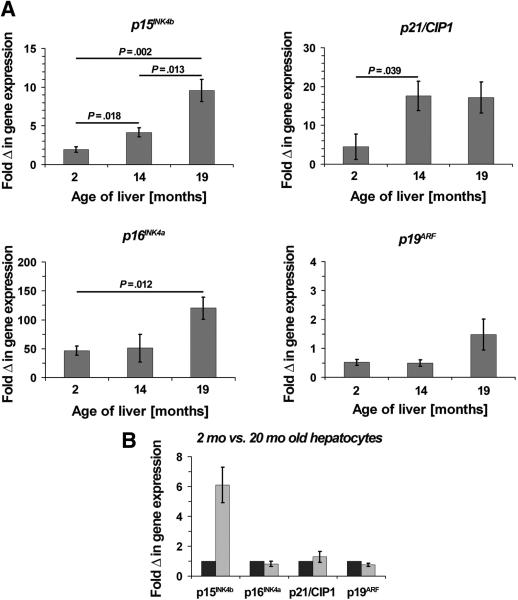
In fetal liver, p15INK4b, p16INK4a, p19ARF and p21/CIP1 mRNAs were expressed at low levels (Fig. 2B). At age 14 and 19 months, p15INK4b and p21/CIP1 expression was increased substantially (Fig. 2B). These results have been confirmed by qRT-PCR (Fig. 3A). Both ED14 fetal liver and 2 month old liver showed low levels of p15INK4b mRNA. However, compared to fetal liver, p15INK4b expression increased by 1.9-, 4.2-, and 9.6-fold in 2, 14, and 19 month old rats, respectively (Fig. 3A). Similarly, p21/CIP1 mRNA expression increased progressively up to 14 months; however, no further increase was observed at 19 months (Fig. 3A). In contrast, p16INK4a and p19ARF mRNA expression was unchanged between 2 and 14 months and became modestly elevated at 19 months (Fig. 3A). Finally, p18INK4c, p19INK4d, p27/KIP1, and p57/KIP2 mRNA expression was readily detected in fetal liver and was unchanged in aging liver tissues (Fig. 2B).
Figure 3.
(A) qRT-PCR analysis of selected genes in aging liver. Values are means ± SEM of 4 liver samples/time point and are expressed as fold differences with respect to fetal liver. The same tissue specimens, as used in Fig. 1, were used in Fig. 2. (B) qRT-PCR analysis of isolated hepatocyte fractions derived from 2 and 20 month old rat livers were analyzed for p15INK4b, p16INK4a, p21/CIP1, and p19ARF expression. Values are means ± SEM of 3 hepatocyte preparations derived from 20 month old livers and are expressed as fold differences with respect to 2 month old hepatocyte fractions. One representative experiment for each gene from at least two replicate experiments is shown.
mRNA expression of selected genes in 2 vs. 20 month old hepatocytes
Since regeneration in normal liver is achieved mainly through proliferation of hepatocytes (12), we subsequently determined whether changes observed in mRNA levels in aging liver (see Figs. 2B & 3A) were hepatocyte-specific. For this purpose, we isolated hepatocyte fractions from 2 and 20 month old rat livers and determined the mRNA expression of p15INK4b, p16INK4a, p19ARF, and p21/CIP1. In 20 month old hepatocytes, we observed a 6.1-fold increased expression of p15INK4b mRNA compared to young hepatocytes (Fig. 3B); however, no differences were found in p21/CIP1, p16INK4a, and p19ARF expression (Fig. 3B).
