Abstract
The glmS ribozyme is a conserved riboswitch found in numerous Gram-positive bacteria and responds to the cellular concentrations of glucosamine-6-phosphate (GlcN6P). GlcN6P binding promotes site-specific self-cleavage in the 5′UTR of the glmS mRNA, resulting in down regulation of gene expression. The glmS ribozyme has previously been shown to lack strong cation specificity when the rate-limiting folding step of the cleavage reaction pathway is measured. This does not provide data regarding cation and ligand specificities of the glmS ribozyme during the rapid ligand binding chemical catalysis events. Prefolding of the ribozyme in Mg2+-containing buffers effectively isolates the rapid ligand binding and catalytic events (kobs > 60 min−1) from rate-limiting folding (kobs<4 min−1). Here we employ this experimental design to assay the cations and ligand requirements for rapid ligand binding and catalysis. We show that molar concentrations of monovalent cations are also capable of inducing the formation of the native GlcN6P binding structure, but are unable to promote ligand binding and catalysis rates >4 min−1. Our data show that the sole obligatory role for divalent cations, for which there is crystallographic evidence, is coordination of the phosphate moiety of GlcN6P in the ligand binding pocket. In further support of this hypothesis, our data show that a non-phosphorylated analog of GlcN6P, glucosamine, is unable to promote rapid ligand binding and catalysis in the presence of divalent cations. Folding of the ribozyme is, therefore, relatively independent of cation identity but the rapid initiation of catalysis upon the addition of ligand is stricter.
Keywords: glmS ribozyme, RNA, Riboswitch, Ribozyme, Gene Expression
Riboswitches are structural genetic control elements that are generally located in the mRNAs that they regulate (1–5). These motifs act as biosensors, sensitive to the intracellular concentrations of specific metabolites. The glmS ribozyme is a conserved riboswitch motif in numerous Gram-positive bacteria and is located upstream of the gene encoding glucosamine-6-phosphate (GlcN6P) synthetase (6). This enzyme catalyzes the conversion of glutamine and fructose-6-phosphate to glutamate and GlcN6P, a compound that is required for sugar metabolism and cell wall biosynthesis (6–8). Binding of GlcN6P triggers a self-cleavage reaction that results in rapid RNase J1-mediated degradation of the 3′cleavage product, including the coding region of the mRNA (9). Degradation of glmS mRNA leads to the down-regulation of the glmS protein expression, thereby decreasing the concentration of available GlcN6P. The glmS gene is believed to be the primary control point for cell wall synthesis in gram positive bacteria including pathogenic species such as Staphylococcus aureus (10), which carries a copy of the glmS ribozyme motif (5).
Through the use of in-line probing (6), x-ray crystallography (11, 12), hydroxyl radical footprinting (13) and fluorescence resonance energy transfer (FRET) (11) it has been determined that the glmS ribozyme’s ligand binding pocket is preformed in the absence of its ligand (1, 11–14). In addition, we and others have determined that the ligand binding and catalysis steps can be experimentally isolated from native tertiary folding (Figure 1) (15, 16). By this kinetic method we identified a slow folding step that precedes ligand binding and catalysis (16). Dissection of the catalytic pathway provides the opportunity to define the intrinsic base sequence and nucleotide functional group requirements as well as the extrinsic temperature, pH and cation requirements for individual steps in the reaction pathway. In addition, experimental designs that isolate native ribozyme folding from ligand binding and catalysis open the door to a more careful examination of ligand specificity.
Figure 1.
A folding and reaction pathway model for the glmS ribozyme. (A) Global folding from the unfolded (U) to a compact native-like intermediate (I) or misfolded (M) form occurs rapidly upon the addition of MgCl2. A second Mg2+ dependent event folds the catalytic RNA into a complex capable of GlcN6P binding (N). GlcN6P binding and catalysis occur on a second timescale once the native (N) structure is formed. (B) Complex initiated experimental design assays the rate of formation of N. (C) Ligand binding and catalysis can be assayed independent of the rate-limiting step by prefolding glmS-Rz-S in cations and triggering reactions through the addition of ligand.
The ligand specificity for the glmS ribozyme is a significant focus of study due in large measure to the potential antibiotic use of GlcN6P analogs to disrupt cell wall synthesis in Gram positive pathogenic bacteria. In addition, significant biochemical and structural data point to the hypothesis that the ligand functions as a coenzyme in the glmS reaction (12, 14, 17, 18). The amine residue of GlcN6P is in a position to serve as a proton donor to the G+1 5′oxygen. This function is unique among naturally occurring ribozymes and impacts our understanding of the enzymatic repertoire of ribozymes since coenzymes provide numerous chemical functionalities not present in RNA (19). Crystallographic data support a ligand binding model where the GlcN6P makes contacts with Mg2+ ions and RNA. Two fully hydrated Mg2+ ions can be modeled into the ligand binding pocket and are hypothesized to make a set of water and outer sphere mediated contacts that bridge the phosphate group of GlcN6P and RNA functional groups. Although present in the catalytic core, these Mg2+ ions are not in a position to directly participate in reaction chemistry (12, 17, 20). In addition, phosphorothioate substitution within the catalytic core of the B. cereus glmS ribozyme were rescued by Mn2+ at sites that mediate ligand phosphate recognition (20). Protonation of the ligand phosphate is expected to negatively impact binding by altering this network of Mg2+ and water interactions and has been proposed to give rise to increase in GlcN6P binding affinity as a function of pH (15). In addition, a hydrogen bonding interaction between a GlcN6P phosphate oxygen and the N1 hydrogen of the highly conserved G1 residue has been proposed (21). Substitution of G1 with adenosine has a large impact on GlcN6P mediated cleavage activity but only a modest affect on activity in the presence of a non-phosphorylated ligand analog glucosamine, GlcN. Since most experiments that have specifically addressed the question of ligand specificity have utilized protocols that do not rule out the impact of slow global folding on their outcome, we have an incomplete picture of the requirements for the very rapid ligand binding that has been observed in the glmS system. Finally, the degree to which divalent cations are required for rapid ligand binding and ligand specificity has not been specifically addressed.
