Abstract
From an acetone extract of the aerial parts of Tidestromia oblongifolia (Amaranthaceae), a new drimane sesquiterpenoid bearing an 11,12,13-trihydroxydrimene skeleton (1) as well as the 11,12 acetonide of 1 were isolated. Three known stigmastane triterpenoids were also isolated. Structures were elucidated with the aid of 1D and 2D NMR spectroscopic techniques. The absolute configuration of 1 was confirmed by single-crystal x-ray crystallography. This is the first report on phytochemical constituents from any plant of the genus Tidestromia and is the first report of the occurrence of drimanes in the Amaranthaceae.
Keywords: drimane, stigmastane, Tidestromia oblongifolia, brine shrimp
The genus Tidestromia (Amaranthaceae) is represented by six to seven species of annual to perennial plants native to desert regions of western and south-western United States (US) and Mexico [1a–c]. Tidestromia oblongifolia (Arizona Honeysweet) is a subshrub which can be found in arid zones of Arizona, Utah, Nevada, Sonora and Baja California [1a, 2]. Previous literature reports on this plant document the effects of various physical and environmental factors on it’s photosynthetic characteristics [3–4]. However, there have been no reports on the phytochemical constituents of T. oblongifolia or of any other plant of the Tidestromia genus. Twigs and herbage of the related species Tidestromia lanuginosa have been used in folkloric medicine by the Seri Indians of Sonora Mexico for head-ache and foot-ache [5]. As part of our program to examine extremophiles of desert regions of the US for novel bioactive natural products, we undertook an investigation of T. oblongifolia. Our investigations resulted in the isolation of two new drimane sesquiterpenoids (1 and 2) as well as three known stigmastane triterpenoids. The structures of the compounds were deduced with the aid of 1D and 2D-NMR spectroscopic techniques as described below.
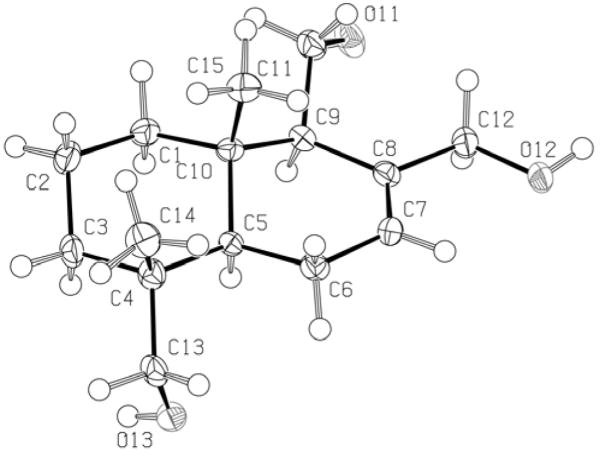
Compound 1 showed an [M+Na]+ peak at m/z 277.1773 in the HREIMS indicating an elemental molecular formula of C15H26O3. The 1H NMR spectrum of 1 showed two methyl singlets at δH 0.79 and 0.82. Six signals in the δH 3.00 – 4.40 ppm region of the spectrum were indicative of protons attached to oxygen-bearing carbon atoms. Two mutually-coupled single proton doublets were observed at δH 3.06 and 3.41 and a similarly coupled pair of signals was seen at δH 3.97 and 4.35 ppm. HSQC analysis indicated that these two pairs of doublets were attached to carbons at δC 71.8 (C-13) and 67.7 (C-12) respectively. Two other protons resonated at δH 3.65 and 3.90 and HSQC analysis confirmed that these protons were both attached to the same carbon atom (C-15, δ 61.6). A vinylic proton signal was seen at δH 5.78 ppm; this proton showed HMBC correlations to δC 23.6 (C-6) and δC 67.7 (C-12). Further inspection of 13C, DEPT, HMBC and HSQC spectral data enabled the assignment of all substituents on the decalin core of1. Thus the gross structure of 1 was determined to be that of 11,12,13-trihydroxydrimene (Fig. 1). To deduce stereochemical assignments, a NOESY experiment was performed. The NOESY spectrum showed key correlations between the H-15 protons (δH 0.79) and the H-11 oxymethylene protons (δH 3.65, 3.90) as well as correlations between the H-13 protons and H-5. H-5 (δH 1.59) in turn showed NOESY cross-peaks to H-9 (δH 2.20). On the basis of this data, compound 1 was assigned the relative stereochemistry depicted in Figure 1. This was corroborated by single-crystal X-ray analysis (Fig. 2).
