Summary
Serotonin (5-HT) and leptin play important roles in the modulation of energy balance. Here we investigated mechanisms by which leptin might interact with CNS 5-HT pathways to influence appetite. Although some leptin receptor (LepRb) neurons lie close to 5-HT neurons in the dorsal raphe (DR), 5-HT neurons do not express LepRb. Indeed, while leptin hyperpolarizes some non-5-HT DR neurons, leptin does not alter the activity of DR 5-HT neurons. Furthermore, 5-HT depletion does not impair the anorectic effects of leptin. The serotonin transporter-cre allele (Sertcre) is expressed in 5-HT (and developmentally in some non-5-HT) neurons. While Sertcre promotes LepRb excision in a few LepRb neurons in the hypothalamus, it is not active in DR LepRb neurons, and neuron-specific Sertcre-mediated LepRb inactivation in mice does not alter body weight or adiposity. Thus, leptin does not directly influence 5-HT neurons and does not meaningfully modulate important appetite-related determinants via 5-HT neuron function.
Keywords: serotonin, leptin, food intake, PCPA, Sert-Cre, electrophysiology, raphe
Introduction
Leptin, a hormone secreted by adipocytes in proportion to fat mass, acts via the long form of the leptin receptor (LepRb) to regulate energy homeostasis (Myers et al., 2009). Neuronal specific deletion of LepR promotes hyperphagia and obesity (Cohen et al., 2001), and restoration of neuronal LepRb in LepR-deficient mice normalizes food intake and body weight (de Luca et al., 2005), indicating that leptin’s effects on energy balance are achieved via brain LepRb.
Within the brain, multiple populations of LepRb-expressing neurons that contribute to energy homeostasis have been identified, including (i) neurons of the hypothalamic arcuate nucleus (ARC) that express pro-opiomelanocortin (POMC) or agouti-related peptide (AgRP) (Balthasar et al., 2004; van de Wall et al., 2008), (ii) SF1-expressing neurons of the ventromedial nucleus of the hypothalamus (Dhillon et al., 2006), (iii) dopaminergic neurons in the ventral tegmental area (Fulton et al., 2006; Hommel et al., 2006), (iv) GABAergic neurons of the lateral hypothalamic area (LHA) (Leinninger et al., 2009), and (v) neurons within the nucleus of the solitary tract (Hayes et al., 2009), among others (Myers et al., 2009; Patterson et al., 2011; Scott et al., 2009).
Leptin also acts in concert with the brain serotonin (5-HT) pathways, although important discrepancies exist regarding the mechanisms underlying this interaction. A variety of data suggest that leptin and 5-HT function independently, but converge in the ARC, although recent publications report a role for direct leptin action via brain 5-HT neurons (Calapai et al., 1999; Harris et al., 1998; von Meyenburg et al., 2003; Yadav et al., 2009; Yadav et al., 2011; Yamada et al., 2003). We thus generated and utilized several novel mouse lines to resolve these issues and clarify the potential role for 5-HT neurons in leptin action.
Results and Discussion
Distinct localization of LepRb and 5-HT in CNS
Analysis using a LepRb riboprobe (Elmquist et al., 1998) revealed that, with the exception of the dorsal raphe nucleus (DR), brain regions containing 5-HT do not express Leprb mRNA in the rat (Figure S1A–F’) or mouse (Figure S1G–L’). Similarly, utilizing LepRbEGFP mice (Patterson et al., 2011), we observed no co-localization of LepRb with 5-HT in the DR (Figures 1A, 1A’, and S1). In contrast, most DR neurons containing calbindin (Calb), a marker of interneurons, expressed LepRb (Figures 1B and 1B’); and, as previously reported, a small population of DR dopamine (DA) neurons also expressed LepRb (Figure S1M and S1M’) (Scott et al., 2009).