Gene expression of TGF-β superfamily members in aging liver
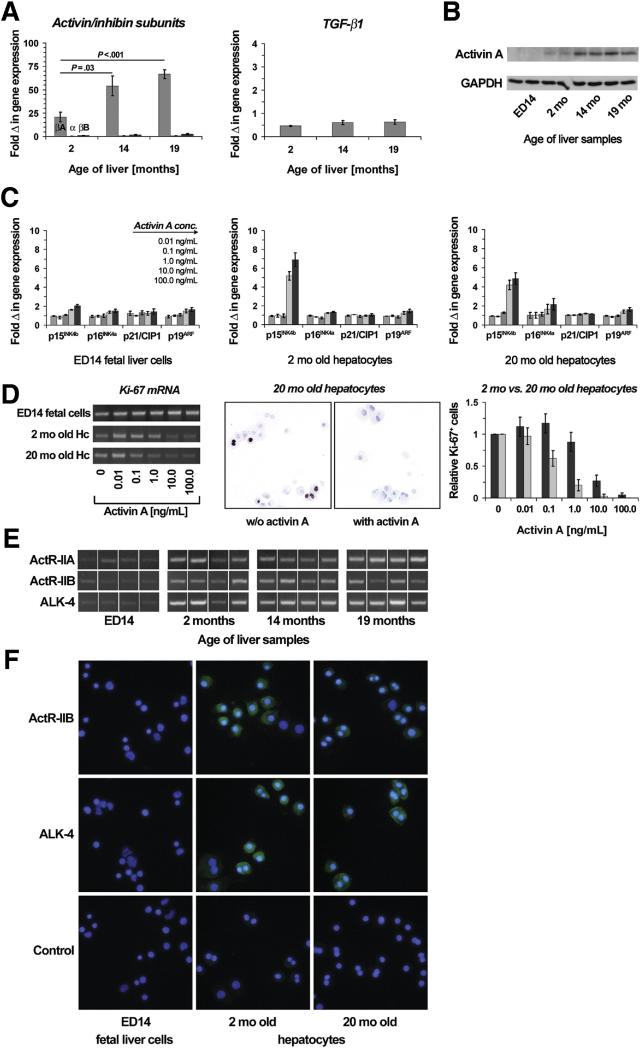
Based on studies reporting that activin A and TGF-β1 are potent regulators of cell proliferation (21,22) and induce p15INK4b and p21/CIP1 expression in various cell types (23-26), we analyzed aging liver for activin A and TGF-β1 gene expression. Using RT- & qRT-PCR, we observed that activin A mRNA increased progressively as rat liver ages (Fig. 4A). Since ED14 rat fetal liver is primarily a hematopoietic organ, we repeated RT-PCR analysis for activin A mRNA, using highly purified Dlk-1+ FLSPC (Supplementary Fig. 1A; 27) vs. hepatocytes from young adult liver and still observed very low activin A expression in both fetal liver cell fractions (Dlk-1−/Dlk-1+) compared to adult hepatocytes (Supplementary Fig. 1B). Western blot analysis for activin A showed a similar expression pattern at the protein level (Fig. 4B). However, TGF-β1 expression in the liver did not increase as rats age (Fig. 4A).
Figure 4.
Role of activin A in aging liver. (A) RNA extracts from fetal and aging liver were analyzed for mRNA expression of βA-, α-, βB-activin/inhibin subunits and TGF-β1 by qRT-PCR. Values are means ± SEM of 4 liver samples/time point and are expressed as fold differences with respect to fetal livers. (B) Western blot for activin A in aging liver, using GAPDH as a loading control. These experiments were repeated. (C) Gene expression in cultured hepatic cells of different ages after activin A treatment. Fetal liver cells (left panel) and young (middle) vs. old hepatocytes (right) were incubated with activin A at various concentrations for 24 hours. Cell RNA extracts were analyzed for mRNA expression using qRT-PCR. Values are expressed as fold changes with respect to cells cultured w/o activin A. Data represent the means ± SEM of 3 independent in vitro experiments. (D) Anti-proliferative effect of activin A on cultured hepatic cells. RNA extracts from fetal and adult hepatocytes were analyzed for Ki-67 mRNA expression (left panel). One representative analysis out of 2 independent analyses is shown. Using immunohistochemistry for Ki-67 (middle), proliferative activity of young vs. old hepatocytes with and without activin A treatment was calculated (right). Values are means ± SEM of % Ki-67+ cells from 5-10 analyzed microscopic fields and are expressed as fold differences with respect to cultured hepatocytes in the absence of activin A. One representative analysis out of 2 independent analyses is shown. (E,F) Activin receptor expression in aging liver. RNA extracts from fetal and adult liver tissues were analyzed for ActR-IIA, ActR-IIB and ALK-4 mRNA expression (E). Immunohistochemistry for ActR-IIB and ALK-4 on isolated fetal liver cells and adult hepatocytes (F). Original magnification ×400.
Growth-inhibitory effects of activin A in vitro
Since we observed increasing activin A concentrations in aging liver, we performed in vitro studies to determine whether fetal liver cells and aging hepatocytes are differentially affected by increasing activin A concentrations . There was a 5 to 7-fold increase in p15INK4b mRNA in hepatocytes after incubation with 10 and 100 ng/mL activin A (Fig. 4C); however, no changes were seen in expression of any senescence-related gene in cultured FLSPC after activin A treatment (Fig. 4C). Using RT-PCR for Ki-67 mRNA expression, activin A showed no effect on proliferation of cultured FLSPC (Fig. 4D, left). However, adult hepatocytes (especially hepatocytes isolated from older rats) were clearly proliferation-inhibited (i.e., exhibited reduced Ki-67 mRNA expression) by increasing activin A concentrations (Fig. 4D, left). Immunohistochemistry also showed a marked reduction in Ki-67 expression when 20 month old hepatocytes were cultured in the presence of activin A (Fig. 4D, middle). Furthermore, 20 month old hepatocytes were 10-fold more sensitive to inhibition of proliferation by activin A than 2 month old hepatocytes (Fig. 4D, right).
