Abstract
BACKGROUND AND PURPOSE
Leucocyte infiltration is a rate-limiting step in the pathophysiology of acute pancreatitis (AP) although the adhesive mechanisms supporting leucocyte-endothelium interactions in the pancreas remain elusive. The aim of this study was to define the role of lymphocyte function antigen-1 (LFA-1) in regulating neutrophil-endothelium interactions and tissue damage in severe AP.
EXPERIMENTAL APPROACH
Pancreatitis was induced by retrograde infusion of sodium taurocholate into the pancreatic duct in mice. LFA-1 gene-targeted mice and an antibody directed against LFA-1 were used to define the role of LFA-1.
KEY RESULTS
Taurocholate challenge caused a clear-cut increase in serum amylase, neutrophil infiltration, CXCL2 (macrophage inflammatory protein-2) formation, trypsinogen activation and tissue damage in the pancreas. Inhibition of LFA-1 function markedly reduced taurocholate-induced amylase levels, accumulation of neutrophils, production of CXC chemokines and tissue damage in the pancreas. Notably, intravital microscopy revealed that inhibition of LFA-1 abolished taurocholate-induced leucocyte adhesion in postcapillary venules of the pancreas. In addition, pulmonary infiltration of neutrophils was attenuated by inhibition of LFA-1 in mice challenged with taurocholate. However, interference with LFA-1 had no effect on taurocholate-induced activation of trypsinogen in the pancreas.
CONCLUSIONS AND IMPLICATIONS
Our novel data suggest that LFA-1 plays a key role in regulating neutrophil recruitment, CXCL2 formation and tissue injury in the pancreas. Moreover, these results suggest that LFA-1-mediated inflammation is a downstream component of trypsinogen activation in the pathophysiology of AP. Thus, we conclude that targeting LFA-1 may be a useful approach to protect against pathological inflammation in the pancreas.
Keywords: adhesion, amylase, inflammation, leucocytes and pancreas
Introduction
The clinical presentation of acute pancreatitis (AP) ranges from a mild and local condition to a severe and systemic disease (Van Laethem et al., 1998). Despite significant investigative efforts specific therapy is not yet available and treatment of patients with AP is largely limited to supportive care, which is related to an incomplete understating of the basic pathophysiology. It is widely held that protease activation, inflammation and impaired microvascular perfusion are involved in the pathophysiology of pancreatitis (Wang et al., 2009; Zhang et al., 2009) although their interrelationships are not well understood. For example, it is not known whether activation of trypsinogen into trypsin in the pancreas is dependent on neutrophil infiltration in the pancreas or not.
Infiltration of neutrophils represents a hallmark in AP (Gukovskaya et al., 2002). Secreted chemokines coordinate leucocyte migration to sites of tissue damage. For example, CXCL2 (macrophage inflammatory protein-2) is a potent neutrophil attractant and one previous study reported that CXCL2 plays an important role in AP (Pastor et al., 2003). Moreover, CXCR2 is the main receptor of CXCL2 and it has been shown that inhibition of CXCR2 protects against AP (Bhatia and Hegde, 2007). In general, leucocyte extravasation is a multistep process including initial rolling along activated endothelial cells followed by firm adhesion and transmigration (Månsson et al., 2000; Riaz et al., 2002). The interactions between leucocytes and endothelium are mediated by specific adhesion molecules of the selectin and integrin families (Butcher, 1991). Numerous studies have shown that leucocyte rolling is supported by P-, E- and L-selectins (Thorlacius et al., 1994; Ridger et al., 2005). Stationary adhesion of leucocytes to the microvascular endothelium is mainly mediated by a group of heterodimeric molecules referred to as β2-integrins, such as lymphocyte function antigen-1 (LFA-1; CD11a/CD18), membrane-activated complex-1 (Mac-1; CD11b/CD18) and p150,95 (CD11c/CD18). The literature is rather complex and partly contradictory with respect to the function of individual β2-integrins in leucocyte adhesion, and the relative importance of specific β2-integrins appears to vary depending on the type of inflammatory stimulus and experimental model (Argenbright et al., 1991; Issekutz and Issekutz, 1992; Rutter et al., 1994; Issekutz, 1995). Notably, by use of LFA-1 gene-targeted mice, we and others have demonstrated that LFA-1 plays an important role in supporting firm leucocyte adhesion in striated muscles (Thorlacius et al., 2000; Dunne et al., 2002), peritoneum (Schmits et al., 1996; Lu et al., 1997), skin (Schramm et al., 2002), colon (Riaz et al., 2002) and liver (Li et al., 2004; Dold et al., 2008). Nonetheless, the importance of β2-integrins for leucocyte recruitment and tissue damage in the pancreas is not known.
Based on these considerations, the aim of this study was to examine the role of LFA-1 in regulating recruitment of neutrophils and tissue damage in the pancreas. In addition, we wanted to determine the impact of inhibiting pancreatic infiltration of neutrophils on protease activation in AP. For this purpose, we used a mouse model of AP based on retrograde infusion of taurocholate into the pancreatic duct.
Methods
Animals
All experiments were done in accordance with the legislation on the protection of animals and were approved by the Regional Ethical Committee for animal experimentation at Lund University, Sweden. Fifty C57BL/6 wild-type and 10 LFA-1 gene-targeted male mice weighing 20–26 g (6–8 weeks) were maintained in a climate-controlled room at 22°C and exposed to a 12:12-h light-dark cycle. Animals were fed standard laboratory diet and given water ad libitum. Mice were anaesthetized by i.p. administration of 7.5 mg of ketamine hydrochloride (Hoffman-La Roche, Basel, Switzerland) and 2.5 mg of xylazine (Janssen Pharmaceutica, Beerse, Belgium) per 100 g body weight in 200 µL saline.
Taurocholate-induced AP
Through a small (1–2 cm) upper midline incision, the second part of duodenum and papilla of Vater were identified. Traction sutures (7–0 prolene) were placed one cm from the papilla. Parallel to the papilla of Vater a small puncture was made through the duodenal wall with a 23 G needle. A non-radiopaque polyethylene catheter (ID 0.28 mm) connected to a micro infusion pump (CMA/100, Carnegie Medicin, Stockholm, Sweden) was inserted through the punctured hole in the duodenum and one mm into the common bile duct. The common hepatic duct was identified at the liver hilum and clamped with a neurobulldog clamp. Infusion of 10 µL of either 5% sodium taurocholate (Sigma-aldrich, USA) or 0.9% sodium chloride (n = 5) for 5 min was performed and after completion, the catheter was withdrawn and the common hepatic duct clamp was removed. The duodenal puncture was closed with a purse-string suture (7–0 monofilament). The traction sutures were removed and the abdomen was closed in two layers. Animals were allowed to wake up and were given free access to food and water. Sham operated animals underwent the same procedure without any infusion into the pancreas (n = 5). Control (5 µg·g−1, rat IgG2a, eBioscience, San Diego, CA, USA) antibody (n = 5) or purified anti-mouse LFA-1 antibody (5 µg·g−1, clone M17/4, rat IgG2a, n = 5, eBioscience, San Diego, CA, USA) was administered i.p. prior to bile duct cannulation. This dose and scheme of administration of the anti-mouse LFA-1 antibody was based on a previous investigation (Asaduzzaman et al., 2008). In addition, LFA-1 gene-deficient (n = 5) and wild-type (n = 5) mice were also challenged with 10 µL of 5% sodium taurocholate. All animals were killed 24 h after pancreatitis induction and assessed for all parameters included in this study. Blood was collected from the tail vein for systemic leucocyte differential counts. Blood samples were also collected from the inferior vena cava for determination of serum amylase levels and measurements of serum CXCL2. Pancreatic tissue was removed and kept in two pieces; one piece was snap frozen in liquid nitrogen for biochemical analysis of myeloperoxidase (MPO), CXCL2 and trypsinogen activation peptide (TAP) and the other piece was fixed in formalin for later histological analysis. Lung tissue was also harvested for MPO measurements.