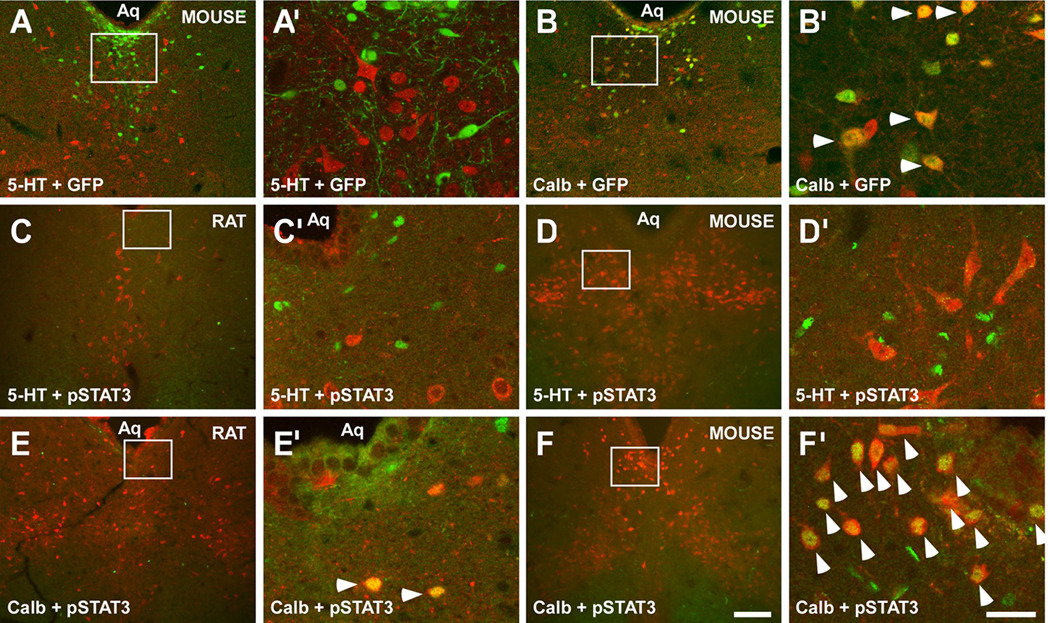
Figure 1. Brain LepRb neurons do not contain 5-HT and leptin treatment does not induce LepRb signaling (pSTAT3) in 5-HT neurons.
(A) LepRb (green), as detected by GFP-IR, was not colocalized with 5-HT (red) in the DR of LepRbEGFP mice. In contrast, DR LepRb neurons (green) also expressed Calb-IR (red in (B)) in LepRbEGFP mice. Accordingly, leptin-induced pSTAT3 (green) was not detected in DR 5-HT neurons (red) in rats (C) treated with 1 mg/kg leptin i.v., or wild-type mice (D) treated with 3 mg/kg leptin i.p. Leptin-induced pSTAT3 was present in calbindin neurons (red) in rats (E) and wild-type mice (F). (A’–F’) are magnifications of the boxed areas in (A–F) respectively. Scale bar in (F) = 200 µm also applies to (A–E). Scale bar in (F’) = 50 µm also applies to (A’–E’). Arrowheads indicate dual-labeled cells. See also Figure S1.
We also assayed leptin-induced STAT3 phosphorylation (pSTAT3), which is directly mediated by activated LepRb, to examine direct leptin action in the midbrain. Leptin-induced pSTAT3 was absent from all 5-HT containing sites, with the exception of the DR, in the rat (Figure S1P–V’) and mouse (Figure S1W-AC’). Within the DR, leptin-induced pSTAT3 was absent from all 5-HT neurons in the rat (Figures 1C, 1C’, and S1P–V’) and mouse (Figures 1D, 1D’, and S1W-AC’). In contrast, most DR Calb neurons in the rat (Figures 1E and 1E’) and mouse (Figures 1F and 1F’) contained leptin-induced pSTAT3, as did a subpopulation of DR DA neurons in the rat (Figure S1N and S1N’) and mouse (Figure S1O and S1O’). Thus, although some Calb- and DA-expressing DR neurons contain functional LepRb, CNS 5-HT neurons do not.
Leptin does not alter the activity of DR 5-HT neurons
We also examined the ability of leptin to influence the membrane potential of identified DR 5-HT neurons in acute slice preparations from wild-type mice. We recorded from 26 DR neurons in current-clamp mode, after which recorded cells were filled with biocytin and slices were subjected to the post hoc IHC identification of 5-HT. None (0/5) of the recorded 5-HT-containing neurons demonstrated detectable changes in membrane potential in response to leptin (Figure 2A–C). Consistent with previous results from DR neurons not characterized post hoc by IHC (Yadav, et al, 2009), leptin inhibited the firing of a subpopulation (29% (6/21)) of DR neurons (Figure 2D–F, p<0.01). None of these leptin-responsive cells were 5-HT positive (Figure 2F).