Using custom-made qRT-PCR arrays, we performed pathway analyses in hepatic cells cultured with 10 ng/mL activin A (see Supplementary Table 2 for complete gene list). Only one gene was significantly changed in fetal liver cells; however, 50 or 39 genes were down- or upregulated in 2 or 20 month old hepatocytes (Table 1). Most of these genes were downregulated and the major pathway affected was cell cycle regulation, in which 23 out of 64 genes tested were downregulated (e.g., cyclins, cdks, cdc6, E2f1, PCNA; Supplementary Fig. 2). In addition, p53 signaling (with cell cycle arrest as major output) and apoptosis (most notably anti-apoptotic genes: Birc5, Chek1, Naip2) showed significant changes (Table 1). Within the upregulated genes, most interestingly p15INK4b and Bhlhe40 (DEC1), recently reported as de novo markers for senescence (17), were upregulated in activin A-treated hepatocytes.
Table 1.
Differential gene expression of hepatic cells cultured for 24 hours with activin A compared to cells cultured in absence of activin A.
| ED14 fetal | 2 mo old | 20 mo old | |||||
|---|---|---|---|---|---|---|---|
| liver cells* | hepatocytes* | ||||||
| Gene symbol |
Pathway** | Fold change |
P value | Fold change |
P value | Fold change |
P value |
| Downregulated genes | |||||||
| Bik | c | −3.11 | 0.004 | ||||
| Birc5 | c | −5.17 | 0.001 | −6.24 | 0.001 | ||
| Bub1 | a | −4.78 | 0.001 | −3.93 | 0.003 | ||
| Casp1 | c | −2.12 | 0.007 | ||||
| Casp14 | c | −3.18 | 0.047 | ||||
| Ccna2 | a | −6.44 | 0.003 | −7.35 | 0.001 | ||
| Ccnb1 | a b | −5.27 | <0.001 | −6.71 | 0.008 | ||
| Ccnb2 | a b | −7.16 | <0.001 | −4.74 | 0.001 | ||
| Ccne1 | a b | −2.53 | 0.003 | −2.62 | 0.019 | ||
| Ccne2 | a b | −3.58 | 0.002 | −3.77 | <0.001 | ||
| Ccnf | −5.03 | 0.006 | |||||
| Cdc2 | a b | −6.07 | 0.001 | −12.90 | 0.004 | ||
| Cdc25b | a e | −4.25 | 0.004 | −3.37 | 0.042 | ||
| Cdc6 | a | −4.09 | 0.001 | −8.40 | 0.005 | ||
| Cdk2 | a b | −2.03 | 0.01 | ||||
| Cdkn2c | a | −4.61 | 0.001 | −5.03 | 0.006 | ||
| Cdkn2d | a | −2.07 | 0.002 | ||||
| Chek1 | a b c | −2.61 | 0.044 | −3.68 | 0.007 | ||
| E2f1 | a | −6.45 | 0.002 | −4.40 | 0.015 | ||
| Faslg | c e | −3.29 | 0.013 | ||||
| Id2 | d | −3.08 | 0.001 | −2.73 | 0.002 | ||
| Inhbe | d | −3.10 | <0.001 | −2.82 | 0.022 | ||
| Klf4 | −3.42 | 0.001 | −2.72 | 0.002 | |||
| Ksr1 | −2.90 | 0.008 | |||||
| Mad2l1 | a | −3.50 | <0.001 | −2.11 | 0.004 | ||
| Mcm2 | a | −3.06 | 0.005 | −2.99 | 0.007 | ||
| Mcm3 | a | −3.99 | 0.003 | −3.43 | 0.003 | ||
| Mcm4 | a | −2.87 | 0.003 | −2.53 | 0.001 | ||
| Mki67 | −2.95 | 0.021 | |||||
| Naip2 | c | −5.10 | 0.032 | ||||
| Nek2 | −5.76 | <0.001 | −6.60 | 0.029 | |||
| Pcna | a | −3.2 | 0.001 | −2.89 | 0.003 | ||
| Pkmyt1 | a | −2.51 | 0.002 | ||||
| Prom1 | −2.58 | 0.001 | |||||
| Rbl1 | a d | −2.76 | 0.004 | ||||
| Skp2 | a | −2.27 | 0.002 | ||||
| Smad9 | d | −2.90 | 0.011 | ||||
| Tnf | c d e | −2.76 | 0.001 | ||||
| Tnfrsf11b | c | −3.06 | 0.014 | ||||
| Tp73 | b | −3.78 | 0.004 | ||||
| Wee1 | a | −2.03 | 0.014 | −2.41 | 0.005 | ||
| Up regulated genes | |||||||
| Acvr1 | d | 2.76 | 0.009 | 2.35 | 0.004 | ||
| Bcl2a1d | c | 2.81 | 0.006 | 2.10 | 0.006 | ||
| Bhlhe40 | 2.72 | 0.007 | 2.58 | 0.003 | |||
| Bmp1 | 2.30 | 0.005 | |||||
| Cdkn2b | a d | 3.84 | 0.032 | 3.11 | 0.002 | ||
| Cish | g | 3.62 | 0.011 | ||||