Cations play important roles in the formation of ribozyme tertiary structure and can participate directly in catalysis (22, 23). In some cases ribozymes that were previously thought to function as metalloenzymes have been found to work well in the absence of cations capable of assisting in active site catalysis (24, 25). As well, the Thermotoga maritima lysine riboswitch has been shown to form near-identical structures in the presence of K+ and Mg2+ ions (26). In many ribozyme systems, high concentrations of monovalent cations can support significant activity (27–30). In most cases however, monovalent cation supported activities are lower than those obtained in divalent cations. These differences have been ascribed to divalent cations, notably Mg2+, being uniquely suited to assist directly in chemical catalysis as seen in group I and II introns (31–33), and guiding the formation of specific tertiary structures required for optimal catalysis (34). These types of experiments are important since they have the potential to define the obligatory roles for specific types of cations in the function of catalytic RNAs. A general lack of cation specificity for catalysis has been observed for the glmS ribozyme. Monovalent cations, several divalent cations and cobalt hexaammine have all been shown to allow for some level of activity. These experiments, however, followed the rate limiting step in the reaction pathway (6, 17, 35). Therefore, in the presence of cations that permit rapid ligand binding, these data probably represent the rate of formation of the native ribozyme structure or unfolding of a non-native intermediate rather than the rate of ligand binding and catalysis. We know little about the range of cations that are able to support the rapid ligand binding a catalytic activity that we and others have observed.
Here we present a ribozyme kinetics study aimed at defining the cation and ligand requirements for fast catalysis by the glmS ribozyme. Our data support the hypothesis that the ligand phosphate is involved in rapid binding and that divalent cations are uniquely suited to provide a rapid GlcN6P binding mode. In addition, we have observed that the glmS ribozyme, folded in the presence of monovalent cations, is indistinguishable from one folded in Mg2+.
EXPERIMENTAL PROCEDURES
RNA preparation
The 19 nucleotide substrate RNA (Figure 2A) was generated on an Applied biosystems DNA/RNA synthesizer using standard phophoramidite chemistry from Glen Research. The RNA products were deprotected and purified by denaturing PAGE and reverse phase HPLC as described previously (36). The glmS-Rz and glmS 3′P RNAs (Figure 2) were made by transcription of double-stranded DNA templates with T7 RNA polymerase. Transcription templates were constructed by annealing two large overlapping DNAs, filling in the single-stranded regions with Klenow fragment DNA polymerase and subsequent PCR amplification as previously described (13). In order to generate glmS-3′P cleavage products, 10 mM GlcN6P was added to glmS-cis transcriptions following the standard transcription incubation of 3 hours and this mixture was allowed to incubate for an additional 1 hour at 37°C. All transcription products were purified by polyacrylamide gel electrophoresis as described previously (37). Radiolabeled RNAs were prepared by phosphorylation of the 5′-terminal hydroxyl with [γ-32P]ATP (ICN) and polynucleotide kinase.
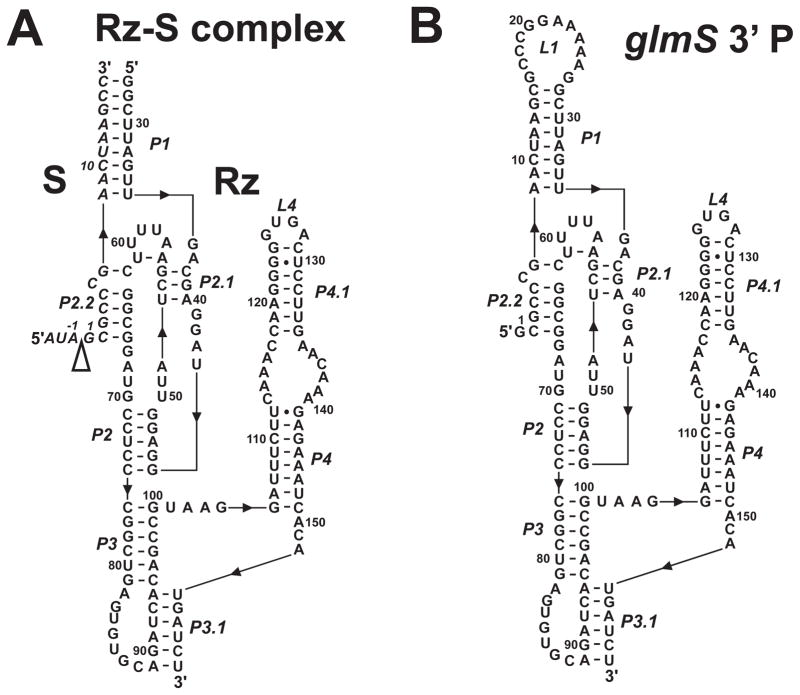
Figure 2.
Secondary structure diagrams of the Bacillus subtilis glmS trans-acting ribozyme-substrate complex (glmS-Rz-S) used in cleavage kinetics experiments (A) and glmS 3′ cleavage product (B) used in hydroxyl radical footprinting. The secondary structures are shown according to existing crystal structures for the glmS ribozyme (12, 14), and numbered according to Winkler and co-workers (6). The 5′ to 3′ direction of the RNA is indicated with black triangles. The cleavage site is indicated with a open triangle.