Figure 1.
Structures of Compounds 1 and 2
Figure 2.
ORTEP drawing of compound 1.
The molecular formula of compound 2, C18H30O3 was indicated by HRESIMS at m/z 295.2291 [M+H]+ suggesting four degrees of unsaturation. The presence of 3 additional carbon atoms (compared to 1) as indicated by the HREIMS was also supported by 13C NMR data. The 1H NMR spectrum of 2 was very similar to that of 1 indicating that they were structurally related. However, major differences as compared to 1 were observed in the methyl region.
Here, there were two nearly coincident methyl singlets at δH 0.86 (distinguished upon spectral expansion as δH 0.858 and 0.863) as well as an additional pair of methyl singlets at δH 1.32 and 1.35. Two geminally coupled doublets (J = 10.9 Hz) resonating at δH 3.11 and 3.36 ppm corresponded closely in chemical shift to protons assigned to H-13 in 1. Signals for the H-11 and H-12 protons were also similar in chemical shift to those of 1, although there were notable upfield shifts (up to 0.22 ppm) for these proton signals in 2. HMBC analysis established the presence of a gem-dimethyl fragment and allowed for formulation of an acetonide sub-structure in 2. Based on the foregoing spectral data and coupled with further HMBC analysis, the complete structure for 2 was elucidated. Examination of the NOESY spectrum of 2, have enabled assignments of it’s stereostructure. Thus similarly to 1, key NOESY cross-peaks were seen between the H-13 protons and H-5 and between H-5 and H-9. NOESY cross-peaks were also seen between the H-11 (δH 3.64, 3.70) and H-15 methyl protons (δH 0.86).
It is possible that compound 2 is an artifact of the extraction or isolation procedure being derived from 1, but further experiments (eg. plant material extraction and chromatography in the absence of acetone, HPLC-MS analysis) are needed to confirm this.
Further column chromatography of fractions derived from the crude acetone extract, yielded the known compounds stigmasterol and β-sitosterol as an inseparable mixture as well as stigmast-22-en-3β-ol.
Drimanes, particularly those containing aldehyde functionalities (such as polygodial, 3) have been reported to possess cytotoxic, antibacterial and antifungal activities [6–8]. As a preliminary evaluation of the bioactivity of 1 and semi-synthetic derivatives obtainable therefrom, we oxidized 1 under Swern conditions to give the trialdehyde 4 and evaluated compounds 1 and 4 in a brine shrimp lethality assay [9]. Compounds 1 and 4 were toxic with LC50 values of and 147.4 μg/ml and 9.0 μg/ml respectively.
A drimane bearing the same gross structure as 1 but with the opposite stereochemistry at C-4, has been isolated from the fairy ring fungus Marasmius oreades [10]. Among higher plants, drimanes are well-known metabolites of the Canellaceae and Winteraceae families, and have been suggested as taxonomic markers of the Canellaceae [11–14]. From the marine environment, drimanes are particularly known to occur in sponges of the Dysidea genus and nudibranchs from the genus Dendrodoris [15,16]. Certain species of fungi are also known to produce these metabolites [10].
This communication is the first report on the occurrence of drimane sesquiterpenes from any plant of the Amaranthaceae family. Further phytochemical and biological evaluations of T. oblongifolia are currently being pursued in our laboratory.
Experimental
Plant material
Plant material was collected in Laughlin, Nevada, United States in Sept. 2006. A voucher specimen has been deposited in the Herbarium at the World Botanical Associates, Bakersfield, California (voucher # Spjut 16041).