| Col1a1 | 2.05 | 0.007 | |||||
| Fst | d | 5.04 | 0.013 | 5.16 | <0.001 | ||
| Inhba | d | 3.95 | 0.009 | ||||
| Inhbb | d | 9.70 | 0.017 | ||||
| Junb | 2.29 | 0.02 | |||||
| Map2k1 | e f | 2.03 | 0.027 | ||||
| Pdgfb | e | 22.20 | 0.024 | 13.48 | 0.018 | ||
| Pmaip1 | b c | 2.49 | 0.004 | 2.32 | 0.002 | ||
| Smad7 | d | 2.30 | <0.001 | 2.17 | 0.015 | ||
| Sphk1 | f | 4.02 | 0.004 | ||||
| Tgfb1 | a d e | 4.33 | 0.013 | 3.84 | 0.014 | ||
Each group consists of 3 independent experiments of cultured hepatic cells in absence or presence of 10 ng/mL activin A. In each group, changes are expressed as fold differences in mRNA expression in activin A-treated cell fractions vs. non-treated cells. Statistical analysis was performed using SA Biosciences Web-Based PCR Array Data Analysis tool (http://www.sabiosciences.com/pcrarraydataanalysis.php) with boundary and P value set at 2 and 0.05, respectively. The mean Ct number of 4 housekeeping genes (Rplp1, Rpl13a, Ldha, Actb) was used to normalize the gene expression levels of analyzed genes.
(a) Cell cycle, (b) p53 signaling pathway, (c) apoptosis, (d) TGF-β signaling pathway, (e) MAPK signaling pathway, (f) VEGF signaling pathway, (g) Jak-Stat signaling pathway.
Finally, we performed RT-PCR and immunohistochemistry to determine expression levels of activin receptors in FLSPC and adult hepatocytes. Expression of ActR-IIA, ActR-IIB, and ALK-4 was markedly reduced in fetal liver tissue (Fig. 4E) and in isolated FLSPC (Fig. 4F) compared to adult hepatocytes. Using highly enriched Dlk-1+ FLSPC vs. 2 mo old hepatocytes, we repeated RT-PCR analysis for ALK-4 mRNA and still observed very low receptor expression in both fetal liver cell fractions (Dlk-1−/Dlk-1+) (Supplementary Fig. 1B).
Gene expression in FLSPC derived cell clusters vs. surrounding host liver
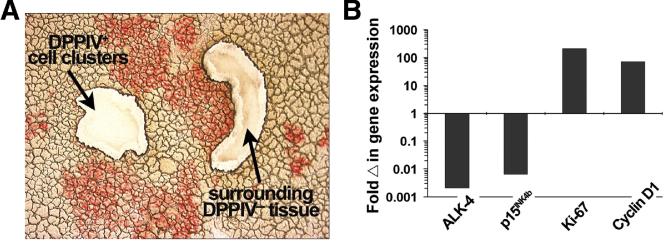
Using laser-captured tissue samples (Fig. 5A), we compared mRNA expression of selected genes relevant to activin A/p15INK4b signaling and cell cycling in transplanted cell clusters vs. surrounding host liver at 6 months after FLSPC cell transplantation into a 14 month old recipient. Transplanted FLSPC that had expanded into cell clusters during liver repopulation showed much lower levels of ALK-4 and p15INK4b and increased Ki-67 and cyclin D1 expression compared to surrounding host liver (Fig. 5B).
Figure 5.
Changes in gene expression between DPPIV+ cell clusters and surrounding DPPIV− host liver at 6 months after FLSPC transplantation into a 14 month old recipient. After DPPIV enzymehistochemistry, DPPIV+ cell clusters and surrounding DPPIV− host parenchyma were laser-captured (A; image for demonstration purposes) and qRT-PCR was performed using amplified RNA (B). Values are expressed as fold changes in DPPIV+ cell clusters vs. surrounding DPPIV− tissue.
Apoptosis in transplanted cell clusters and surrounding host parenchyma
We measured the apoptosis rates in both DPPIV+ repopulating clusters and surrounding DPPIV− host liver, using TUNEL assay, as reported previously (5). In 14 month old rats vs. 2 month old rats, we observed a 3-fold greater level of apoptosis in host cells surrounding transplanted cell clusters (Table 2). For specific examples of augmented apoptosis in aging host liver adjacent to FLSPC derived cell clusters, see Supplementary Fig. 3.