Systemic leucocyte counts
Tail vein blood was mixed with Turks solution (0.2 mg gentian violet in 1 mL glacial acetic acid, 6.25% v/v) in a 1:20 dilution. Leucocytes were identified as monomorphonuclear and polymorphonuclear cells in a Burker chamber.
Serum amylase
Amylase was quantified in serum with a commercially available assay (Reflotron®, Roche Diagnostics GmbH, Mannheim, Germany).
MPO assay
Frozen pancreatic and lung tissue were pre-weighed and homogenized in 1-mL mixture (4:1) of PBS and aprotinin 10 000 KIE·mL−1 (Trasylol®, Bayer HealthCare AG, Leverkusen, Germany) for 1 min. The homogenate was centrifuged (153 39×g, 10 min) and the supernatant was stored at −20°C and the pellet was used for MPO assay as previously described (Laschke et al., 2007). In brief, the pellet was mixed with 1 mL of 0.5% hexadecyltrimethylammonium bromide. Next, the sample was frozen for 24 h and then thawed, sonicated for 90 s, put in a water bath 60°C for 2 h, after which the MPO activity of the supernatant was measured. The enzyme activity was determined spectrophotometrically as the MPO-catalysed change in absorbance in the redox reaction of H2O2 (450 nm, with a reference filter 540 nm, 25°C). Values are expressed as MPO units·g−1 tissue.
Flow cytometry
Blood was collected (1:10 acid citrate dextrose) from wild-type and LFA-1 gene-targeted mice. To block Fcg III/II receptors and reduce non-specific labelling samples were incubated with an anti-CD16/CD32 for 5 min. Then samples were stained with a PE-conjugated anti-Gr-1 (clone RB6-8C5, eBioscience, San Diego, CA, USA) antibody and with a FITC-conjugated anti-LFA-1 (clone 2D7, BD Biosciences Pharmingen, San Jose, CA) antibody at 4°C for 30 min. Erythrocytes were lysed and Cells were fixed. Cells were recovered following centrifugation before being analysed with a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA). A viable gate was used to exclude dead and fragmented cells. After gating the neutrophil population based on forward and side scatter characteristics, LFA-1 expression was determined on cells positive for Gr-1, which is a neutrophil marker.
CXCL2 levels
Tissue levels of CXCL2 were determined in serum and pancreatic tissue by using double-antibody Quantikine enzyme linked immunosorbent assay kits (R & D Systems Europe, Abingdon, UK) using recombinant murine CXCL2 as standard. The minimal detectable protein concentration is less than 0.5 pg·mL−1.
Histology
Pancreas samples were fixed in 4% formaldehyde phosphate buffer overnight and then dehydrated and paraffin embedded. Six micrometre sections were stained (haematoxylin and eosin) and examined by light microscopy. The severity of pancreatitis was evaluated in a blinded manner by use of a pre-existing scoring system including oedema, acinar cell necrosis, haemorrhage and neutrophil infiltrate on a 0 (absent) to four (extensive) scale as previously described in detail (Schmidt et al., 1992).
TAP levels
Trypsinogen is activated to trypsin in a reaction where TAP is cleaved off and thus can be used as marker of trypsinogen activation (Chen et al., 2003). The RIA was performed as described previously (Lindkvist et al., 2008). A 0.1 M Tris HCL buffer (pH 7.5) containing 0.15 M NaCl, 0.005 M EDTA and 2 g·L−1 bovine serum albumin (Sigma, St Louis, USA) was used as assay buffer. Samples of 100 µL diluted in assay buffer were incubated (16 h, 4°C) with 200 µL of I 125Tyr-TAP (=20 000 counts·min−1) in assay buffer and 200 µL of antiserum diluted 1/750 in assay buffer. Parallel incubations with the synthetic activation peptides TAP1 diluted in assay buffer in a series of concentrations from 0.078 to 20 nM, were used as standards in the assays. Free and bound radioactivities were separated by means of a second step antibody precipitation; 100 µL of a cellulose coupled anti-mouse IgG suspension (Sc-Cel® IDA, Boldon, England) was added to the samples. After 30 min of incubation 1 mL of water was added and tubes were centrifuged (704×g, 5 min, room temperature). The supernatant was decanted and radioactivity of the precipitate was counted in a γ-spectrophotometer (1 min).
Reverse-transcription polymerase chain reaction
Total RNA was extracted from the blood samples of the knockout mice and wild-type mice using RNeasy Mini-kit (Qiagen Gmbh, Hilden, Germany) and treated with RNease-free DNease (Amersham Pharmacia Biotech AB, Sollentuna, Sweden) to remove potential genomic DNA contaminants according to manufacturer's handbook. RNA concentrations were determined by measuring the absorbance at 260 nm spectrophotometrically. Reverse-transcription polymerase chain reaction (RT-PCR) was performed with Superscript One-Step RT-PCR system (Gibco BRL Life Technologies, Grand Islands, NY). The RT-PCR profile was one cycle of cDNA synthesis at 50°C for 30 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 10 min. After RT-PCR, aliquots of the RT-PCR products were separated on 2% agarose gel containing ethidium bromide and photographed. The primers sequences were as follows: CD11a (f) 5′-AGA TCG AGT CCG GAC CCA CAG-3′, CD11a (r) 5′-GGC AGT GAT AGA GGC CTC CCG-3′, β-actin (f) 5′-ATG TTT GAG ACC TTC AAC ACC-3′, β-actin (r) 5′-TCT CCA GGG AGG AAG AGG AT-3′. β-Actin served as a house keeping gene to control for the loading amount of cDNA.
Intravital microscopy
A 5-min equilibration time was allowed before analysis of leucocyte rolling and adhesion was performed in postcapillary venules (19–51 µm) in the pancreas. Contrast enhancement by i.v. injection of fluorescein isothiocyanate-labelled dextran 150 000 (0.05 mL, 5 mg·mL−1, Sigma Chemical Co.) and in vivo labelling of leucocytes with rhodamine 6 G (0.1 mL, 0.5 mg·mL−1, Sigma Chemical Co.) enabled analysis of leucocyte–endothelium interactions in the microvascular bed. For observations of the microcirculation, we used a modified Olympus microscope (BX50WI, Olympus Optical Co. GmbH, Hamburg, Germany) and recorded videos on a computer for later off-line analysis of leucocyte-endothelium interactions. Twenty-five C57BL/6 wild-type and five LFA-1 gene-targeted male mice were used, two to six postcapillary venules were evaluated in each animal and leucocyte rolling was measured by counting the number of cells rolling along the endothelial lining for 20 s and is expressed as cells·min−1. Leucocyte adhesion was measured by counting the number of cells that adhered and remained stationary for more than 30 s during the observation time and is expressed as cells·mm−2. Certain animals received an anti-P-selectin antibody (40 µg, i.v., clone RB40.34, BD Biosciences Pharmingen) immediately before capturing microphotographs of the postcapillary venules in the pancreas in order to abolish leucocyte rolling and thereby enable visualization of the remaining leucocytes that were firmly adherent to the endothelium.