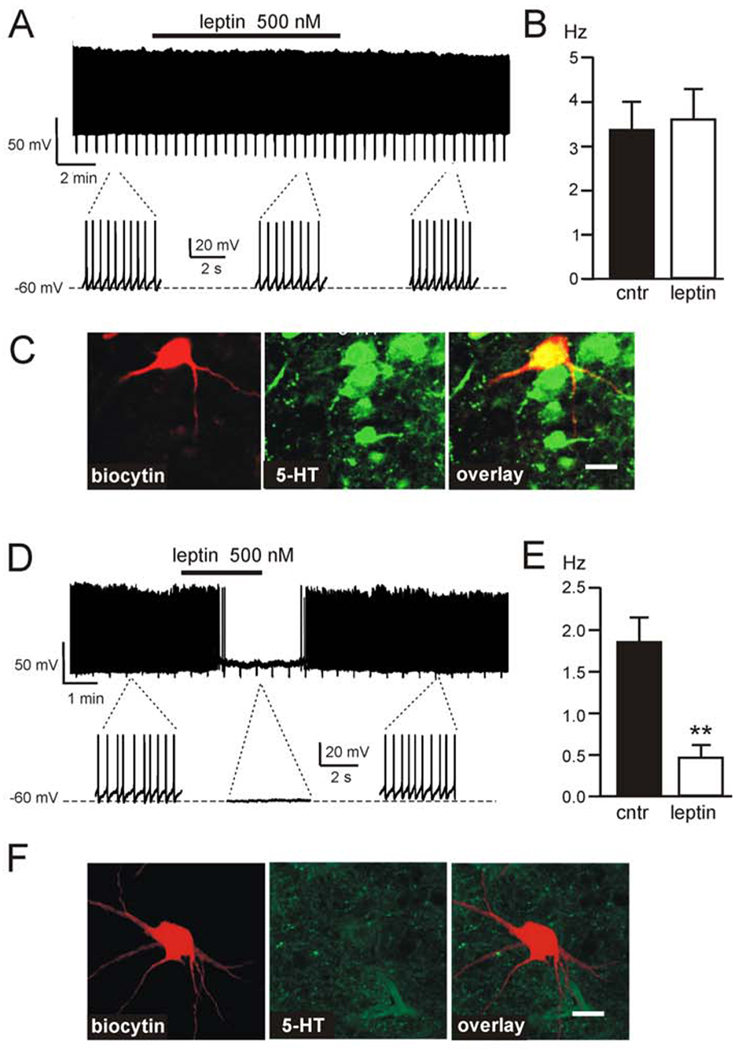
Figure 2. Leptin does not alter the electrical activity of DR 5-HT neurons.
(A) Representative firing trace of DR 5-HT cell in response to 500nM leptin. Expanded traces highlight the firing rate before, during and after application. (B) Average firing rate of DR 5-HT cells before and after 500 nM leptin treatment; 100% (n=5) of identified DR 5-HT neurons are unaffected by 500 nM leptin (mean ± SEM). (C) Immunofluorescence imaging of the cell shown in A, identified by biocytin staining (red); the cell contains 5-HT (green), overlay is shown in yellow. (D) Representative firing trace of DR non-5-HT cell demonstrating an inhibitory response to 500 nM leptin. Expanded traces highlight the firing rate before, during and after application. (E) Non-5-HT DR neurons inhibited by leptin, showing average firing rate before and after 500 nM leptin treatment (n=6; mean ± SEM, **p<0.01). (F) Immunofluorescence imaging of the cell shown in (D), identified by biocytin staining (red); the cell does not contain 5-HT (green). Scale bar in (C) and (F) = 30 µm. See also Figure S2.
Yadav, et al., reported increased expression of DR tryptophan hydroxylase 2 (Tph2), which mediates 5-HT synthesis, in old, but not young, Lepob/ob mice (Yadav et al., 2009), which suggests that Tph2 expression changes in Lepob/ob mice are related to age, rather than to direct leptin effects. Indeed, unlike Yadav, et al (2009), we were not able to detect an effect of leptin on DR Tph2 in wild-type mice, either by acute peripheral (0.5, 2.0, or 5.0 mg/kg, IP) or central (5 µg ICV) leptin treatment (Figure S2A, S2B). Similarly, we were unable to detect any changes in DR Tph2 in fasted rats receiving continuous leptin treatment (a paradigm in which leptin induces changes in other appetite-associated neuropeptides (Ahima et al., 1999)) (Figure S2C). Our data suggest that leptin does not directly or significantly modulate DR Tph2 expression.
Leptin-induced hypophagia is independent of serotonin bioavailability
While leptin does not directly control CNS 5-HT neurons, we investigated potential indirect interactions between 5-HT and LepRb-expressing cells using a transgenic mouse line in which LepRb neurons throughout the brain express the transneuronal tracer wheat germ agglutinin (Figure S3). Our observations indicate that an unidentified population of LepRb neurons lie in synaptic contact with DR 5-HT neurons (Figure S3).