Ribozyme Cleavage Kinetics
A 25 mM TAPS/HEPES/Cacodylate/Sodium Acetate (THCA) buffer was utilized as previously described at all pH values so that buffer-specific effects could be minimized (16). All buffer reagents were purchase from Sigma. THCA was pH adjusted by adding NaOH to each buffer stock. The final concentration of Na+ ions was, therefore, different at each pH. This difference was adjusted by the addition of NaCl to reaction buffers above pH 6 so that the final concentration of Na+ was equal to 60 mM for all reactions. Ligand stocks were adjusted to the pH of the reaction to which they were added. All RNAs were subjected to a 2 minute incubation at 70°C in 25 mM THCA with 0.1 mM EDTA followed by a 5 minute bench top cooling time. This incubation served to release the RNAs from any non-productive conformations present upon thawing. All ribozyme kinetics assays were single-turnover experiments where the concentration of glmS-Rz was 0.5 μM and labeled substrate was present at less than 10 nM. Reaction time course were generally continued for 7 hrs for reaction rates < 0.1 min−1, and 1 hour for reaction rates > 0.1 min−1.
Complex Initiated Reactions
RNAs were incubated in 25mM THCA, pH 7.5, in the presence of various mono-, di-, and multivalent cations and 15 mM ligand at 25°C for 1.5 h. Cleavage reactions were initiated by adding an equal volume of solution containing 25 mM THCA and specified concentrations of glmS-Rz, cation, and GlcN6P. Reactions were quenched by adding 1 μL of the reaction mixture to 9 μL of loading buffer, 95% (v/v) formamide, 25 mM EDTA, 0.01% (w/v) bromophenol blue, and 0.01% (w/v) xylene cyanol. Reactions performed in 3 M NaCl were quenched by adding 1 μL of the reaction mixture to 49 μL of loading buffer. The reaction products were separated on denaturing 20% polyacrylamide gels and the data were quantified using BioRad phosphorimager analysis and Quantity One 1-D software. The resulting data points were plotted and fitted using Synergy’s Kalidegraph (version 3.6) software to the double exponential equation y = yo = A1(1−e−t/τ1) + A2(1−e−t/τ1), yielding cleavage rate constants ki = 1/τ1 and amplitudes Ai. Rapid quench reactions were carried out on a three syringe Kintek rapid quench mixer. Equal volumes from the two sample ports containing RNA and buffer components as described were pushed into the mixing chamber to initiate reactions. The push buffers were identical in composition to the samples that they pushed minus the RNA and GlcN6P. The quench syringe contained rapid quench buffer (RQB) 86% (v/v) formamide, 35 mM EDTA and 1X Tris-borate-EDTA (TBE) buffer. Reactions containing a monovalent cation background were expelled into microcentrifuge tubes containing 400 μL of additional RQB, as listed above.
Ligand Initiated Reactions
RNAs were incubated in 25 mM THCA, pH 7.5, in the presence of 3 M NaCl and 1 mM EDTA and incubated at 25°C for 1.5 h. Cleavage reactions were initiated by adding an equal volume of solution containing 25mM THCA and specified concentrations of cation, and GlcN6P. Reactions were quenched by adding 1 μL of the reaction mixture to 49 μL of loading buffer, 95% (v/v) formamide, 25 mM EDTA, 0.01% (w/v) bromophenol blue, and 0.01% (w/v) xylene cyanol. The reaction products were separated on denaturing 20% polyacrylamide gels and the data was quantified and fit as described above. Rapid quench reactions were carried out as described above and quenched into 400 μL of additional RQB as described above.
Measurement of the rate of GlcN6P independent folding
Unlabeled ribozyme and 5′end labeled substrate were incubated separately in 25 mM THCA in the presence of 15 mM MgCl2 for 1.5 h at 25°C. Folding reactions were initiated by adding equal volumes of ribozyme and substrate solutions together. At various time points a 15 mM GlcN6P chase was added to the reaction and the mixture was incubated for 5 seconds before quenching. Time points faster than 10 second reactions were conducted on a three-syringe Kintek rapid quench mixer. The right sample syringe contained 5′-end-labeled cleavable substrate in 25 mM THCA pH 7.5 and 15 mM MgCl2. The left sample syringe contained cold ribozyme in the presence of 25 mM THCA pH 7.5 and 15 mM MgCl2. Buffer syringes contained 25 mM THCA and 15 mM MgCl2. The quench syringe contained equal concentrations of buffer and Mg2+ in addition to saturating concentrations of GlcN6P. Individual reactions proceeded with an initial mixing of equal volumes of the two sample syringes. This mixture was allowed to fold for a specified time at 25°C prior to rapid mixing with GlcN6P from the quench syringe. When the quench syringe solution is introduced to the reaction, GlcN6P binds native Rz-S complex resulting in cleavage of the RNA backbone. This solution is pushed into an empty microcentrifuge tube, allowed to incubate for 5 seconds and then quenched with 400 μL RQB. Substrate and product were separated on 20% denaturing polyacrylamide gels and analyzed with a Bio-Rad phosphorimager as described above.
pH Dependence of Ligand Initiated Reactions
RNAs were incubated in 25mM THCA, pH 5.5 to pH8, in the presence of 15 mM MgCl2 or 3 M NaCl and 1 mM EDTA and incubated at 25°C for 1.5 h. Cleavage reactions were initiated by adding an equal volume of solution containing 25 mM THCA and specified concentrations of cation, and saturating concentrations of either GlcN6P or GlcN (Supporting Information Figures S3 and S4). Reactions containing MgCl2 were quenched by adding 1 μL of the reaction mixture to 9 μL of loading buffer, 95% (v/v) formamide, 25mM EDTA, 0.01% (w/v) bromophenol blue, and 0.01% (w/v) xylene cyanol. Reactions containing a 3 M NaCl background were quenched by adding 1 μL of the reaction mixture to 49 μL of loading buffer. The reaction products were separated on denaturing 20% polyacrylamide gels and the individual cleavage rate were quantified and fit using the double exponential equation described above. Cleavage rates were plotted as a function of pH and fit to a single ionization equation: kobs = kmax/(1 + 10(pka-pH)), where kmax is the intrinsic rate of the cleavage reaction and pKa is the acid dissociation constant of a single titrating functional group. Rapid quench reactions were carried out as described above and reactions containing 3 M NaCl were additionally quenched into 400 μL of RQB, as described above.