Statistics
Data are presented as mean values ± SEM. Statistical evaluations were performed by using non-parametrical tests (Mann–Whitney). P < 0.05 was considered significant and n represents the number of animals.
Results
Role of LFA-1 in taurocholate-induced tissue damage in the pancreas
First, we examined LFA-1 expression at the mRNA and protein level in the LFA-1 gene-targeted mice used herein and found that these animals completely lacked LFA-1 (Figure S1). Retrograde infusion of sodium taurocholate into the pancreatic duct enhanced serum amylase levels by nearly 16-fold (Figure 1). Taurocholate-induced serum levels of amylase were reduced by more than 70% in LFA-1-deficient animals and in mice treated with an antibody against LFA-1 (Figure 1). Tissue damage was evaluated by quantification of acinar cell necrosis, oedema formation and haemorrhage in the pancreas. Taurocholate-induced acinar cell necrosis, oedema formation and interstitial haemorrhage were markedly attenuated in LFA-1 gene-targeted animals and in mice treated with the anti-LFA-1 antibody (Figure 2). Moreover, morphological examination of the pancreas revealed normal microstructure in control animals, whereas taurocholate challenge caused severe destruction of the pancreatic tissue structure characterized by extensive cell necrosis, oedema and massive infiltration of neutrophils (Figure 3). However, the structure of the pancreas was protected in LFA-1-deficent and in anti-LFA-1 antibody-treated mice challenged with taurocholate (Figure 3).
Figure 1.
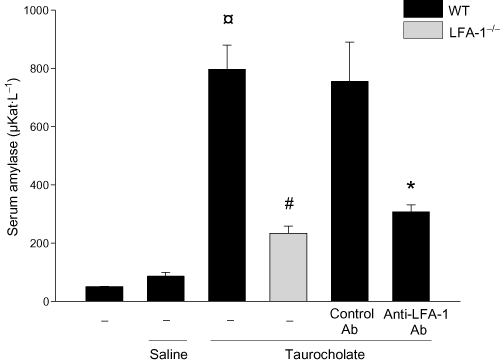
Serum amylase (µKat·L−1) in wild-type (WT) and lymphocyte function antigen-1 (LFA-1)-deficient mice. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. Control mice received saline alone. Certain mice received a control antibody (Ab) or an Ab against LFA-1 prior to pancreatitis induction. Blood samples were obtained 24 h after induction of pancreatitis. Data represent means ± SEM and n = 5. ¤P < 0.05 versus saline control, #P < 0.05 versus WT and *P < 0.05 versus control Ab.
Figure 2.
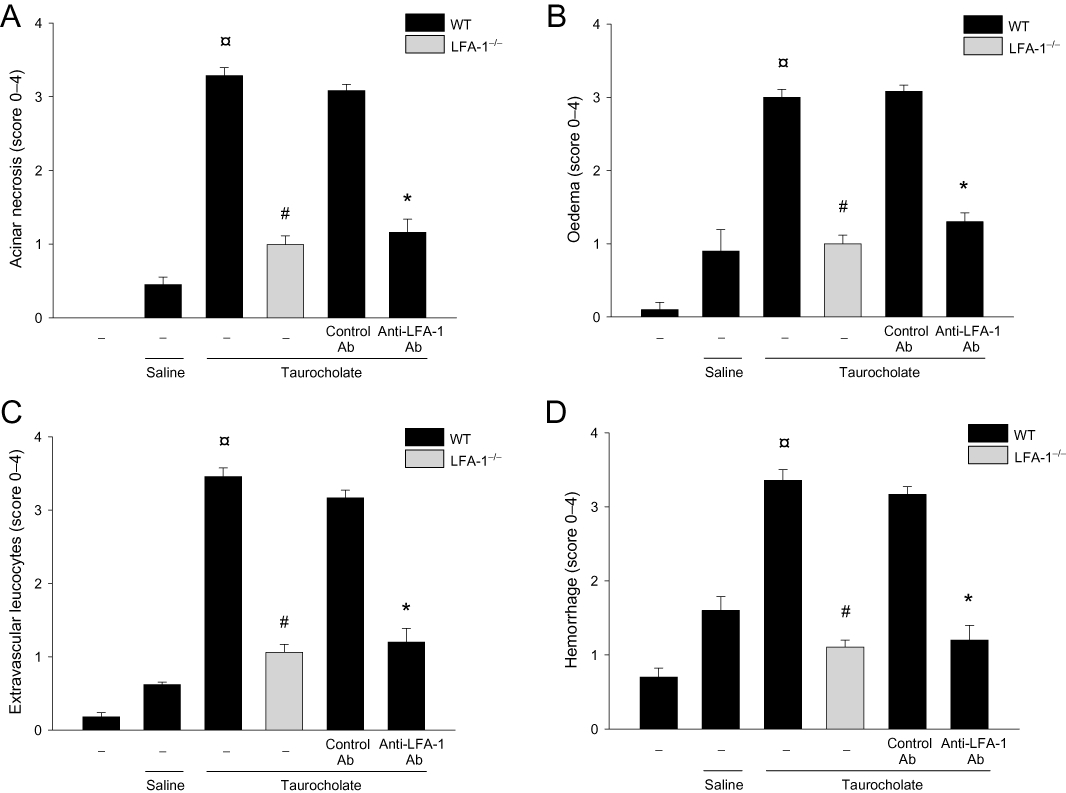
Taurocholate-induced tissue damage in the pancreas. (A) Acinar cell necrosis (B) oedema formation (C) infiltration of neutrophils in the pancreas and (D) haemorrhage. Control mice received saline alone. Certain mice received a control antibody (Ab) or an Ab against LFA-1 prior to pancreatitis induction. Blood samples were obtained 24 h after induction of pancreatitis. Data represent means ± SEM and n = 5. ¤P < 0.05 versus saline control, #P < 0.05 versus WT and *P < 0.05 versus control Ab.
Figure 3.