In order to address the possibility of 5-HT participation in leptin action via such an indirect mechanism, we utilized PCPA to selectively deplete brain 5-HT, and by consequence its metabolite 5-hydroxyindoleacetic acid, by ~80% (Figure 3A). Noradrenaline (NA) and DA were not affected (Figure 3B–C). This profound depletion of 5-HT did not interfere with the ability of leptin (5 mg/kg, i.p.) to attenuate food intake (Figure 3D). Thus, 5-HT is not required for the anorectic action of leptin. These data are consistent with the notion that leptin does not mediate its effects on appetite via LepRb neurons synapsing on 5-HT cells and also with previous reports. Specifically, leptin exhibits normal anorectic efficacy in mice null for 5-HT2CR (Nonogaki et al., 1998), and mice null for both leptin and 5-HT2CR show greater hyperphagia than mice with either mutation alone (Wade et al., 2008). Furthermore, 5-HT requires the melanocortin pathway to influence food intake, whereas leptin affects appetite through both melanocortin-dependent and -independent pathways (Balthasar et al., 2004; Dhillon et al., 2006; Heisler et al., 2002; Lam et al., 2008; Marsh et al., 1999). Thus, leptin’s effects on appetite and body weight are unlikely to be substantially 5-HT-dependent.
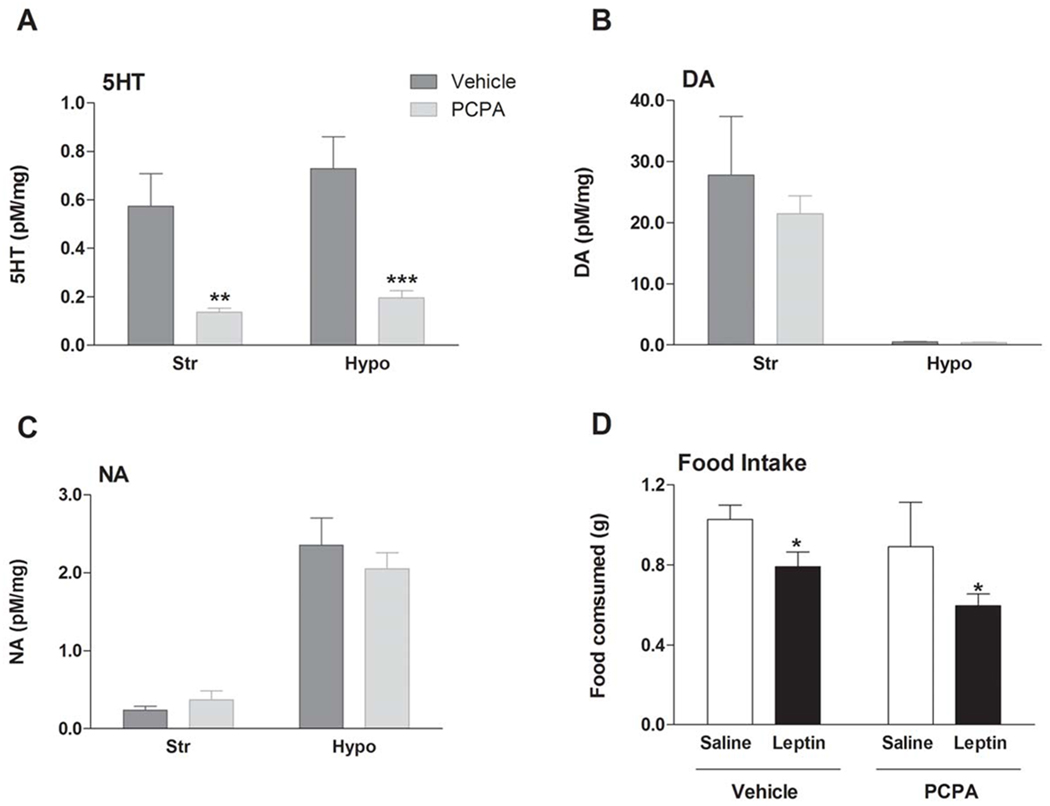
Figure 3. Leptin-induced hypophagia is independent of serotonin bioavailability.
Pre-treatment with the tryptophan hydroxylase inhibitor PCPA (200 mg/kg i.p. twice daily for three days) (A) significantly depleted 5-HT (mean ± SEM), but did not influence (B) DA (mean ± SEM) or (C) NA (mean ± SEM) in the brain (striatum (Str) and hypothalamus (Hypo) presented). Levels of neurotransmitters were measured by HPLC. (D) This substantial 5-HT depletion did not impair leptin’s effects on 90 min dark cycle food intake (n=11 per treatment group, mean ± SEM). *p<0.05; **p<0.01, ***p<0.001. See also Figure S3.