Table 2.
Apoptotic cells in DPPIV+ clusters vs. surrounding parenchyma in young vs. old rats 6 months after FLSPC transplantation.
| Age of recipients at the time of cell transplantation |
||
|---|---|---|
| 2 months | 14 months | |
| DPPIV− surrounding parenchyma | ||
| Total cell number analyzed | 23028 | 25489 |
| TUNEL+ cells | 56 | 178 |
| % | 0.23 ± 0.03A,B | 0.71 ± 0.05B,C |
| DPPIV+ clusters | ||
| Total cell number analyzed | 5149 | 13360 |
| TUNEL+ cells | 4 | 16 |
| % | 0.06 ± 0.3A | 0.13 ± 0.04C |
Using TUNEL assay for apoptosis in conjunction with DPPIV immunohistochemistry, 27 or 30 microscopic fields containing DPPIV+ cell clusters from liver sections of 4 or 3 animals at 6 months after fetal liver cell transplantation into 2 month or 14 month old recipients were examined and the percentage of apoptotic cells was determined.
P < 0.001 (Mann-Whitney Rank Sum test). Data are presented as mean ± SEM.
DISCUSSION
FLSPC are the only cells identified to date that efficiently repopulate the normal rat liver after their transplantation. Therefore, this model represents an excellent tool to study novel cell transplantation strategies and mechanisms necessary for successful tissue replacement. In the present study, we have made three major observations. First, the level of long-term tissue repopulation obtained in older rats transplanted with FLSPC is dramatically increased compared to that observed in younger rats. Second, several CKIs (most importantly p15INK4b) are elevated in aging rat liver. Third, activin A is significantly increased in aging rat liver and exerts differential effects on FLSPC and hepatocytes. Furthermore, we have obtained strong evidence that activin A is a key mediator leading to reduced liver regenerative capacity. Taken together, these observations provide important insights into the mechanism(s) through which FLSPC repopulate the recipient liver and more effectively repopulate the aging liver.
In previous studies, we obtained evidence that a proliferative differential exists between transplanted FLSPC and young host hepatic cells, causing replacement of host hepatocytes by FLSPC (5). Subsequently, Pasciu et al. showed that, in contrast to young recipients, transplanted hepatocytes in old recipients are capable of expanding and they suggested that this might be due to changes in the tissue microenvironment in older rats (28). In the present study, we observed that repopulation levels after FLSPC transplantation into older rats far exceed those obtained in younger rats and we have identified specific changes in the aging liver microenvironment that are responsible for increased repopulation by transplanted FLSPC.
During normal liver development, fetal liver cells undergo dramatic changes in their proliferative activity mediated by cell cycle regulators (29,30). A reduction in mRNA expression of several cdks and cyclins in 2 month old liver compared to fetal liver (Fig. 2A) indicates that hepatic cells have entered into a non-proliferative (quiescent) state in the young liver, which is defined by temporary arrest of cell proliferation (31). Since the percentage of epithelial liver cells capable of proliferating decreases from 79% in young rats to 30% in old rats (15), this suggests that hepatocytes become senescent, which represents a non-proliferative state characterized by permanent arrest of cell proliferation (32), altered responsiveness to apoptotic stimuli, morphologic transformation and altered protein expression (33,34). Telomere attrition has been identified as a critical factor in cellular senescence (35,36). Telomere-independent senescence has also been reported (17,34) and can be mediated by CKIs, especially p16INK4a and p21/CIP1, that have been shown to execute and maintain cell cycle arrest in senescent cells through the INK4a-RB and the ARF-p53 pathways (37).
In the present study, 3 genes of the CKI families (p15INK4b, p16INK4a, and p21/CIP1) are expressed at very low levels in ED14 fetal liver and increase in aging liver tissue. The continued increase of p15INK4b expression during liver aging (Fig. 3) results in accumulation of senescent cells in aging liver that can lead to progressively reduced regenerative capacity (38). Although p16INK4a expression was increased after birth compared to fetal liver, it showed only a modest increase at 19 months compared to 2 months (Fig. 3A), whereas increased repopulation by FLSPC occurred much earlier (i.e., in rats transplanted at 6 months of age). p21/CIP1 mRNA expression was elevated at 14 months; however, there was no additional increase between 14 and 19 months (Fig. 3A), and no difference was observed in p21/CIP1 expression with isolated hepatocytes from 20 vs. 2 month old rats (Fig. 3B). Since high expression of CKIs maintains cells in a non-proliferative state (31), our data suggest that p15INK4b is the major cell cycle regulator throughout the life span of rats.