Extraction and Isolation
Air-dried aerial parts of T. oblongifolia (397 g) were extracted with hexanes (3 × L). The hexanes was evaporated in vacuo to give 5.2 g of crude hexane extract. The marc from the hexane extraction was then extracted with acetone (3 × L), providing 6.0 g of crude acetone extract after evaporation of the solvent in vacuo. A methanol extract (22.1 g) was similarly prepared by extraction (3 × L) of the marc from the acetone extraction. Fractionation of the acetone extract in 1L portions of increasing polarities of acetone-hexane mixtures (10% – 100% acetone) afforded 10 fractions (1A–1J). The fractions eluted in 30% – 40% acetone-hexanes (1C and 1D) were pooled on the basis of tlc profiles and subjected to repeated flash column chromatography affording compounds 1 and 2. Repeated column chromatography of fraction 1B gave stigmasterol and β-sitosterol as an intractable mixture and stigmast-22-en-3β-ol (spectroscopic data as previously reported) [18].
Brine Shrimp Assay
The brine shrimp (Artemia salina) lethality assay was conducted as described by McLaughlin et al [9]. Briefly, brine shrimp were added to artificial salt water (ASW) suspensions of the test compound to give final concentrations of 1000, 100, 10, 5 and 1 μg/ml (conducted in triplicate plus ASW controls). After 24 hours the number of surviving shrimp was counted and the percentage lethality calculated. LC50 values were determined by statistical analysis of the data (log concentration vs. percentage lethality) using Graphpad Prism.
Compound 1
White needles (acetone-hexanes) MP: 126–129 °C.
[α]D25: −14.7 (c 2.00, MeOH).
IR (film): 3398, 2926, 1643, 1442, 1384, 1038, 989 cm−1.
1H NMR (500 MHz, CDCl3): see Table 1
Table 1.
NMR spectral data for compounds 1 and 2a in CDCl3
| 1 |
2 |
|||
|---|---|---|---|---|
| Position | 1H | 13C | 1H | 13C |
| 1 | 1.19, ddd (12.5, 12.5, 5.1) | 39.1 | 1.16, ddd (12.8, 12.8, 4.6) | 39.0 |
| 1.93, m | 1.90, m | |||
| 2 | 1.50, m | 18.3 | 1.54, m | 18.1 |
| 3 | 1.20, m | 35.5 | 1.40, ddd (12.8, 4.4) | 35.3 |
| 1.50, ddd (12.7, 12.7, 4.4) | 1.52, m | |||
| 4 | 35.6 | 34.6 | ||
| 5 | 1.59, dd (12.0, 4.9) | 42.8 | 1.47, dd (11.2, 5.5) | 43.1 |
| 6 | 1.91, m | 23.6 | 1.96, m | 23.3 |
| 1.99, m | 2.00, m | |||
| 7 | 5.78, dd (2.6, 2.6) | 127.3 | 5.61, br. s | 125.0 |
| 8 | 137.3 | 137.8 | ||
| 9 | 2.20, br. d (4.1) | 54.7 | 2.10, br. d | 54.1 |
| 10 | 34.8 | 37.4 | ||
| 11 | 3.65, dd (10.9, 8.4) | 61.6 | 3.64, dd (11.2, 11.2) | 60.7 |
| 3.90, dd (10.9, 1.7) | 3.70, dd (11.2, 4.3) | |||
| 12 | 3.97, d (12.2) | 67.7 | 3.75, d (13.2) | 67.1 |
| 4.35, dd (12.2) | 4.28, d (13.2) | |||
| 13 | 3.41, dd (11.1) | 71.8 | 3.36, d (10.9) | 72.0 |
| 3.06, dd (11.1) | 3.11, d (10.9) | |||
| 14 | 0.82, s | 18.0 | 0.86, s | 18.0 |
| 15 | 0.79, s | 15.3 | 0.86, s | 15.4 |
| 11′ | 101.4 | |||
| 11′-Me | 1.35, s | 24.9 | ||
| 11′-Me | 1.32, s | 29.7 | ||
500 MHz for 1H NMR and 125 MHz for 13C NMR; coupling constants (Hz) in brackets
13C NMR (125 MHz, CDCl3): see Table 1
HREIMS: m/z [M + Na]+ calcd for C15H26O3: 254.1882; found: 254.1773.
Compound 2
Colorless oil
[α]D25: −2.3 (c 0.13, MeOH).
IR (film): 3390, 2925, 1644, 1040 cm−1.