Deletion of LepRb from Sert-cre neurons does not affect body weight
A recent paper by Yadav et al. reported that LepRb in 5-HT neurons is crucial for most leptin action (Yadav et al., 2009). Clearly, this conclusion is incompatible with the lack of LepRb/5-HT co-expression, the lack of direct leptin effect on DR 5-HT neurons, and the irrelevance of brain 5-HT content to the anorexic action of leptin that we have demonstrated here. Methodological factors may have promoted non-specific signal in the LepRb localization studies performed by Yadav et al., as their depiction of LepRb abundance in the median raphe (MnR) as well as the DR is not consistent with previous reports of LepRb distribution (Elmquist et al., 1998; Patterson et al., 2011; Scott et al., 2009).
Yadav et al. reported that mice lacking LepRb in 5-HT transporter-cre (Sertcre)-expressing neurons are hyperphagic and obese (Yadav et al., 2009). Sert is broadly expressed in both 5-HT and non-5-HT neurons during development (Lebrand et al., 1998;Narboux-Neme et al., 2008). We therefore investigated the possibility that the Sertcre eliminated LepRb in crucial populations of non-5-HT neurons (Figure 4). We crossed the Sertcre allele utilized by Yadav et al. onto the conditional Leprfl background and the ROSA26-reporter alleles that express EGFP/EYFP following cre-dependent excision (Mao et al., 1999; McMinn et al., 2005; Narboux-Neme et al., 2008). Sertcre;ROSA26-reporter mice displayed Sertcre-mediated recombination in non-5-HT neurons within the DR and elsewhere in the brain (Figure S4).
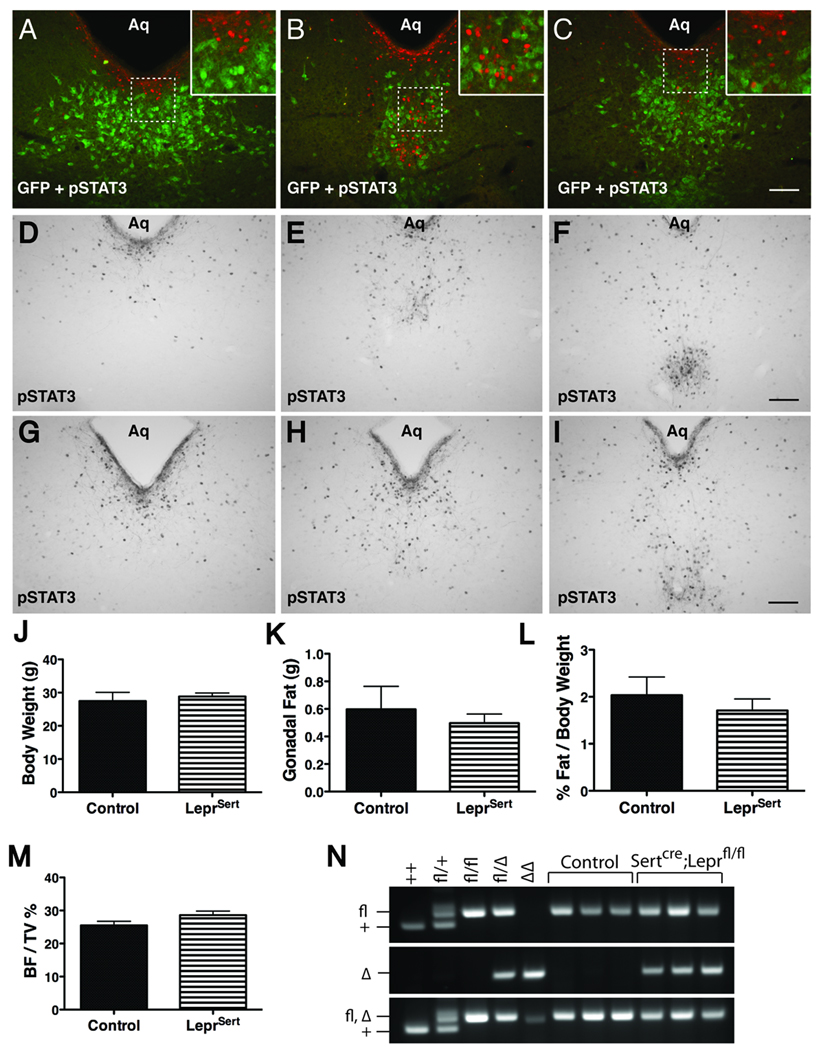
Figure 4. Characterization of Sertcre/LepRb interaction.