Furthermore, activin A is rapidly upregulated in 2 month old compared to fetal liver tissue and increases progressively in aging liver. Activin A is involved in several physiological processes, including cell proliferation, differentiation, apoptosis, metabolism and homeostasis (21). In adult hepatocytes, activin A has been shown to induce growth arrest and block DNA synthesis (39,40). Our in vitro studies demonstrate 1) that FLSPC are resistant to growth-inhibitory effects of activin A, resulting from reduced expression of activin A receptors that are essential for activin A signaling (21). 2) Differentiated hepatocytes are strongly growth-inhibited by activin A through downregulation of cell cycle-related genes (Table 1), which is consistent with our finding in vivo that mRNAs for several cdks and cyclins in 2 month old liver are reduced compared to fetal liver, whereas activin A expression is increased. 3) Old hepatocytes are 3-fold less proliferative (data not shown) and more susceptible to induced growth-inhibition than young hepatocytes. This is due to augmented p15INK4b expression in aging hepatocytes (Fig. 3B), consistent with a report showing that p15(−/−) MEFs are more rapidly growing and more resistant to growth-inhibitory effects than wt MEFs (41). 4) Cell cycle arrest in aging hepatocytes, mediated by increased expression of p15INK4b, is induced by high concentrations of activin A, suggesting a new mechanism for induced senescence by a secreted protein in the liver (42). Taken together, these data suggest that resistance of fetal liver cells to activin A-induced growth inhibition leads to their ability to repopulate the young and more effectively repopulate the aging host liver, which is progressively impaired in regenerative capacity by augmented activin A expression.
Although we observed strong evidence that activin A is a key regulator in liver regenerative capacity, we cannot exclude the possibility that activin A-independent events in aging recipients contribute to repopulation by transplanted FLSPC. Iakova et al. showed that reduced proliferative activity in old animals is caused by a switch from C/EBPα mediated growth arrest to repression of E2F transcription and subsequent block of c-myc induction (43). Furthermore, reduced expression of FoxM1 contributes to diminished proliferation of aging hepatocytes associated with a decrease of several M-phase promoting genes (cyclins A2, B1, and B2, cdc2, cdc25b) (44). Since we observed significant downregulation of these cell cycle-related genes in activin A-treated hepatocytes (Table 1), we subsequently evaluated FoxM1 mRNA expression and observed a 3-fold decrease in activin A-treated hepatocytes (data not shown), suggesting that activin A is involved in FoxM1 gene regulation. Furthermore, additional studies are required to determine the role of senescent non-parenchymal cells in secreting degrative enzymes and growth factors, which could promote the proliferation of neighboring transplanted FLSPC (34). As we reported previously, cell competition between more highly proliferative FLSPC and less actively cycling hepatocytes is coupled with increased apoptosis in host cells (5). In the present study, we observed a 3-fold higher level of apoptosis in host cells surrounding transplanted cell clusters after FLSPC transplantation into older compared to younger recipients. Furthermore, there is strong evidence that activin A is capable of inducing apoptosis in hepatocytes (45,46) and we observed a strong downregulation of anti-apoptotic genes (Birc5, Chek1, Naip2) in hepatocytes cultured with activin A (Table 1).
In a recent review (8), it was stressed that cell competition is context-dependent, i.e., it depends on factors in a given tissue microenvironment that favor expansion of one cell population over another. In normal quiescent adult liver, most hepatocytes are maintained in a non-proliferative state (12,13). However, in response to a liver regenerative stimulus, hepatocytes enter the cell cycle, proliferate rapidly and restore liver mass. Once liver mass returns to normal, hepatocytes return to quiescence. Activin A is expressed in the normal adult liver and its expression undoubtedly helps to maintain hepatocytes in a quiescent state. However, when FLSPC are transplanted into the liver under conditions in which a regenerative stimulus is also present (e.g., PH), both the transplanted cells and host hepatocytes proliferate to restore hepatic mass. Once hepatic mass returns to normal, mature hepatocytes return to quiescence, but transplanted FLSPC continue to proliferate because, lacking expression of activin receptors, they are resistant to activin A mediated quiescence signaling. As activin A levels increase during normal liver aging in the rat, this creates a tissue microenvironment in which there is increased competition between transplanted FLSPC and host hepatocytes.
Observations made in the present study have broad therapeutic implications. Since life expectancy has increased significantly in recent years (47), end-stage liver diseases are becoming more prevalent in the elderly (48). Although, the observed increase in repopulation by FLSPC in aged rats in the present study needs to be reproduced in humans, the present work suggests that cell transplantation might be performed more successfully in elderly patients. In this regard, increased levels of activin A, as well as p15INK4b, have been reported in human liver diseases, e.g. liver fibrosis and cirrhosis, chronic viral hepatitis, acute liver failure, non-alcoholic fatty liver disease and hepatocellular carcinoma (49-54). Therefore, patients with liver diseases under appropriate circumstances might be very good candidates for therapeutic repopulation by transplanted cells based on the principles of cell competition.