Equilibrium hydroxyl-radical footprinting reactions
End-labeled glmS 3′cleavage product (Figure 2A) was incubated in a buffer containing 25 mM Na-cacodylate, pH 7 and a range of NaCl and MgCl2 concentrations for 2 minutes at 70°C followed by 90 minutes at room temperature and then probed using hydroxyl-radicals as described previously (13). Polyacrylamide gel electrophoretic separation of the cleavage products and quantification of the results was conducted according to our previously published methods (13). Protected fractions at each site were normalized to the highest level of protection observed in individual experiments and plotted as a function of cation concentration. Magnitudes of protection matched our previously reported range (13).
RESULTS
Our objective for this work was to define the cation specificity and ligand requirements for the rapid ligand binding and catalysis that we and others have observed upon addition of ligand to prefolded ribozymes (Figure 1) (15, 16). We have previously demonstrated that the glmS ribozyme rapidly collapses into a near native tertiary structure as shown by time resolved hydroxyl radical footprinting (16). These experiments, performed both in the presence and absence of GlcN6P, indicate a rapid compaction (~1 s−1) of the ribozyme into a (near) native tertiary structure without any evidence of a distinct conformational rearrangement. In contrast, the glmS ribozyme achieves a catalytically active conformation on a much slower timescale, ~3 min−1, in the presence of saturating levels of MgCl2 and GlcN6P. This slow step is cation dependent and must be completed prior to catalysis (Figure 1A). These experiments were performed in the presence of GlcN6P and, therefore, do not provide data for the potential of ligand induced behavior during this transition. Therefore, before examining the requirements for rapid ligand binding and catalysis we wanted to be able to rule-out ligand assisted prefolding of the native complex.
To monitor the rate limiting folding event in the absence of ligand, we performed ‘GlcN6P chase’ experiments. Briefly, trans ribozyme-substrate complex [glmS Rz-S (Figure 2A)] was prefolded in MgCl2 in the absence of GlcN6P for varying amounts of time. After this folding time we introduced a saturating concentration of GlcN6P into solution and allowed the reaction to proceed for 5 seconds before quenching (Figure 3A). The 5 second chase time was chosen for these reactions due to the observation that when the ribozyme-substrate complex was folded in MgCl2 containing solutions prior to initiation by adding a saturating concentration of GlcN6P, >80% substrates are cleaved during this time (16). In addition, the 5-second chase time can be accurately and reproducibly made by hand mixing. Although cation dependent folding is ongoing during the short chase time, we did not expect this to significantly increase the apparent rate of this slow step in the reaction. Thus, we measured the fraction of molecules that are folded into a catalytically active conformation as a function of folding time. Experiments were performed in the rapid quench and via hand-held mixing. We observed global folding rates equal, within error, in the presence and absence of GlcN6P [(3.5 min−1 ± 0.1 vs 3.4 min−1 ± 0.26, respectively) (Figure 3B)]. These findings support our previous data indicating that the rate-limiting step is cation dependent with no detectable influence of GlcN6P. We have also demonstrated, through the use of two distinct experimental designs, that the ligand binding and catalysis steps of the reaction pathway can be isolated from this slow, rate-limiting, folding step by pre-incubating the RNA in MgCl2 prior to initiation of catalysis (Figure 1) (16).
Figure 3.
Determining the global folding rate of the glmS ribozyme in the absence of GlcN6P. (A) Experimental design used to determine the rate of global folding in the absence of GlcN6P. Ribozyme and substrate strands were mixed in the presence of MgCl2 and allowed to fold for a specific time. The level of native folding was then assayed by addition of GlcN6P for a fixed length of time, 5 seconds. (B) Comparison of a representative complex initiated reaction (open circles) in the presence of GlcN6P previously reported (16) and three replicate GlcN6P chase reactions (closed circles with error bars set at one standard deviation). The fraction of substrate cleaved is plotted as a function of time and were fit to a double-exponential equation as described in Experimental Procedures.
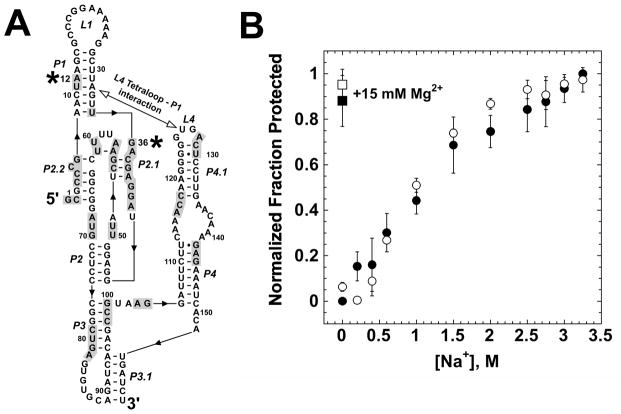
In order to gain a better understanding of the cation specificity of the glmS ribozyme, we sought to elucidate the role that cations play in the process of folding, ligand binding and catalysis. We utilized the glmS-Rz-S construct (Figure 2B) and performed single turnover kinetic complex-initiated experiments, as previously described (16) (Figure 1B) in the presence of mono-, di- and multivalent cations. This assay measures the rate-limiting step of the catalytic pathway. Our findings support previous data showing a lack of strong cation specificity as measured in this manner (17). Mn2+and Ca2+ ions promote rates of self-cleavage that are similar (within 4-fold difference) to those performed in the presence of Mg2+ (Figure 4, Table 1). Co(NH3)63+, an exchange-inert structural mimic of hexahydrated Mg2+, associates with RNA only through outer sphere and electrostatic interactions (38). This cation promotes a cleavage rate an order of magnitude slower than that achieved in MgCl2. In addition, we observed that the use of molar concentrations of NaCl resulted in cleavage rates within 3-fold of the rate achieved in MgCl2 (Figure 4) (17). These data supports previous findings indicating the glmS ribozyme lacks an essential role for divalent metal ions in either the folding process or to promote catalysis (17). K+ ions have been found to promote catalysis at rates similar to molar concentrations of Na+ ions (17). Next, we wanted to determine if molar concentrations of NaCl are sufficient to fold the glmS motif into its native tertiary structure. Equilibrium hydroyxl radical footprinting experiments were performed using the glmS 3′-cleavage product (Figure 2B) in increasing concentrations of NaCl. Figure 5 shows the comparison solvent protection as a function of NaCl concentration and 15 mM MgCl2 at two sites on the ribozyme backbone. A12 is protected by a long-range tertiary interaction between the L4 tetraloop and P1 (Figure 2). G36 is protected by formation of the core double pseudoknot structure (14). The results from the quantification of these two sites are representative of the entire protection pattern in molar NaCl and 15 mM MgCl2. These experiments revealed that molar concentrations of Na+ ions are sufficient to promote equivalent solvent protection at the same RNA sites as obtained in the presence of MgCl2 (Figure 5).