(A–C) Immunofluorescent analysis of leptin-stimulated pSTAT3 (red) and Sertcre (GFP, green) in DR sections from Sertcre;ROSA26-EGFP mice. (D–I) Immunohistochemical detection of ICV leptin-induced pSTAT3 in DR sections of control (D–F) and LeprSert mice (G–I). (J–M) Body weight (8 weeks of age, J, mean ± SEM), gonadal fat pad weight (9 weeks of age, K, mean ± SEM), gonadal fat pad weight as a percentage of body weight (L, mean ± SEM) and vertebral trabecular bone density (9 weeks of age, M, mean ± SEM) from male control (black bars) and LeprSert (hashed bars) mice. (N) Genotyping of Lepr in three representative control (Leprfl/fl) and LeprSert mice compared to animals of known genotypes. Top panel, Lepr+ and Leprfl bands; middle panel, LeprΔ; lower panel, multiplex analysis fails to distinguish Leprfl from LeprΔ. Scalebars in (C,F,I) = 100 µm. Insets are digital enlargements. See also Figure S4.
We examined the potential colocalization of Sertcre-induced EGFP with leptin-stimulated pSTAT3 in Sertcre;ROSA26-EGFP mice (Figure 4A–C); this revealed no colocalization of pSTAT3 with EGFP in the DR, although a few Sertcre/LepRb neurons were observed in the LHA (Figure S4). Given the modest number of Sertcre/LepRb neurons revealed by this analysis, we were surprised by the strong reported phenotype of Sertcre;Leprfl/fl (LeprSert) mice (Yadav et al., 2009), and therefore bred Sertcre;Leprfl/+ animals to independently-derived Leprfl/fl animals to generate LeprSert animals and littermate Leprfl/fl controls for analysis. Examination of leptin-stimulated pSTAT3 in the DR of control (Figure 4D–F) and LeprSert (Figure 4G–I) animals revealed similar levels and patterns of leptin-induced pSTAT3-IR in LeprSert and control animals, consistent with the lack of Sertcre colocalization with LepRb in the DR. We also examined the body weight and adiposity phenotypes of LeprSert and littermate control animals (Figure 4J–L). Body and fat pad weights did not differ between LeprSert and control animals, in contrast to the previously reported approximately 60% increase in body weight and 3–4-fold increase in fat pad weight/body weight at similar ages (Yadav et al., 2009). Vertebral bones from LeprSert mice were also similar to controls (Figure 4M).
As the Leprfl allele is prone to germline excision in a number of cre strains (generating the null LeprΔ allele) ((van de Wall et al., 2008) and our unpublished data), we thus examined the possibility of such events in the LeprSert and littermate control animals from Figure 4 (Figure 4N). While LeprΔ is not distinguished from Leprfl by the standard 3-primer multiplex genotyping paradigm to detect Leprfl and Lepr (Figure 4N, bottom panel) (McMinn et al., 2005), separation of the primer sets into two reactions discerns Leprfl from the germline deleted (LeprΔ) allele (Figure 4N, top and middle panels). Our analysis revealed the presence of one germline-deleted LeprΔ allele in all of the LeprSert mice that we examined (i.e., our LeprSert mice were uniformly Sertcre;Leprfl/Δ while controls were Leprfl/fl), suggesting that germline deletion of Leprfl occurs uniformly in gametes containing both Sertcre and Leprfl.
Given our present findings and the dramatic phenotype of the LeprSert mice (closely resembling mice completely devoid of leptin action) reported by Yadav, et al., we surmise that this tendency toward germline deletion combined with subsequent inbreeding yielded LeprSert mice of the Sertcre;LeprΔ/Δ genotype (globally Lepr deficient) in their hands. While the LeprSert animals studied by Yadav, et al. did not demonstrate overt diabetes as is often observed in animals devoid of LepRb, genetic background effects can suppress hyperglycemia in such animals, and this may have been the case in the mixed genetic background animals studied (Moritani et al., 2006; Yadav et al., 2009). With regards to the more recent study in which LepRb was deleted in adults by means of an inducible Tph2-cre (for which no recombination mapping data were provided) (Yadav et al., 2011), we postulate ectopic excision by this allele, given the substantial data demonstrating the lack of LepRb expression in 5HT neurons and the failure of Sertcre-mediated LepRb deletion to alter body energy balance.
Neuron-specific LepRb deletion data have revealed no single pathway purported to mediate the majority of appetitive leptin action, with the exception of the recently suggested 5-HT system (Yadav et al., 2009; Yadav et al., 2011). Here we clarify that brain 5-HT neurons do not express LepRb and do not, therefore, directly respond to leptin. Furthermore, our data reveal that brain 5-HT is not required for the anorectic action of leptin, and that neuron-specific excision of LepRb by Sertcre does not disrupt DR leptin action or the ability of leptin to regulate energy homeostasis. We conclude that leptin does not substantially influence energy balance via brain 5-HT neurons, and that the crucial population(s) of LepRb neurons for the control of energy balance remain to be precisely identified.