1H NMR (500 MHz, CDCl3): see Table 1
13C NMR (125 MHz, CDCl3): see Table 1
HRESIMS: m/z [M + H]+ calcd for C18H30O3: 295.2273; found: 295.2291
Preparation of Compound 4
To a solution of DMSO (28 μl, 1.94 mmol) in CH2Cl2 (2 ml) was added oxalyl chloride (66 μl, 0.78 mmol) at −78 °C. After stirring the solution for 15 min, a solution of 1 (49 mg, 0.19 mmol) in CH2Cl2 (2 ml) was added over 5 min. The mixture was stirred for 45 min at −78 °C and then triethylamine (0.64 ml, 0.20 mmol) was added. The resulting solution was stirred for 30 min at room temperature and then was diluted with 20 ml water and 20 ml ether. The phases were separated and the aqueous phase extracted with a further 20 ml of ether. The organic layers were combined, dried (Na2SO4) and concentrated. Column chromatography in 1% MeOH-CH2Cl2 gave 32 mg (0.13 mmol, 68%) of the trialdehyde 4 as a colorless solid.
Compound 4
Colorless solid MP: 106–109 °C.
[α]D25: +24.4 (c 4.50, MeOH).
IR (film): 2930, 1667, 1639, 1390, 1187, 1067, 1033, 922 cm−1.
1H NMR (500 MHz, CDCl3): 1.00 (3H, s), 1.21 (3H, s), 1.4–1.5 (4H, m), 1.58–1.64 (2H, m), 1.66–1.71 (1H, m), 1.82 (1H, dd, J = 4.3, 12.2 Hz), 1.92 (1H, br. d), 2.13 (1H, m), 2.28 (1H, m), 2.93 (1H, m), 7.08 (1H, ddd, J = 2.2, 2.8, 5.0 Hz), 9.23 (1H, s), 9.47 (1H, s), 9.53 (1H, d, J = 4.3 Hz).
13C NMR (125 MHz, CDCl3): 14.9, 15.8, 16.4, 26.2, 32.7, 35.8, 38.6, 40.9, 48.6, 59.7, 138.3, 152.8, 193.1, 201.1, 204.7.
HRESIMS: m/z [M + H]+ calcd. for C16H22O3: 262.1569; found: 262.1569.
Single-Crystal X-ray Diffraction of 1 [17]
The intensity data for 1 were measured on an Bruker-Nonius KappaCCD diffractometer (graphite-monochromated Mo Kα radiation, λ = 0.71073 Å φ-ω scans) at 100 (1) K. The data were not corrected for absorption. Details of the solution and refinements for this compound are presented below:
C15H26O3 (1)
The crystal of 1, with approximate dimensions 0.10 × 0.20 × 0.25 mm, were monoclinic with space group P21. The final unit-cell constants of 1 were a = 9.154(2), b = 8.388(2), c = 9.819(2) Å, β = 110.40(3)°, V = 702.4(3) Å3, Z = 2, ρ = 1.203 g cm−1, μ = 0.081 mm−1, formula weight = 254.36. The structure of 1 was solved with SHELXS-97 and refined by full-matrix least squares on F2 with SHELXL-97. The hydrogen atoms were included in the structure-factor calculations, but their parameters were not refined. The final discrepancy indices for the 1704 reflections (θ < 27.50°) were R = 0.0512 (calculated on F) and Rw = 0.0919 (calculated on F2) with 168 parameters varied. The final difference map peaks are < 0.20 e Å−3.
Figure 3.

Structures of polygodial (3) and compound 4.