Figure 4.
Complex initiated trans-cleavage reactions in various cations. Complex initiated cleavage assays were conducted as described previously (16), utilizing glmS-Rz-S construct, at pH 7.5. Experiments were carried out over a range of cation concentrations using saturating amounts of GlcN6P. Reaction rates were plotted as a function of cation concentration on a log scale. The symbols used for each cation are shown in the graph as follows, Mg2+ filled circles, Ca2+ open circles, Mn2+ filled squares, Co(NH3)63+ open squares, Na+ filled diamonds. All data points are the average of three replicate experiments with error bars set at one standard deviation from the mean. Individual titrations were fit to the Hill equation as described in the Experimental Procedures. kobsmax, Kdapp and the Hill cofficient, n, for each cation are reported in Table 1.
Table 1.
Apparent Kds and maximum cleavage rates (kobsmax) for complex-initiated reactionsa employing a variety of cations at pH 7.5
| Cation | Kdapp b | kobsmax, min1 | n |
|---|---|---|---|
| Na+ | 1.5 M | 1.2 | 2.7 |
| Mg2+ | 10 mM | 3.6 | 2.0 |
| Ca2+ | 6 mM | 3.4 | 2.5 |
| Mn2+ | 2.5 mM | 0.77 | 1.5 |
| Co(NH3)63+ | 0.28 mM | 0.33 | 1.3 |
The complex-initiated experimental design is outlined in Figure 1B and described in Experimental Procedures.
Kd values were determined by fitting kobs vs cation concentration plots to the Hill equation, as described in Experimental Procedures, where the value for the Hill coefficient (n) is shown above for each cation tested.
Figure 5.
Global folding of the glmS-3′P as a function of Na+ concentration. End-labeled glmS-3′P was folded in the indicated concentration of Na+ or 15 mM Mg2+ and then probed with hydroxyl-radical as described in Experimental Procedures. (A) The secondary structure of the glmS-3′P with the sites of hydroxyl radical protection shaded. Positions 12 and 36 are indicated with asterixes. (B) Quantification of the solvent protection at one site of core tertiary structure, G36 (open symbols), and one site of peripheral tertiary interaction, A12 (filled symbols) in the presence of 15 mM MgCl2 (squares) and a range of NaCl (circles) concentrations.
Since molar concentrations of NaCl are capable of prefolding a native tertiary structure, we sought to determine if 3 M NaCl could promote rapid ligand binding and chemical catalysis in a GlcN6P-initiated experimental design (Figure 1C). Briefly, the ribozyme and substrate were preincubated together in 3 M Na+ for 90 minutes. This time of incubation is sufficient to allow Rz-S complexes to reach a prefolded equilibrium endpoint. Reactions were then initiated by the addition of a saturating concentration of GlcN6P in the presence or absence of 15 mM MgCl2. The background concentration of NaCl was maintained at 3 M for the initiation of the reactions. To ensure saturation of the system with GlcN6P in a background of 3 M NaCl, a shallow titration of ligand was conducted using a 3 M NaCl background to prefold the ribozyme-substrate complex and various cations and GlcN6P to initiate the reaction (See Supporting Information Figure S3). Little variation in the concentration of ligand required to saturate the reaction among the various cations was observed in these experiments. We compared the results of experiments performed in 3 M NaCL, plus or minus 15 mM MgCl2, with identical experiments where prefolding and GlcN6P-initiation were carried out in 15 mM MgCl2 (Figure 6A). Reactions initiated with GlcN6P in 3 M NaCl plus 15 mM MgCl2 proceeded with the same rate as reactions initiated with GlcN6P and 15 mM MgCl2 (Figure 6A). However, reactions that were initiated in the presence of 3 M NaCl as the sole cation proceeded 18-fold slower than those initiated in the presence of 15 mM MgCl2 (Figure 6A). These findings indicate that molar concentrations of monovalent cations are sufficient to induce the formation of the native coenzyme binding structure, but do not result in the rapid burst of catalysis in the presence of GlcN6P (Figure 6A). Overall, our data suggest that the ribozyme-substrate complex can be prefolded in 3 M NaCl into a native structure equivalent to that produced in MgCl2.
Figure 6.
GlcN6P initiated trans-cleavage demonstrate the requirement for both divalent cations and a ligand phosphate for fast catalysis. GlcN6P initiated cleavage reactions were conducted at pH 7.5, utilizing the glmS-Rz-S construct as described previously (16). (A) Reaction rates determined as described in Experimental Procedures are plotted as a function of the folding and initiating cation, 15mM MgCl2 and 3 M NaCl in the presence of saturating concentrations of GlcN6P. (B) Loss of rapid cleavage kinetics in the absence of ligand phosphate group. Reactions were initiated using various cations and saturating levels of GlcN6P (open bars) or GlcN (filled bars) after prefolding glmS-Rz-S in 3 M NaCl for 90 minutes. Concentrations of cations used were as follows; 15mM of MgCl2, CaCl2 and MnCl2, 10mM Co(NH3)63+ and 3 M NaCl, maintained in a buffer containing 3 M NaCl. All reaction rates are the average of three replicate experiments and error bars indicate one standard deviation from the mean.