EXPERIMENTAL PROCEDURES
Animals
LepRbEGFP mice were generated and propagated as previously described (Leinninger, et al., 2009). For studies with wild type mice and rats, adult male pathogen-free C57BL/6 mice (27–30 g) or Sprague-Dawley rats (250–300 g) were used (Taconic or Charles River). All animals were individually housed with water and chow pellets available ad libitum (unless otherwise stated) in a light- (12 hours on/12 hours off) and temperature-controlled (21.5°C to 22.5°C) environment. Sertcre and Leprfl mice were the generous gifts of Xiaoxi Zhuang (University of Chicago) and Streamson Chua (Albert Einstein College of Medicine), respectively. ROSA26-EGFP and ROSA26-EYFP animals were from the Jackson Laboratory. All procedures performed in the USA were in accordance with NIH guidelines on animal care and use and with the approval of the UCUCA; those performed in the UK were approved by the UK Home Office.
Sertcre mice were genotyped via qPCR, using primers and probes for Cre and Ngf, as described (Leinninger et al., 2009). Leprfl was genotyped by conventional PCR (mLepR-105, mLepR-65A for Leprfl and Lepr+) in addition to or in combination with mLepR-105 and mLepR-106 for LeprΔ (McMinn et al., 2005).
Animal perfusion and Immunohistochemistry (IHC)
For pSTAT3 assessment in rats, femoral vein catheters were inserted as done previously (Elias et al., 1998). Five days later, rats were injected with saline or recombinant rat leptin (Sigma, 0.25 mg/kg or 1.0 mg/kg, i.v.) (n=4 per dose). For pSTAT3 assessment in C57BL/6 or Sertcre;reporter mice, saline or recombinant murine leptin (Peprotech or a generous gift of Amylin Pharmaceuticals) were administered (n=3/genotype). Animals were deeply anesthetized and perfused transcardially with 0.9% saline followed by 10% formalin (Sigma); brain tissue was collected as previously described (Elias et al., 1998; Heisler et al., 2002; Leinninger et al., 2009; Munzberg et al., 2007) (see Supplemental Methods for details).
For dual-label IHC, primary antibodies for pSTAT3 (1:250, rabbit, Cell Signaling), GFP (1:1,000, rabbit, Molecular Probes or 1:000, chicken, Abcam), 5-HT (1:1,000, goat, Immunostar), Calb (1:500, mouse, Sigma), or TH (1:1,000, mouse, Chemicon) were followed by secondary antibodies (Molecular Probes and Jackson Immunoresearch): FITC-conjugated donkey anti-chicken (for GFP, 1:200), Alexa Fluor 594 conjugated to donkey anti-goat (for 5-HT, 1:500), or Alexa Fluor 488 conjugated to donkey anti-mouse or anti-rabbit (for Calb, TH, and GFP, 1:500).
Characterization of LeprSert mice
For the analysis of LeprSert and control mice, 8 wk-old male LeprSert (n=9) and control littermate (n=5) mice were weighed. A subset of mice (LeprSert=5, control=4) were implanted with ICV cannulae as described (Leinninger et al., 2009). Coordinates to the lateral ventricle were, relative to Bregma, A/P −0.34; M/L −1.0; and D/V −2.4. For treatment, the dummy was replaced with an injector to deliver 3 µL of PBS or leptin (1 mg/mL; Amylin Pharmaceuticals) at a rate of 1 µL per minute. Mice were perfused 1 hr after treatment and brains were sectioned and analyzed for pSTAT3 and GFP as described above. Mice that were not cannulated were also perfused at 9 wks of age. Gonadal fat was dissected and weighed post-perfusion. Analysis of vertebral bones was carried out as described in the supplemental methods.
5-HT depletion and leptin-induced hypophagia
Tryptophan hydroxylase inhibitor p-chlorophenylalanine (PCPA) treatment was done as previously described (O'Leary et al., 2007). Briefly, mice were treated with 200 mg/kg i.p. PCPA methyl ester hydrochloride (Sigma) or water twice daily (at 9:00 am and 4:00 pm) for three days (n=44). Mice and food were weighed daily to ensure no adverse effect on body weight or appetite. On the fourth day, mice received mouse recombinant leptin (Merck, 5 mg/kg, i.p.) or vehicle 120 min and 30 min prior to the onset of the dark cycle (lights off at 7:00 pm) and food was removed. At the onset of the dark cycle, food was returned and subsequent 90 min intake was assessed. On completion of the study, animals were sacrificed (n=11 per group) and brains rapidly removed and frozen. Brains were sectioned coronally at 150 µm on a cryostat and mounted onto glass slides. Micro-punches were taken from the hypothalamus and striatum and homogenized in 60 µl of 0.2M perchloric acid by an ultrasonic cell disruptor. Levels of 5-HT, 5-HIAA, DA and NA in the supernatant were determined by HPLC and electrochemical detection, as described previously (Dalley et al., 2002).