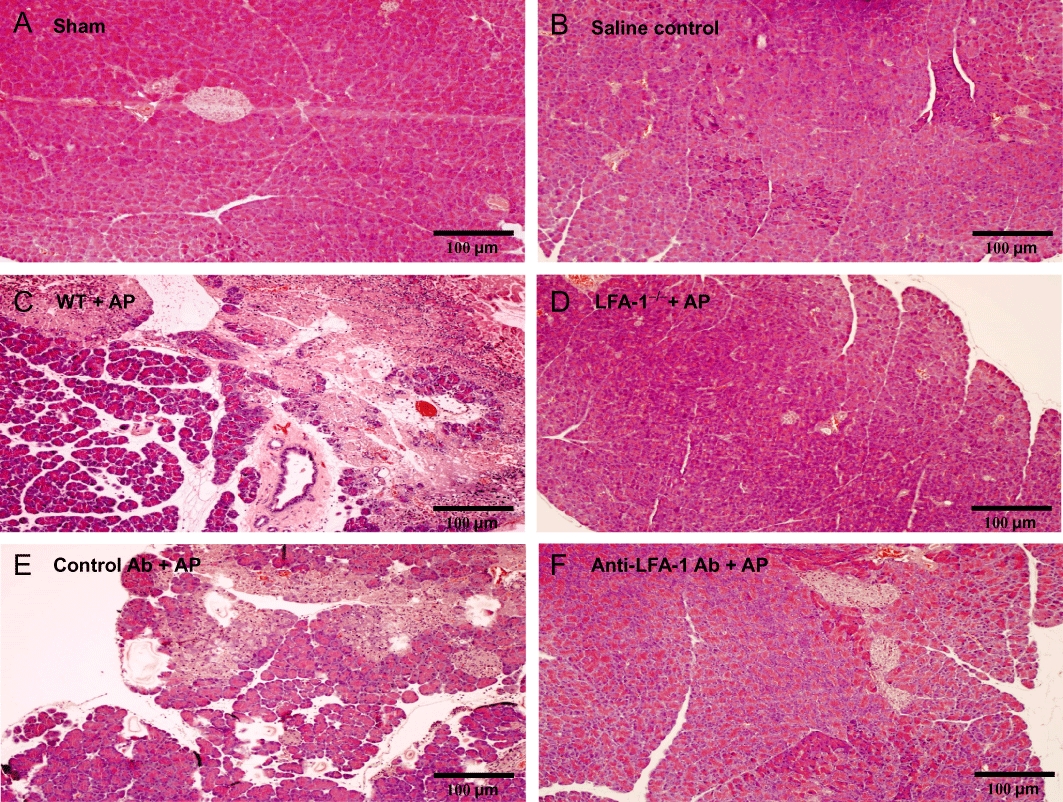
Representative haematoxylin & eosin sections of the pancreas from wild-type (WT) of (A) sham (B) saline infused to the pancreas (C) pancreatitis (D) lymphocyte function antigen-1 (LFA-1)-deficient mice with pancreatitis (E) control antibody (Ab) with pancreatitis and (F) anti-LFA-1 Ab with pancreatitis. Pancreatitis was induced by infusion sodium taurocholate into the pancreatic duct. Control mice received saline alone. Certain mice received a control Ab or an Ab against LFA-1 prior to pancreatitis induction. Samples were obtained 24 h after induction of pancreatitis. Bars represent 100 µm.
Role of LFA-1 in taurocholate-induced neutrophil recruitment in the pancreas
Levels of MPO, a neutrophil indicator, in the pancreas peaked 24 h after taurocholate challenge (21-fold increase). Pancreatic MPO activity was reduced by more than 80% in LFA-1 gene-targeted mice and in animals receiving the anti-LFA-1 antibody (Figure 4A). Similarly, histological quantification of taurocholate-provoked neutrophil infiltration revealed markedly reduced numbers of pancreatic neutrophils in LFA-1-deficient and in anti-LFA-1 antibody-treated mice (Figure 2C). Systemic inflammation such as pulmonary infiltration of neutrophils is a central feature of severe AP. Challenge with taurocholate provoked a clear-cut increase in MPO activity in the lung (Figure 4B). Taurocholate-induced pulmonary levels of MPO were markedly reduced in LFA-1-deficient animals and in mice treated with an antibody against LFA-1 (Figure 4B). We used intravital microscopy of the pancreatic microcirculation in order to study the role of LFA-1 in leucocyte-endothelium interactions in AP. Taurocholate challenge triggered a clear-cut increase in leucocyte-endothelium interactions in the pancreas (Figure 5 and Video S1A,B). It was found that taurocholate challenge increased leucocyte rolling and adhesion by threefold and sevenfold respectively, in postcapillary venules of the pancreas (Figure 5C,D). Notably administration of taurocholate did not enhance leucocyte interactions or trapping in the pancreatic capillaries (Video S2). Inhibition of LFA-1 function did not reduce taurocholate-induced leucocyte rolling (Figure 5C and Video S1B). In contrast, it was observed that taurocholate-induced leucocyte adhesion was decreased by 61% in animals treated with the anti-LFA-1 antibody (Figure 5D). Moreover, the number of firmly adherent leucocytes in taurocholate-treated mice deficient in LFA-1 was reduced by 75% (Figure 5D and Video S1B). We found also that the number of circulating mononuclear leucocytes and neutrophils increased in severe AP, indicating systemic activation in this model (Table 1).
Figure 4.
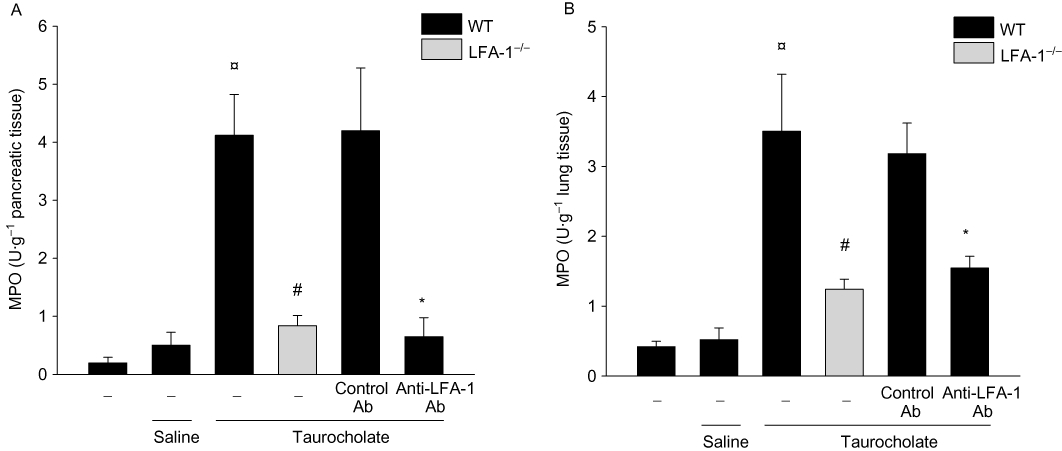
Myeloperoxidase (MPO) levels (U·g−1 tissue) for (A) pancreas and (B) lung in wild-type (WT) and lymphocyte function antigen-1 (LFA-1)-deficient mice. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. Control mice received saline alone. Certain mice received a control antibody (Ab) or an Ab against LFA-1 prior to pancreatitis induction. Samples were obtained 24 h after induction of pancreatitis. Data represent means ± SEM and n = 5. ¤P < 0.05 versus saline control, #P < 0.05 versus WT and *P < 0.05 versus control Ab.
Figure 5.