Having previously demonstrated a lack of cation specificity through the slow-folding step of the reaction pathway (Figure 4), we wanted to explore the ability of a variety of cations to promote fast ligand binding and reaction chemistry. To do this, we utilized the ability of the ribozyme to fold to a native ligand-unbound, or apo, conformation in 3 M NaCL. Reactions were initiated with saturating concentrations of both GlcN6P and the cations listed while maintaining 3 M NaCl (Figure 6B, open bars). Our data shows that the identity of the cation affects the ability of the glmS ribozyme to undergo rapid catalysis in a background of monovalent cation. Of the divalent cations tested, Mg2+ was most efficient in promoting rapid catalysis in the presence of ligand, with MnCl2 and CaCl2 promoting similar catalytic rates under these conditions (Figure 6B). The reduced catalytic rate in the presence of Mn2+ and Ca2+ ions could be the result of conformational or structural limitations of the active site. Although Mn2+ and Ca2+ are the smallest and largest of the divalent cations tested, at 0.67Å and 1.12Å, respectively, they exhibit essentially no difference in their ability to promote catalysis in the presence of ligand. These data suggest an optimal cation size necessary to effectively position the ligand and promote catalysis (39). Co(NH3)63+, a structural analog of fully hydrated Mg2+, was very inefficient at promoting catalysis in the presence of ligand, with a cleavage rate that was only slightly higher than the rate of cleavage triggered by ligand and 3 M NaCl alone (Figure 6B). Unlike the associated water ligands of Mg2+, Ca2+ and Mn2+, the ammine ligands of Co(NH3)63+ cannot be displaced to make inner sphere contacts (38). This indicates that in order to achieve rapid catalysis, there may be a requirement for water-mediated coordination to achieve appropriate or rapid placement of GlcN6P within the binding pocket. Overall, our data suggest that divalent cations are more effective than monovalents or Co(NH3)63+ at positioning GlcN6P within the ligand binding pocket. Alternatively, Co(NH3)63+ may induce ribozyme conformation that is not optimal for rapid catalysis.
Crystallographic structures indicate the presence of two fully hydrated magnesium ions within the ligand binding pocket of the glmS ribozyme (12). The magnesium ions coordinate the phosphate group of GlcN6P in addition to making contacts with the RNA backbone and multiple functional groups within the active site. In order to test the hypothesis that rapid ligand binding and catalysis is determined in part by interactions between Mg2+ and the ligand phosphate we examined the ability of glucosamine (GlcN), a non-phosphorylated analog, to promote ligand initiated reactions. Substitution of GlcN for GlcN6P largely eliminates the cation-dependent variability in ligand-initiated cleavage rates observed with GlcN6P (Figure 6B). Indeed, all GlcN initiated reactions rates are within approximately 2-fold of one another and are all below 4.5 min−1. It should be noted that under all conditions we have employed GlcN at a concentration beyond which we see no further rate enhancement (See Supporting Information Figures S3 and S4). These data support the hypothesis that an interaction between divalent cations and the phosphate moiety of GlcN6P increases the ligand binding rate.
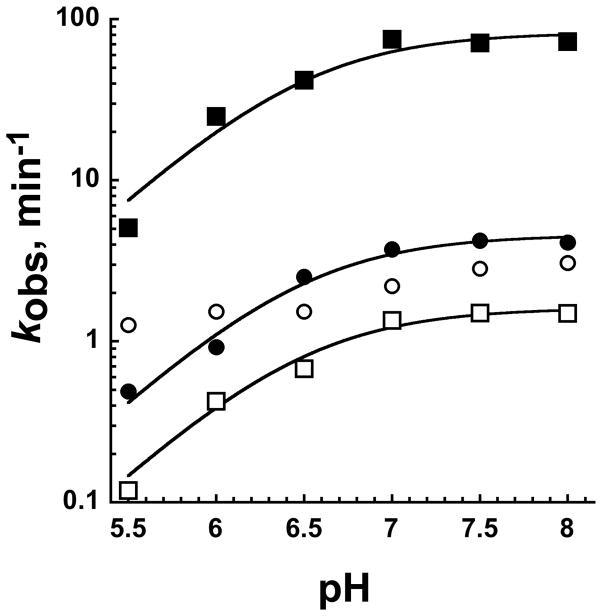
In addition to interacting with the phosphate group of GlcN6P, crystallographic structures also show the Mg2+ ions making contact with nucleobase functional groups within the ligand-binding pocket. Additionally, previous experiments have shown that GlcN6P binding is weak at low pHs, which has been postulated to be due to neutralization of the phosphate moiety and loss of the ability to coordinate Mg2+ (15). The pKa of the phosphate group has been determined, via NMR, to be ~6.1 (15). In order to examine the possibility of Mg2+-dependent pH effects we determined the rate/pH profiles of GlcN6P-initiated reactions in MgCl2 and 3 M NaCl (Figure 7). When the reaction is triggered with MgCl2 and GlcN6P, we observed a base-activation rate/pH profile with a kmax = 70 and a pKa = 6.2 when fitting to an equation assuming a single titratable group (Figure 7). The pKa for this experiment is very close to previous findings (15) (Figure 7). Reactions initiated with 3 M NaCl and GlcN6P display pKa = 6.4, and also fit to an equation assuming a single titratable group, but with a 18 fold lower kmax. When the same reactions were initiated with GlcN we observed the same pH dependent reactions in the presence of MgCl2 but not NaCl. In 3 M NaCl, GlcN-initiated reactions displayed only a two-fold increase in the cleavage rate over the pH5.5–8 range (Figure 7). These data indicates that removal of the phosphate moiety in the presence of Mg2+ ions does not eliminate the pH dependence of the catalytic reaction, as observed with Na+ as the sole cation.