Electrophysiology
Acute coronal brain slices (250 µm thick) containing the DR were prepared from 22–44 day old mice and standard whole-cell patch-clamp recordings were performed (Burdakov and Ashcroft, 2002); see Supplemental Methods. As in previous studies (Yadav et al., 2009), to facilitate the firing of serotonin neurons, we supplemented aCSF (see Supplemental Methods for details) with phenylephrine (3 µM, Tocris). The following synaptic blockers were also added to aCSF: AP5 (50 µM), CNQX (10 µM), picrotoxin (50 µM), strychnine (3 µM), and CGP52432 (10 µM) (Tocris). Data collection and analysis was performed using Pulse (HEKA). To visualize neurons after recordings, biocytin (0.5 mg/ml, Tocris) was added to the pipette solution (see Supplemental Methods for details), and the slices were fixed in 10% formalin. Immunohistochemical processing for the detection of 5-HT was performed as detailed above and images were acquired using an Olympus BX61WI confocal microscope.
Data Analysis
The effect of drug treatment on cell firing rate was assessed using t-test. The effects of PCPA on 5-HT, 5-HIAA, DA and NA levels in the striatum and hypothalamus were analyzed with t-test. The effect of PCPA pretreatment and leptin treatment on food intake was assessed with a two-way ANOVA, followed by Tukey HSD post hoc test. The effect of genotype on body weight, gonadal fat, and bone density were analyzed with t-test. For all analyses, significance was assigned at the p ≤ 0.05 level.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the Gates Cambridge Trust (D.D.L); the Wellcome Trust WT081713 (L.K.H); the Marilyn H. Vincent Foundation and American Diabetes Association (M.G.M.); NIH (HD061539 to C.E.; DK065171 to L.K.H.; DK057768 and DK078056 to M.G.M.); and the Cambridge Medical Research Council Centre for Study of Obesity and Related Disorders (L.K.H., D.B., and M.L.E.). We thank Drs. Xiaoxi Zhuang and Streamson Chua for the gift of Sertcre and Leprfl mice, respectively. We thank Amylin Pharmaceuticals for the generous gift of leptin. Core support was provided by NIH DK20572.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140:4923–4931. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976;192:382–385. doi: 10.1126/science.130678. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Ashcroft FM. Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-Type potassium channels. J Neurosci. 2002;22:6380–6387. doi: 10.1523/JNEUROSCI.22-15-06380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G, Corica F, Corsonello A, Sautebin L, Di Rosa M, Campo GM, Buemi M, Mauro VN, Caputi AP. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104:975–982. doi: 10.1172/JCI5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, Zachwieja JJ. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema Is required for energy balance regulation. Cell Metab. 2009;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Kanarek RB, Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol Biochem Behav. 1997;58:767–773. doi: 10.1016/s0091-3057(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O'Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Liu H, Dragatsis I, Dietrich P, Ludwig T, Boozer CN, Chua SC., Jr Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab. 2005;289:E403–E411. doi: 10.1152/ajpendo.00535.2004. [DOI] [PubMed] [Google Scholar]
- Moritani M, Togawa K, Yaguchi H, Fujita Y, Yamaguchi Y, Inoue H, Kamatani N, Itakura M. Identification of diabetes susceptibility loci in db mice by combined quantitative trait loci analysis and haplotype mapping. Genomics. 2006;88:719–730. doi: 10.1016/j.ygeno.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Bjornholm M, Elmquist J, Sleeman M, Cowley MA, et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narboux-Neme N, Pavone LM, Avallone L, Zhuang X, Gaspar P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs) Neuropharmacology. 2008;55:994–1005. doi: 10.1016/j.neuropharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology. 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Leshan RL, Jones JC, Myers MG., Jr Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28. doi: 10.1016/j.brainres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120:2931–2941. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg C, Langhans W, Hrupka BJ. Evidence for a role of the 5-HT2C receptor in central lipopolysaccharide-, interleukin-1 beta-, and leptin-induced anorexia. Pharmacol Biochem Behav. 2003;74:1025–1031. doi: 10.1016/s0091-3057(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Wade JM, Juneja P, MacKay AW, Graham J, Havel PJ, Tecott LH, Goulding EH. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology. 2008;149:955–961. doi: 10.1210/en.2007-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Tanaka K, Thomas T, Wang Y, Cremers S, Hen R, Krust A, Chambon P, Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208:41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Hirose H, Kajiwara Y. Role of serotonergic mechanisms in leptin-induced suppression of milk intake in mice. Neurosci Lett. 2003;348:195–197. doi: 10.1016/s0304-3940(03)00772-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.