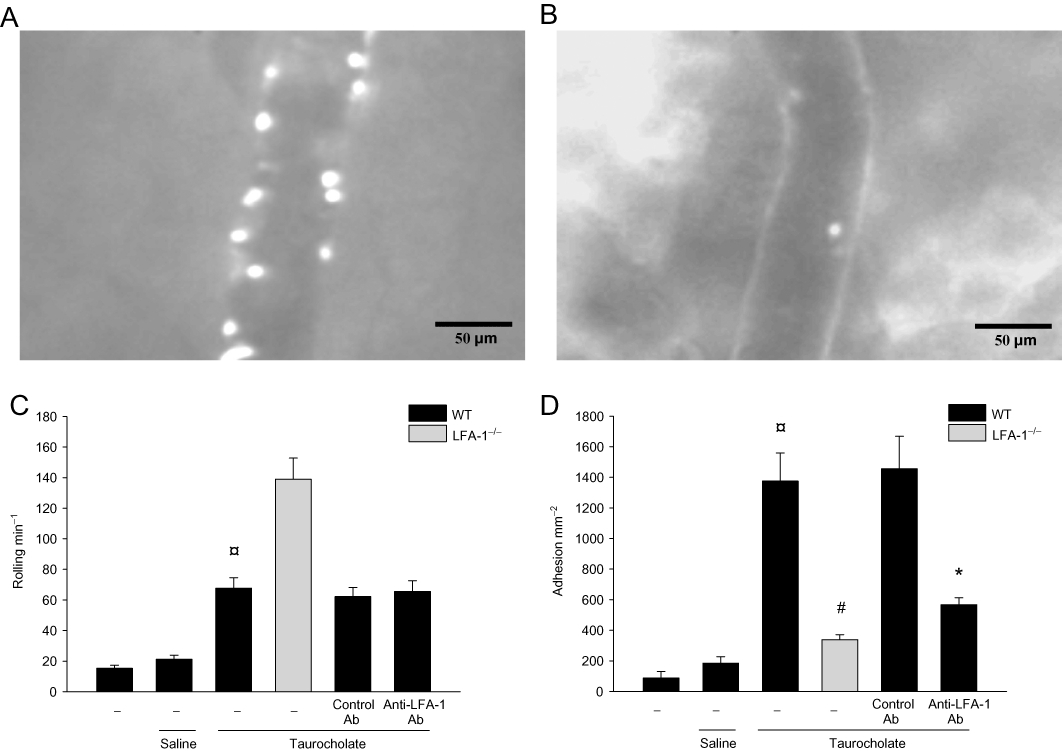
Leucocyte-endothelium interactions in the pancreas. Intravital photos of postcapillary venules in the pancreas after pancreatitis in (A) wild-type (WT) and (B) lymphocyte function antigen-1 (LFA-1)-deficient animals after i.v. administration of an anti-P-selectin antibody (40 µg). The anti-P-selectin antibody was given to abolish leucocyte rolling and leave the remaining firmly adherent leucocytes visible. Quantification of (C) leucocyte rolling (cells·min−1) and (D) adhesion (cells·mm−2) in WT and lymphocyte function antigen-1 (LFA-1)-deficient mice. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. Control mice received saline alone. Certain mice received a control antibody (Ab) or an Ab against LFA-1 prior to pancreatitis induction. Samples were obtained 24 h after induction of pancreatitis. Data represent means ± SEM and n = 5. ¤P < 0.05 versus saline control, #P < 0.05 versus WT and *P < 0.05 versus control Ab.
Table 1.
Systemic leucocyte differential counts
| PMNL | MNL | Total | |
|---|---|---|---|
| WT: sham | 0.8 ± 0.1 | 4.5 ± 0.1 | 5.3 ± 0.2 |
| WT: saline | 0.8 ± 0.1 | 4.6 ± 0.1 | 5.4 ± 0.2 |
| WT: taurocholate | 1.8 ± 0.2¤ | 6.7 ± 0.6¤ | 8.5 ± 0.8¤ |
| LFA-1-deficient: taurocholate | 3.9 ± 0.4 | 11.8 ± 0.3 | 15.7 ± 0.7 |
| WT + control Ab: taurocholate | 1.9 ± 0.1 | 5.9 ± 0.1 | 7.8 ± 0.2 |
| WT + anti-LFA-1 Ab: taurocholate | 1.3 ± 0.3 | 6.4 ± 0.1 | 7.7 ± 0.4 |
Blood samples were collected from wild-type (WT) and lymphocyte function antigen-1 (LFA-1) gene-targeted mice. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. Control mice received saline alone. Certain mice received a control antibody (Ab) or an Ab against LFA-1 prior to pancreatitis induction. Samples were obtained 24 h after induction of pancreatitis. Cells were identified as monomorphonuclear leucocytes (MNL) and polymorphonuclear leucocytes (PMNL). Data represent means ± SEM, 106 cells·mL−1 and n = 5.
P < 0.05 versus saline control.
Role of LFA-1 in taurocholate-induced chemokine formation in the pancreas and serum
At baseline levels of CXCL2 in the pancreas were 0.2 ng·pg−1. Administration of taurocholate caused a 13-fold and eightfold increase in the levels of CXCL2 in the pancreas (Figure 6A) and serum (Figure 6B) respectively. It was observed that immunoneutralization of LFA-1 decreased taurocholate-induced production of CXCL2 in the pancreas by 64% (Figure 6A). Also, we found that taurocholate-provoked formation in the serum was reduced by 63% in LFA-1-deficient animals (Figure 6B).
Figure 6.
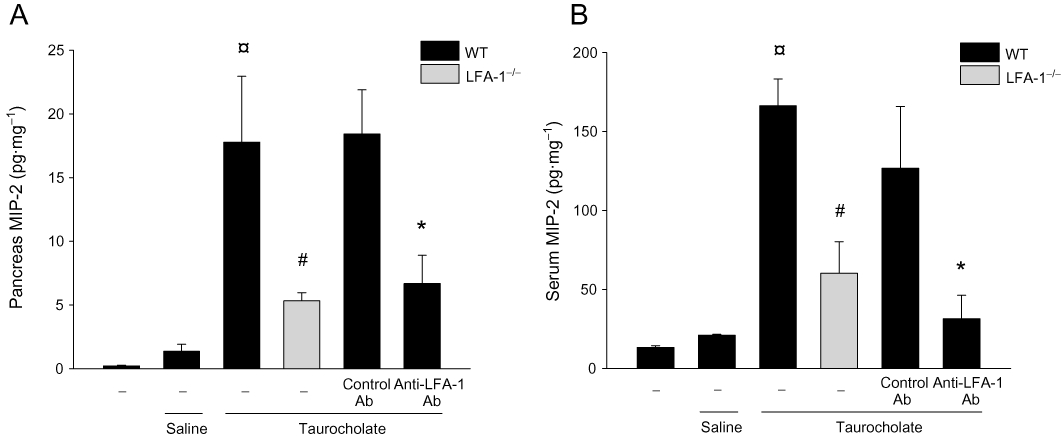
CXCL2 in the (A) pancreas and (B) serum in wild-type (WT) and lymphocyte function antigen-1 (LFA-1)-deficient mice. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. Control mice received saline alone. Certain mice received a control (Ab) or an Ab against LFA-1 prior to pancreatitis induction. Samples were obtained 24 h after induction of pancreatitis. Data represent means ± SEM and n = 5. ¤P < 0.05 versus saline control, #P < 0.05 versus WT and *P < 0.05 versus control Ab.
Role of LFA-1 in taurocholate-induced protease activation in the pancreas
Trypsinogen activation into trypsin was determined by measuring pancreatic levels of TAP. Taurocholate administration significantly enhanced trypsinogen activation reflected by a more than twofold increase in TAP levels in the pancreas (Figure 7). However, it was observed that taurocholate-induced activation of trypsinogen was not changed in LFA-1 gene-targeted animals (P > 0.05 vs. wild-type, Figure 7) or in mice treated with the anti-LFA-1 antibody (P > 0.05 vs. control antibody, Figure 7).
Figure 7.