Figure 7.
pH dependence of the glmS ribozyme trans cleavage in the presence of GlcN6P or GlcN. Reaction rates determined as described in Experimental Procedures are plotted as a function of pH (5.5–8). The glmS-Rz-S was prefolded in MgCl2 (squares) or 3 M NaCl (circles) for 90 minutes prior to initiating the reaction with saturating concentrations (See Supplementary Data) of GlcN6P (filled symbols) or GlcN (open symbols). The data were fit to a single ionization equation as described in Experimental Procedures. Each data point is the average of three replicate experiments and error bars indicating one standard deviation from the mean are shown, but are smaller than the symbols.
DISCUSSION
The rate of the glmS ribozyme reaction pathway is limited by a slow folding step that precedes formation of a native structure capable of ligand binding (16). Having previously shown that the pathway can be dissected such that the combined rate of the ligand binding and catalysis steps can be studied in isolation from this rate-limiting step, our goal in the current work was to define the cation and ligand requirements for these rapid steps (16). All previous examinations of the cation requirements for the glmS ribozyme have used experimental designs that assay the rate-limiting step in the pathway (6, 17). Here we have shown that in molar concentrations of Na+ ions, the ribozyme is able to fold to the native state and catalyze reactions through the rate-limiting step at a rate similar to that observed in MgCl2-containing solutions (Figures 4 and 5). We define “through the rate-limiting step” as complex-initiated reactions where the ribozyme-substrate complex must fold into its native three-dimensional structure during the assay. More importantly, high NaCl allows the ribozyme to fold into a complex that is competent to bind ligand, preincubated in MgCl2, rapidly (≥70 min−1) and execute chemical catalysis (Fig. 6A). These data also indicate that the reduction in water activity in 3 M NaCl compared to dilute salt conditions does not strongly influence the folding of the ribozyme (Figure 5). We cannot exclude the possibility that this solution property plays a role in the low catalytic rate of the glmS ribozyme in 3M NaCl. However, our data fit more closely to a model where Mg2+ ions are particularly well suited to promote rapid ligand binding and catalysis.
Molar concentrations of monovalent cations, such as Na+, K+, Li+ and NH4+ have been employed in a number of ribozyme systems in order to define the specific requirements for Mg2+ in ribozyme structure and catalysis (22, 40, 41). Though molar concentrations of monovalents are not physiological, they can serve to define structural and catalytic roles uniquely suited to divalent cations (28, 30, 42). We have observed Na+-mediated native folding of the glmS ribozyme into a tertiary structure that is able to bind GlcN6P and Mg2+, without any apparent conformational change, and direct catalysis very rapidly. This suggests that the sole obligatory role for Mg2+ ions in the glmS system is to assist in ligand binding. We are limited in this interpretation in one respect only. If a Mg2+-dependent conformational change is required of the 3 M NaCl folded ribozyme that is faster than the rate of ligand binding, we would not observe this event in our catalytic assays. We expect that if such a conformational change exists, however, it is a short-range change in structure to accommodate ligand binding. Nevertheless the observation that the native glmS tertiary structure can be form in the complete absence of divalent cations is unique among ribozyme systems. The Tetrahymena group I intron ribozyme can be folded to a near native three-dimensional conformation in high monovalent cation concentrations (30, 43), but requires divalent cations to activate the active site chemistry and to fold the catalytic core. In the case of the glmS ribozyme folding in 3 M NaCl is sufficient to form all hydroxyl-radical protected sites to the same magnitude observed in MgCl2. High NaCl is able to mediate native tertiary contacts in the S. mansoni hammerhead ribozyme, but does not alone permit efficient catalysis to occur (27). Furthermore, when molar concentrations are present, the cleavage rate in MgCl2 is reduced 4600-fold suggesting that monovalent cations are able to compete for divalent cation binding or shield the RNA from divalent cations (27). In contrast we find that 3 M NaCl has no observable effect on the ligand-initiated cleavage rate in the presence of 15 mM MgCl2 (Figure 6A). This result indicates that Na+ is itself capable of folding the RNA to the native conformation, but requires the presence of Mg2+ ions to activate catalysis. In addition, it our data indicate that high salt-induced changes to the electrostatic potential of the RNA does not rapid ligand binding and catalysis observed in the presence of Mg2+. MgCl2 mediated reactions in the HDV ribozyme system can be inhibited by up to 10-fold the addition of high concentrations of monovalent cations, but this inhibition can be partially reversed by increasing the MgCl2 concentration (44), suggesting that Mg2+ ions can preferentially occupy specific binding sites related to catalytic activity. Our results also argue that there is no functional difference between the structure prefolded in MgCl2 or NaCl since both are able to achieve the same cleavage rate enhancement upon the addition of GlcN6P and MgCl2. The Thermotoga maritima lysine riboswitch has also been shown to form near- identical structures in the presence of K+ and Mg2+ ions (26). We conclude that folding of the ribozyme is relatively independent of cation identity but the rapid initiation of catalysis upon the addition of ligand is stricter.
Of the three divalent metals tested, Mg2+ was most effective at ~65 min−1, while Ca2+ and Mn2+ were almost 3-fold slower at ~20 min−1 and ~27 min−1, respectively, indicating specific requirements to achieve rapid catalysis. The reduced catalytic rate in the presence of Mn2+ and Ca2+ could be the result of conformational or structural limitations of the active site. Although Mn2+ and Ca2+ are the smallest and largest of the divalent cations tested, at 0.67Å and 1.12Å, respectively, they exhibit essentially no difference in their ability to promote catalysis in the presence of ligand. These data may indicate an optimal cation size requirement necessary to effectively position the ligand and promote catalysis (39). To achieve maximum catalytic activity, it is not unusual for certain ribozymes to discriminate against closely related cations. For example, naturally occurring Tetrahymena and Azoarcus group I ribozymes exhibit a strong dependence on Mg2+ for effective activity and display no detectable activity in vitro with Ca2+ as the sole divalent (45–48). Additionally, the genomic and antigenomic forms of the HDV ribozyme also exhibit an interesting Mg2+/Ca2+ switch, in which the genomic form is catalytically efficient in Mg2+ whereas the antigenomic form cleaves faster in Ca2+ (49). The difference in rates for the glmS ribozyme could also be due to distinct metal ions coordinating GlcN6P in slightly different manners within the ligand binding pocket, thus promoting a different binding mode that may result in less efficient catalysis (39).