Recent studies in both rodents and humans suggest that hepatic epithelial stem cells exist in adult liver (55,56). It would be interesting to determine whether these cells exhibit properties of fetal liver stem/progenitor cells regarding activin A/p15INK4b signaling and activin receptor expression and whether these cells also produce long-term liver repopulation after their transplantation. Ultimately to derive human iPS cells that express these same properties would represent a most significant advance in the use of human hepatic cells for therapeutic repopulation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Drs. Nicholas Baker (Dept. of Genetics) and Liang Zhu (Dept. of Developmental & Molecular Biology) for their critical reading of this manuscript and helpful comments and discussions.
Financial support: Research reported from the author’s laboratories was supported in part by NIH grants R01 DK17609 and P30 DK41296 to D.A.S. and AFAR Research Grant from the American Federation for Aging Research (AFAR) to M.O.
Footnotes
Disclosures: The authors indicate no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Perera MT, Mirza DF, Elias E. Liver transplantation: Issues for the next 20 years. J Gastroenterol Hepatol. 2009;24(Suppl 3):S124–131. doi: 10.1111/j.1440-1746.2009.06081.x. [DOI] [PubMed] [Google Scholar]
- 2.Tosh D, Strain A. Liver stem cells-prospects for clinical use. J Hepatol. 2005;42:S75–S84. doi: 10.1016/j.jhep.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Kawashita Y, Guha C, Yamanouchi K, et al. Liver repopulation: a new concept of hepatocyte transplantation. Surg Today. 2005;35:705–710. doi: 10.1007/s00595-005-3024-5. [DOI] [PubMed] [Google Scholar]
- 4.Sandhu JS, Petkov PM, Dabeva MD, et al. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am J Pathol. 2001;159:1323–1334. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oertel M, Menthena A, Dabeva MD, et al. Cell competition leads to high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130:507–520. doi: 10.1053/j.gastro.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 6.Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 7.de la Cova C, Abril M, Bellosta P, et al. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 8.Johnston LA. Competitive interactions between cells: death, growth, and geography. Science. 2009;324:1679–1682. doi: 10.1126/science.1163862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmucker DL. Age-related changes in liver structure and function: Implications for disease? Exp Gerontol. 2005;40:650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20:171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Bucher NRL, Malt RA. Regeneration of Liver and Kidney. Vol. 17. Little, Brown and Co.; Boston, MA: 1971. p. 17649. [Google Scholar]
- 12.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 13.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bucher NL, Swaffield MN, Ditroia JF. The influence of age upon the incorporation of thymidine-2-C14 into the DNA of regenerating rat liver. Cancer Res. 1964;24:509–512. [PubMed] [Google Scholar]
- 15.Stocker E, Heine WD. Regeneration of liver parenchyma under normal and pathological conditions. Beitr Pathol. 1971;144:400–408. [PubMed] [Google Scholar]
- 16.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 17.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 18.Zindy F, Quelle DE, Roussel MF, et al. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timchenko NA, Wilde M, Kosai KI, et al. Regenerating livers of old rats contain high levels of C/EBPalpha that correlate with altered expression of cell cycle associated proteins. Nucl Acids Res. 1998;26:3293–3299. doi: 10.1093/nar/26.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YG, Wang Q, Lin SL, et al. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (Maywood) 2006;231:534–544. doi: 10.1177/153537020623100507. [DOI] [PubMed] [Google Scholar]
- 22.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 23.Hannon GJ, Beach D. p15INK4b is a potential effector of TGFβ-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 24.Ho J, de Guise C, Kim C, et al. Activin induces hepatocyte cell growth arrest through induction of the cyclin-dependent kinase inhibitor p15INK4B and Sp1. Cell Signal. 2004;16:693–701. doi: 10.1016/j.cellsig.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Zauberman A, Oren M, Zipori D. Involvement of p21(WAF1/Cip1), CDK4 and Rb in activin A mediated signaling leading to hepatoma cell growth inhibition. Oncogene. 1997;15:1705–1711. doi: 10.1038/sj.onc.1201348. [DOI] [PubMed] [Google Scholar]
- 26.Reynisdóttir I, Polyak K, Iavarone A, et al. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 27.Oertel M, Menthena A, Chen Y-Q, et al. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for the normal adult rat liver. Gastroenterology. 2008;134:823–832. doi: 10.1053/j.gastro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Pasciu D, Montisci S, Greco M, et al. Aging is associated with increased clonogenic potential in rat liver in vivo. Aging Cell. 2006;5:373–377. doi: 10.1111/j.1474-9726.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 29.Awad MM, Gruppuso PA. Cell cycle control during liver development in the rat: evidence indicating a role for cyclin D1 posttranscriptional regulation. Cell Growth Differ. 2000;11:325–334. [PubMed] [Google Scholar]