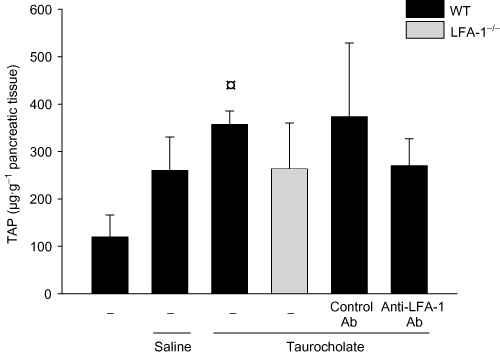
Trypsinogen activation peptide (TAP) levels (µg·g−1 pancreatic tissue) in wild-type (WT) and lymphocyte function antigen-1 (LFA-1)-deficient mice. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. Control mice received saline alone. Certain mice received a control antibody (Ab) or an Ab against LFA-1 prior to pancreatitis induction. Samples were obtained 24 h after induction of pancreatitis. Data represent means ± SEM and n = 5. ¤P < 0.05 versus saline control.
Discussion and conclusions
This study documents an important role of LFA-1 in AP. Our findings show that LFA-1 is a key regulator of neutrophil infiltration into the pancreas by regulating firm adhesion in postcapillary venules. Interference with LFA-1 not only decreased adhesion and recruitment of neutrophils but also protected against tissue damage in AP. However, these data show that trypsinogen activation into trypsin is not apparently dependent on LFA-1 in AP, suggesting that LFA-1-mediated inflammation is a downstream component of protease activation in the pathophysiology of AP. Taken together; these novel results indicate that targeting LFA-1 may be an effective approach to ameliorate pathological inflammation in AP.
It is well recognized that leucocyte recruitment is a fundamental feature in inflammatory diseases. Numerous mechanisms of neutrophil-mediated tissue injury have been forwarded. For example, neutrophils are potent producers of reactive oxygen species (ROS), such as hydroxyl radicals and superoxide, which can exert harmful effects on tissue and endothelial cells in the pancreas (Mossman, 2003). Indeed, several studies have demonstrated that depletion of neutrophils protects against tissue injury in AP (Frossard et al., 1999; Gukovskaya et al., 2002). LFA-1 has been shown to mediate neutrophil adhesion and tissue recruitment (Ding et al., 1999; Thorlacius et al., 2000) but the role of LFA-1 in AP is not known. Our data show that LFA-1-deficient mice exhibited significantly reduced acinar cell necrosis, tissue oedema and haemorrhage as well as serum amylase, indicating that LFA-1 plays an important role in mediating organ damage in AP. This notion was confirmed by our findings that immunoneutralization of LFA-1 markedly decreased taurocholate-induced pancreatic tissue destruction and serum amylase levels. Thus, these data suggest for the first time that LFA-1 is a key regulator of pancreatic injury in AP. This adds AP to the list of conditions in which LFA-1 has turned out to be a significant target; these include septic and cholestatic liver injury (Li et al., 2004; Dold et al., 2008), alcoholic liver disease (Ohki et al., 1998), viral hepatitis (Matsumoto et al., 2002), endotoxaemia (Li et al., 2004), graft-versus-host disease (Kimura et al., 1996; Sato et al., 2006) and colonic ischaemia-reperfusion (Wan et al., 2003). In this context, it should be mentioned that a previous study reported that depletion of neutrophils increases pancreatic haemorrhage in response to taurocholate challenge (Ryschich et al., 2009), suggesting that leucocytes protect against haemorrhage in AP. This is in contrast to previous studies showing that neutrophil depletion reduces tissue damage in AP (Weiss, 1989; Gukovskaya et al., 2002) and we have also recently depleted mice of neutrophils and found no signs of increased haemorrhage but instead a clear-cut decrease in taurocholate-induced haemorrhage in the pancreas, suggesting that neutrophils do not protect against tissue haemorrhage in AP (data not shown). In fact, this notion is also supported by our present findings showing that inhibition of neutrophil accumulation in the pancreas by targeting LFA-1 function also reduced taurocholate-induced haemorrhage in the pancreas.
The extravasation of leucocytes is a multistep process supported by a sequential engagement of adhesive receptors, such as selectins and integrins (Butcher, 1991). Although the function of these receptors has been extensively studied in certain organs, the role of specific adhesion molecules in pancreatic infiltration of leucocytes is virtually unknown. Two previous studies have reported that LFA-1 expression is increased on the surface of circulating neutrophils in pancreatitis (Sun et al., 2006; 2007;). We have extended these observations and demonstrated herein that genetic deficiency or functional inhibition of LFA-1 greatly reduces pancreatic infiltration of neutrophils, suggesting that LFA-1 mediate tissue accumulation of neutrophils in AP. This finding is also supported by a previous study showing that chemoattractant-induced leucocyte recruitment in the pancreas is mediated by LFA-1 (Ryschich et al., 2009). By use of intravital microscopy, we could document a direct and dominating role of LFA-1 in supporting firm adhesion of leucocytes in the postcapillary venules of the microcirculation in AP. Systemic depletion of neutrophils abolished leucocyte-endothelium interactions in the pancreas, suggesting that neutrophils constitute the main leucocyte subtype interacting with the microvascular endothelium in AP (not shown). Although our findings show that LFA-1 is the predominant adhesion molecule supporting pancreatic adhesion and infiltration of neutrophils, these data do not exclude the possibility that other β2-integrins may also be important in AP. For example, Hentzen et al. (2000) have shown that Mac-1 and LFA-1 cooperate for optimal recruitment of inflammatory cells, that is, LFA-1 initiates first stable contact and Mac-1 establishes a more sustainable adhesion onto the endothelium of inflamed organs. In this context, it is interesting to note that one previous study has reported that inhibition of LFA-1 decreases neutrophil formation of ROS in AP (Inoue et al., 1996). Thus, considered collectively, these data suggest that LFA-1 may be of importance at several steps in the pathophysiology of AP, including both tissue leucocyte recruitment and ROS-mediated organ damage. Activation and extravascular navigation of neutrophils are orchestrated by secreted CXC chemokines, such as CXCL2 (Bacon and Oppenheim, 1998). In the present study, we found that both pancreatic and systemic levels of CXCL2 were markedly enhanced after taurocholate challenge. Interestingly, taurocholate-induced formation of CXCL2 was significantly decreased in LFA-1-deficient animals. Similarly, inhibition of LFA-1 function also attenuated tissue formation of CXCL2 in AP. These observations are somewhat surprising considering that CXC chemokines are largely secreted by cells resident in the tissue of the pancreas (Bradley et al., 1999). Nonetheless, our findings indicate that LFA-1 exerts an early feature in the pathophysiology of pancreatitis upstream of CXC chemokine formation. Thus, our data suggest that LFA-1-mediated functions regulate subsequent formation of CXCL2 in AP. The link between LFA-1 function and CXCL2 production is speculative but may be related to pro-inflammatory compounds secreted from activated leucocytes, which in turn may activate tissue-resident cells in the pancreas. For example, LFA-1-depedent formation of ROS may be involved as ROS have been shown to have the capacity to stimulate chemokine formation (Riaz et al., 2003; Kina et al., 2009).