Acknowledgments
The authors thank Drs. Richard Spjut (World Botanical Associates), Cliff Soll and Matthew Devany for plant material, mass and NMR spectroscopic data collection respectively. This publication was made possible by Grant Number RR03037 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. It’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.a) Everitt JH, Lonard RL, Little CR. Weeds in South Texas and Northern Mexico. Lubbock: Texas Tech University Press; 2007. [Google Scholar]; b) Sánchez-del Pino I, Clemants SE. Flora of North America. 2003;4:439–443. [Google Scholar]; c) Wiggins IL. Flora of Baja California. Stanford: Stanford University Press; 1980. [Google Scholar]
- 2.Information available online at http://plants.usda.gov
- 3.Pike CS, Berry JA. Membrane phospholipid phase separations in plant adapted to or acclimated to different thermal regimes. Plant Physiology. 1980;66:238–241. doi: 10.1104/pp.66.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murata N, Fork DC. Temperature dependence of chlorophyll a fluorescence in relation to the physical phase of membrane lipids in algae and higher plants. Plant Physiology. 1975;56:791–796. doi: 10.1104/pp.56.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felger RS, Moser MB. Seri Indian Pharmacopoeia. Economic Botany. 1973;28:415–436. [Google Scholar]
- 6.Echeverri F, Luis JG, Torres F, Quinones W, Alzate F, Cardona G, Archhold R, Roldan J, Lahlou EH. Danilol, a new drimane sesquiterpene from Polygonum punctatum leaves. Natural Product Letters. 1997;10:285–301. [Google Scholar]
- 7.a) Lunde CS, Kubo I. Effect of Polygodial on the Mitochondrial ATPase of Saccharomyces cerevisiae. Antimicrobial Agents and Chemotherapy. 2000;44:1943–1953. doi: 10.1128/aac.44.7.1943-1953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kubo I, Fujita K, Lee SH. Antifungal mechanism of polygodial. Journal of Agricultural and Food Chemistry. 2001;49:1607–1611. doi: 10.1021/jf000136g. [DOI] [PubMed] [Google Scholar]
- 8.Anke H, Sterner O. Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Planta Medica. 1991;57:344–346. doi: 10.1055/s-2006-960114. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin JL. Bench-top bioassays for the discovery of bioactive compounds in higher plants. Brenesia. 1991;34:1–14. [Google Scholar]
- 10.Ayer WA, Craw PA. Metabolites of the fairy ring fungus, Marasmius oreades. Part 2. Norsesquiterpenes, further sesquiterpenes, and agrocybin. Canadian Journal of Chemistry. 1989;67:1371–1380. [Google Scholar]
- 11.Seeram NP, Francis LS, Needham OL, Jacobs H, McLean S, Reynolds WF. Drimane and bisabolane sesquiterpenoids from Cinnamodendron corticosum (Canellaceae) Biochemical Systematics and Ecology. 2003;31:637–640. [Google Scholar]
- 12.Bastos JK, Kaplan MAC, Gottlieb OR. Drimane-type sesquiterpenes as chemosystematic markers of Canellaceae. Journal of the Brazilian Chemical Society. 1999;10:136–139. [Google Scholar]
- 13.Fotsop DF, Roussi F, Le Callonec C, Bousserouel H, Litaudon M, Gueritte F. Isolation and characterization of two new drimanes from Zygogynum baillonii and synthesis of analogues. Tetrahedron. 2008;64:2192–2197. [Google Scholar]
- 14.Malheiros A, Filho VC, Schmidt CB, Santos ARS, Scheidt C, Calixto JB, Monache FD, Yunes RA. A sesquiterpene drimane with antinociceptive activity from Drimys winterii bark. Phytochemistry. 2001;57:103–107. doi: 10.1016/s0031-9422(00)00515-x. [DOI] [PubMed] [Google Scholar]
- 15.Montagnac A, Martin MT, Debitus C, Pais M. Drimane sesquiterpenes from the sponge Dysidea fusca. Journal of Natural Products. 1996;59:866–68. [Google Scholar]
- 16.Sakio Y, Hirano YJ, Hayashi M, Komiyama K, Ishibashi M. Dendocarbins A-N, new drimane sesquiterpenes from the Nuibranch Dendrodoris carbunculosa. Journal of Natural Products. 2001;64:726–731. doi: 10.1021/np000639g. [DOI] [PubMed] [Google Scholar]
- 17.Cystallographic data for 1 have been deposited with the Cambridge Crystallographic Data Center as deposition number CCDC 694622. Copies of data can be obtained free of charge on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax +44 –(0)1223–336033 or email: deposit@ccdc.cam.ac.uk)
- 18.Rubinstein I, Goad LJ, Clague ADH, Mulheirn LJ. The 220 MHz spectra of phytosterols. Phytochemistry. 1976;15:195–200. [Google Scholar]