- 30.Awad MM, Sanders JA, Gruppuso PA. A potential role for p15(Ink4b) and p57(Kip2) in liver development. FEBS Lett. 2000;483:160–164. doi: 10.1016/s0014-5793(00)02108-6. [DOI] [PubMed] [Google Scholar]
- 31.Pajalunga D, Mazzola A, Salzano AM, et al. Critical requirement for cell cycle inhibitors in sustaining nonproliferative states. J Cell Biol. 2007;176:807–818. doi: 10.1083/jcb.200608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 33.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Campisi J, d′Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 35.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 36.Aikata H, Takaishi H, Kawakami Y, et al. Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res. 2000;256:578–582. doi: 10.1006/excr.2000.4862. [DOI] [PubMed] [Google Scholar]
- 37.Herbig U, Sedivy JM. Regulation of growth arrest in senescence: telomere damage is not the end of the story. Mech Ageing Dev. 2006;127:16–24. doi: 10.1016/j.mad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Cánepa ET, Scassa ME, Ceruti JM, et al. INK4 proteins, a family of mammalian CDK inhibitors with novel biological functions. IUBMB Life. 2007;59:419–426. doi: 10.1080/15216540701488358. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda H, Mine T, Shibata H, et al. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Invest. 1993;92:1491–1496. doi: 10.1172/JCI116727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niimi S, Horikawa M, Seki T, et al. Effect of activins AB and B on DNA synthesis stimulated by epidermal growth factor in primary cultured rat hepatocytes. Biol Pharm Bull. 2002;25:437–440. doi: 10.1248/bpb.25.437. [DOI] [PubMed] [Google Scholar]
- 41.Koyama M, Matsuzaki Y, Yogosawa S, et al. ZD1839 induces p15INK4b and causes G1 arrest by inhibiting the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. Mol Cancer Ther. 2007;6:1579–1587. doi: 10.1158/1535-7163.MCT-06-0814. [DOI] [PubMed] [Google Scholar]
- 42.Ozturk M, Arslan-Ergul A, Bagislar S, et al. Senescence and immortality in hepatocellular carcinoma. Cancer Lett. 2009;286:103–113. doi: 10.1016/j.canlet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 43.Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495–506. doi: 10.1016/s0092-8674(03)00318-0. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Quail E, Hung NJ, et al. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci USA. 2001;98:11468–11473. doi: 10.1073/pnas.201360898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwall RH, Robbins K, Jardieu P, et al. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993;18:347–356. doi: 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- 46.Hully JR, Chang L, Schwall RH, et al. Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology. 1994;20:854–862. doi: 10.1002/hep.1840200413. [DOI] [PubMed] [Google Scholar]
- 47.Kung HC, Hoyert DL, Xu J, et al. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 48.Premoli A, Paschetta E, Hvalryg M, et al. Characteristics of liver diseases in the elderly: a review. Minerva Gastroenterol Dietol. 2009;55:71–78. [PubMed] [Google Scholar]
- 49.Funakoshi F, Masaki T, Kita Y, et al. Proliferative capability of hepatocytes and expression of G1-related cell cycle molecules in the development of liver cirrhosis in rats. Int J Mol Med. 2004;13:779–787. [PubMed] [Google Scholar]
- 50.Sugiyama M, Ichida T, Sato T, et al. Expression of activin A is increased in cirrhotic and fibrotic rat livers. Gastroenterology. 1998;114:550–558. doi: 10.1016/s0016-5085(98)70539-6. [DOI] [PubMed] [Google Scholar]
- 51.Yuen MF, Norris S, Evans LW, et al. Transforming growth factor-beta 1, activin and follistatin in patients with hepatocellular carcinoma and patients with alcoholic cirrhosis. Scand J Gastroenterol. 2002;37:233–238. doi: 10.1080/003655202753416939. [DOI] [PubMed] [Google Scholar]
- 52.Patella S, Phillips DJ, de Kretser DM, et al. Characterization of serum activin-A and follistatin and their relation to virological and histological determinants in chronic viral hepatitis. J Hepatol. 2001;34:576–583. doi: 10.1016/s0168-8278(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 53.Hughes RD, Evans LW. Activin A and follistatin in acute liver failure. Eur J Gastroenterol Hepatol. 2003;15:127–131. doi: 10.1097/00042737-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Yndestad A, Haukeland JW, Dahl TB, et al. A complex role of activin A in non-alcoholic fatty liver disease. Am J Gastroenterol. 2009;104:2196–2205. doi: 10.1038/ajg.2009.318. [DOI] [PubMed] [Google Scholar]
- 55.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 56.Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.