Inflammation and trypsinogen activation are recognized as central components in the pathophysiology of AP. However, the relationship between neutrophil recruitment on one hand and protease activation on the other hand in the pancreas is not known. Since we found that LFA-1 was an important regulator of pancreatic infiltration of neutrophils, we next asked whether LFA-1 controls activation of trypsinogen into trypsin, which is associated with the formation of TAP. Notably, a previous investigation has shown that levels of TAP correlate with disease severity in the early phases of AP (Frossard, 2001). Indeed, we observed that taurocholate challenge markedly increased the levels of TAP in the pancreas. However, taurocholate-induced levels of TAP were not altered in LFA-1 gene-targeted animals or in mice treated with a blocking antibody directed against LFA-1. These findings indicate that trypsinogen activation is independent of LFA-1-mediated neutrophil accumulation in the pancreas. Thus, pancreatic infiltration of neutrophils seems not to be a precondition for protease activation in AP. Whether neutrophils may exert intravascular functions, such as secretion of pro-inflammatory compounds, which may trigger intrapancreatic activation of trypsinogen cannot be excluded by our present findings. It should be noted that trypsin is a potent activator of proteinase-activated receptor-2 (PAR2), which is a 7-transmembrane G-protein-coupled receptor expressed by pancreatic acinar and ductal cells (Nguyen et al., 1999). A recent study reported that taurocholate-triggered calcium transients, kinase activation and acinar cell injury is markedly reduced in isolated pancreatic acini from PAR2 gene-deficient mice, suggesting that PAR2 activation may support acinar cell damage in AP (Laukkarinen et al., 2008). Whether trypsin-mediated activation of PAR2 may explain LFA-1-independent effects, such as trypsinogen activation, in the present study is a matter of future studies. Nonetheless, considering that activation of trypsinogen seems to be an early process, neutrophil recruitment and inflammation in the pancreas persists longer and targeting LFA-1 might be a more favourable strategy for specific therapeutic interventions (Regnér et al., 2008).
In conclusion, our novel data demonstrate not only that neutrophil adhesion and infiltration in AP are mediated by LFA-1 but also that LFA-1-dependant recruitment of neutrophils regulates tissue damage in the pancreas. In addition, these findings also indicate that trypsinogen activation is independent of LFA-1-mediated neutrophil accumulation in the pancreas. Taken together, we conclude that LFA-1 may be a useful target to antagonize pathological inflammation in AP.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (2009-4872), Crafoordska stiftelsen, Einar och Inga Nilssons stiftelse, Harald och Greta Jaenssons stiftelse, Greta och Johan Kocks stiftelser, Fröken Agnes Nilssons stiftelse, Franke och Margareta Bergqvists stiftelse för främjande av cancerforskning, Magnus Bergvalls stiftelse, Lundgrens stiftelse, Mossfelts stiftelse, Nanna Svartz stiftelse, Ruth och Richard Julins stiftelse, Svenska Läkaresällskapet, Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder, Malmö University Hospital and Lund University. Awla D and Abdulla A are supported by a fellowship from the Kurdistan regional government and the Nanakali group.
Glossary
Abbreviations
- AP
acute pancreatitis
- MNL
monomorphonuclear leucocytes
- MPO
myeloperoxidase
- PMNL
polymorphonuclear leucocytes
- RIA
radioimmunoassay
- ROS
reactive oxygen species
- TAP
trypsinogen activation peptide
Conflict of interest
The authors state no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 (A) mRNA and (B) protein expression of CD11a mRNA in peripheral neutrophils from wild-type (WT) and lymphocyte function antigen-1-deficient (LFA-1−/−) mice. β-Actin serves as a housekeeping gene. The results presented are from one experiment, which is representative of four others.
Video S1 Intravital fluorescence clip of a postcapillary venule in a) wild-type and b) lymphocyte function antigen-l-deficient mouse. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. The video was recorded 24 h after induction of pancreatitis.
Video S2 Intravital fluorescence clip of capillaries in a wild-type mouse. Pancreatitis was induced by infusion of sodium taurocholate into the pancreatic duct. The video was recorded 24 h after induction of pancreatitis.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Argenbright LW, Letts LG, Rothlein R. Monoclonal antibodies to the leukocyte membrane CD18 glycoprotein complex and to intercellular adhesion molecule-1 inhibit leukocyte-endothelial adhesion in rabbits. J Leukoc Biol. 1991;49:253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- Asaduzzaman M, Zhang S, Lavasani S, Wang Y, Thorlacius H. LFA-1 and MAC-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock. 2008;30:254–259. doi: 10.1097/shk.0b013e318162c567. [DOI] [PubMed] [Google Scholar]
- Bacon KB, Oppenheim JJ. Chemokines in disease models and pathogenesis. Cytokine Growth Factor Rev. 1998;9:167–173. doi: 10.1016/s1359-6101(98)00005-7. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Hegde A. Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul Pept. 2007;138:40–48. doi: 10.1016/j.regpep.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Bradley LM, Asensio VC, Schioetz LK, Harbertson J, Krahl T, Patstone G, et al. Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J Immunol. 1999;162:2511–2520. [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Chen JM, Kukor Z, Le Marechal C, Toth M, Tsakiris L, Raguenes O, et al. Evolution of trypsinogen activation peptides. Mol Biol Evol. 2003;20:1767–1777. doi: 10.1093/molbev/msg183. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- Dold S, Laschke MW, Lavasani S, Menger MD, Thorlacius H. Cholestatic liver damage is mediated by lymphocyte function antigen-1-dependent recruitment of leukocytes. Surgery. 2008;144:385–393. doi: 10.1016/j.surg.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- Frossard JL. Trypsin activation peptide (TAP) in acute pancreatitis: from pathophysiology to clinical usefulness. JOP. 2001;2:69–77. [PubMed] [Google Scholar]
- Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, et al. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- Hentzen ER, Neelamegham S, Kansas GS, Benanti JA, McIntire LV, Smith CW, et al. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood. 2000;95:911–920. [PubMed] [Google Scholar]
- Inoue S, Nakao A, Kishimoto W, Murakami H, Harada A, Nonami T, et al. LFA-1 (CD11a/CD18) and ICAM-1 (CD54) antibodies attenuate superoxide anion release from polymorphonuclear leukocytes in rats with experimental acute pancreatitis. Pancreas. 1996;12:183–188. doi: 10.1097/00006676-199603000-00013. [DOI] [PubMed] [Google Scholar]
- Issekutz TB. In vivo blood monocyte migration to acute inflammatory reactions, IL-1 alpha, TNF-alpha, IFN-gamma, and C5a utilizes LFA-1, Mac-1, and VLA-4. The relative importance of each integrin. J Immunol. 1995;154:6533–6540. [PubMed] [Google Scholar]
- Issekutz AC, Issekutz TB. The contribution of LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18) to the in vivo migration of polymorphonuclear leucocytes to inflammatory reactions in the rat. Immunology. 1992;76:655–661. [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Suzuki K, Inada S, Hayashi A, Isobe M, Matsuzaki Y, et al. Monoclonal antibody against lymphocyte function-associated antigen 1 inhibits the formation of primary biliary cirrhosis-like lesions induced by murine graft-versus-host reaction. Hepatology. 1996;24:888–894. doi: 10.1053/jhep.1996.v24.pm0008855193. [DOI] [PubMed] [Google Scholar]
- Kina S, Nakasone T, Takemoto H, Matayoshi A, Makishi S, Sunagawa N, et al. Regulation of chemokine production via oxidative pathway in HeLa cells. Mediators Inflamm. 2009;2009:183760. doi: 10.1155/2009/183760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschke MW, Menger MD, Wang Y, Lindell G, Jeppsson B, Thorlacius H. Sepsis-associated cholestasis is critically dependent on P-selectin-dependent leukocyte recruitment in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1396–G1402. doi: 10.1152/ajpgi.00539.2006. [DOI] [PubMed] [Google Scholar]
- Laukkarinen JM, Weiss ER, van Acker GJ, Steer ML, Perides G. Protease-activated receptor-2 exerts contrasting model-specific effects on acute experimental pancreatitis. J Biol Chem. 2008;283:20703–20712. doi: 10.1074/jbc.M801779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Klintman D, Weitz-Schmidt G, Schramm R, Thorlacius H. Lymphocyte function antigen-1 mediates leukocyte adhesion and subsequent liver damage in endotoxemic mice. Br J Pharmacol. 2004;141:709–716. doi: 10.1038/sj.bjp.0705634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindkvist B, Wierup N, Sundler F, Borgstrom A. Long-term nicotine exposure causes increased concentrations of trypsinogens and amylase in pancreatic extracts in the rat. Pancreas. 2008;37:288–294. doi: 10.1097/MPA.0b013e31816a7744. [DOI] [PubMed] [Google Scholar]
- Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, et al. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson P, Zhang XW, Jeppsson B, Johnell O, Thorlacius H. Critical role of P-selectin-dependent rolling in tumor necrosis factor-alpha-induced leukocyte adhesion and extravascular recruitment in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:190–196. doi: 10.1007/s002100000268. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Tsunematsu S, Tsukinoki K, Ohmi Y, Iwamiya M, Oliveira-dos-Santos A, et al. Essential role of the adhesion receptor LFA-1 for T cell-dependent fulminant hepatitis. J Immunol. 2002;169:7087–7096. doi: 10.4049/jimmunol.169.12.7087. [DOI] [PubMed] [Google Scholar]
- Mossman BT. Introduction to serial reviews on the role of reactive oxygen and nitrogen species (ROS/RNS) in lung injury and diseases. Free Radic Biol Med. 2003;34:1115–1116. doi: 10.1016/s0891-5849(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Moody MW, Steinhoff M, Okolo C, Koh DS, Bunnett NW. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J Clin Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki E, Kato S, Ohgo H, Mizukami T, Fukuda M, Tamai H, et al. Effect of chronic ethanol feeding on endotoxin-induced hepatic injury: role of adhesion molecules on leukocytes and hepatic sinusoid. Alcohol Clin Exp Res. 1998;22(Suppl):129S–132S. doi: 10.1111/acer.1998.22.s3_part1.129s. [DOI] [PubMed] [Google Scholar]
- Pastor CM, Rubbia-Brandt L, Hadengue A, Jordan M, Morel P, Frossard JL. Role of macrophage inflammatory peptide-2 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Lab Invest. 2003;83:471–478. doi: 10.1097/01.lab.0000063928.91314.9f. [DOI] [PubMed] [Google Scholar]
- Regnér S, Manjer J, Appelros S, Hjalmarsson C, Sadic J, Borgstrom A. Protease activation, pancreatic leakage, and inflammation in acute pancreatitis: differences between mild and severe cases and changes over the first three days. Pancreatology. 2008;8:600–607. doi: 10.1159/000161011. [DOI] [PubMed] [Google Scholar]
- Riaz AA, Wan MX, Schaefer T, Schramm R, Ekberg H, Menger MD. Fundamental and distinct roles of P-selectin and LFA-1 in ischemia/reperfusion-induced leukocyte-endothelium interactions in the mouse colon. Ann Surg. 2002;236:777–784. doi: 10.1097/00000658-200212000-00010. discussion 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz AA, Schramm R, Sato T, Menger MD, Jeppsson B, Thorlacius H. Oxygen radical-dependent expression of CXC chemokines regulate ischemia/reperfusion-induced leukocyte adhesion in the mouse colon. Free Radic Biol Med. 2003;35:782–789. doi: 10.1016/s0891-5849(03)00405-2. [DOI] [PubMed] [Google Scholar]
- Ridger VC, Hellewell PG, Norman KE. L- and P-selectins collaborate to support leukocyte rolling in vivo when high-affinity P-selectin-P-selectin glycoprotein ligand-1 interaction is inhibited. Am J Pathol. 2005;166:945–952. doi: 10.1016/S0002-9440(10)62314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J, James TJ, Howat D, Shock A, Andrew D, De Baetselier P, et al. The in vivo and in vitro effects of antibodies against rabbit beta 2-integrins. J Immunol. 1994;153:3724–3733. [PubMed] [Google Scholar]
- Ryschich E, Kerkadze V, Deduchovas O, Salnikova O, Parseliunas A, Märten A, et al. Intracapillary leukocyte accumulation as a novel antihemorrhagic mechanism in acute pancreatitis in mice. Gut. 2009;58:1508–1516. doi: 10.1136/gut.2008.170001. [DOI] [PubMed] [Google Scholar]
- Sato T, Habtezion A, Beilhack A, Schulz S, Butcher E, Thorlacius H. Short-term homing assay reveals a critical role for lymphocyte function-associated antigen-1 in the hepatic recruitment of lymphocytes in graft-versus-host disease. J Hepatol. 2006;44:1132–1140. doi: 10.1016/j.jhep.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmits R, Kundig TM, Baker DM, Shumaker G, Simard JJ, Duncan G, et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm R, Schaefer T, Menger MD, Thorlacius H. Acute mast cell-dependent neutrophil recruitment in the skin is mediated by KC and LFA-1: inhibitory mechanisms of dexamethasone. J Leukoc Biol. 2002;72:1122–1132. [PubMed] [Google Scholar]
- Sun W, Watanabe Y, Wang ZQ. Expression and significance of ICAM-1 and its counter receptors LFA-1 and Mac-1 in experimental acute pancreatitis of rats. World J Gastroenterol. 2006;12:5005–5009. doi: 10.3748/wjg.v12.i31.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Watanabe Y, Toki A, Wang ZQ. Beneficial effects of hydrocortisone in induced acute pancreatitis of rats. Chin Med J (Engl) 2007;120:1757–1761. [PubMed] [Google Scholar]
- Thorlacius H, Raud J, Rosengren-Beezley S, Forrest MJ, Hedqvist P, Lindbom L. Mast cell activation induces P-selectin-dependent leukocyte rolling and adhesion in postcapillary venules in vivo. Biochem Biophys Res Commun. 1994;203:1043–1049. doi: 10.1006/bbrc.1994.2287. [DOI] [PubMed] [Google Scholar]
- Thorlacius H, Vollmar B, Guo Y, Mak TW, Pfreundschuh MM, Menger MD, et al. Lymphocyte function antigen 1 (LFA-1) mediates early tumour necrosis factor alpha-induced leucocyte adhesion in venules. Br J Haematol. 2000;110:424–429. doi: 10.1046/j.1365-2141.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- Van Laethem JL, Eskinazi R, Louis H, Rickaert F, Robberecht P, Deviere J. Multisystemic production of interleukin 10 limits the severity of acute pancreatitis in mice. Gut. 1998;43:408–413. doi: 10.1136/gut.43.3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MX, Schramm R, Klintman D, Welzenbach K, Weitz-Schmidt G, Thorlacius H. A statin-based inhibitor of lymphocyte function antigen-1 protects against ischemia/reperfusion-induced leukocyte adhesion in the colon. Br J Pharmacol. 2003;140:395–401. doi: 10.1038/sj.bjp.0705432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15:1427–1430. doi: 10.3748/wjg.15.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Li ZJ, Zhang J. Inflammatory mediators and microcirculatory disturbance in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:351–357. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