Finally, altered outer sphere coordination could be responsible for reduced catalytic efficiency when compared to Mg2+. Although all three of the divalent cations tested are hexahydrated, Mg2+ strongly prefers an octahedral geometry while Ca2+ is more flexible and has a greater range of coordination numbers and also exhibits a greater range of bond distances (50). Ca2+ and Mn2+, although hexahydrated, may not be able to create or maintain the water-mediated contacts necessary for optimal catalysis, due to the orientation/geometry of their surrounding water molecules. Additionally, the coordination geometry and the distance between coordinated ligands may be best suited for Mg2+ (40). Co(NH3)63+, a structural mimic of Mg2+, was very inefficient at promoting catalysis in the presence of ligand, with a cleavage rate only slightly above the cleavage rate triggered by ligand and Na+ alone (Figure 6A). Unlike the associated water ligands of Mg2+, Ca2+ and Mn2+, the ammine ligands of Co(NH3)63+ cannot be displaced to make inner sphere contacts (38). The crystal structure indicates that the Mg2+ ions make both outer sphere and water mediated contacts within the ligand binding pocket. The ammine groups of Co(NH3)63+ should be capable of creating appropriate outer sphere contacts, but would be incapable of creating the water mediated contacts to the nucleobase functional groups within the ligand binding pocket. These data indicates that in order to achieve rapid catalysis, there may be a requirement for a specific geometry of water-mediated coordination to achieve appropriate placement of GlcN6P within the binding pocket to promote rapid catalysis. Overall, our data indicates that divalent cations are more effective than monovalents or Co(NH3)63+ at positioning GlcN6P within the ligand binding pocket to promote rapid catalysis.
Our evidence supports the hypothesis that this Mg2+ or divalent cation requirement is due to the need for divalent cations to bind to GlcN6P and assist in ligand binding to the RNA. This hypothesis has been presented previously on the basis of crystallographic structures that model two hydrated Mg2+ ions coordinated between RNA functional groups and the phosphate moiety of GlcN6P within the ligand binding pocket (12, 14). Substitution of glucosamine (GlcN), an analogue of GlcN6P lacking the phosphate group for GlcN6P, eliminates all fast ligand triggered reaction regardless of the cation employed. All cations tested promote catalysis at ~2/min (Figure 6B), supporting the idea that ligand binding is strongly assisted by divalent cations and that the phosphate moiety of GlcN6P is required for rapid positioning and/or efficient catalytic activity within the ligand-binding pocket (Figure 7). The phosphate group of GlcN6P may help to ‘lock’ the ligand into the ligand-binding pocket. The phosphate edge of the ligand binding pocket is solvent exposed. Both Mg2+ ions and water molecules act to screen the negative charge of the RNA phosphate backbone from the phosphate group of GlcN6P (12), while the remaining portion of the ligand is buried within the active site. Glucose 6-Phosphate (Glc6P), an analogue of GlcN6P, with a hydroxyl group in place of the primary amine, is a competitive inhibitor of the cleavage reaction (18). Crystal structures indicate that Glc6P occupies the ligand binding pocket of the active site in a manner almost identical to GlcN6P binding. The glmS ribozyme recognizes both the phosphate and sugar moieties of Glc6P (14), making a set of contacts that is similar to those made by GlcN6P (15). Even following cleavage, the glmS ribozyme retains affinity for GlcN6P, as post cleavage crystallographic structures identify density for at least the phosphate atom of GlcN6P (15). Our observation of similar reaction rates in GlcN, where the ligand phosphate is absent, or in the presence of 3 M Na+ as a sole cation suggests that the same reaction pathway step is limiting. Under all conditions that we have tested ligand titrations have been conducted and the reported rates are from experiments where ligand binding is saturating (see Supporting Information Figure S3 and S4). The intrinsic rate of ligand association with the ribozyme, however, has not been determined. We do not, therefore, have direct evidence that ligand binding is rate-limiting under these conditions. The flavin mononucleotide (FMN) riboswitch also utilizes a Mg2+-dependent mechanism for coordinating the ligand phosphate in the binding pocket and biochemical studies have shown a strong preference for binding phosphorylated ligands and the presence of Mg2+ (51). In addition, the glycine riboswitch has been shown to form significant native tertiary structure in the presence of molar concentrations of NaCl, but requires specific divalent cations to support ligand binding (52, 53).
Evidence for the importance of the phosphate moiety of GlcN6P towards rapid ligand binding has also come from experiments showing that the highest pKa for a non-bridging phosphate oxygen, as determined by NMR (~pH 6.1), correlates well with the K1/2 of the ligand, determined catalytically (15). The implication is that protonation of a single ligand non-bridging phosphate oxygen impairs ligand binding due to loss of the Mg2+ coordination between the ligand and RNA. Thus, the ribozyme is activated under basic conditions. The same conclusion has been made recently on the basis of molecular dynamics simulations (54). We determined the pH reactivity profiles determined at saturating concentrations of GlcN6P or GlcN in MgCl2 or 3 M NaCl in order to examine this idea in greater detail (Figure 7). In both Na+ and Mg2+ GlcN6P-initiated reactions have rate/pH profiles and fit well to a single ionization equation and yield pKa’s of pH 6.4 and 6.5, respectively (Figure 7). This does not fit with the Mg2+ requirement in this model, but does support the proposal that the non-bridging oxygen can make a hydrogen bond with the N1 hydrogen of the invariant guanosine at position 1, G1, in MgCl2 and 3 M NaCl (21). Phosphate protonation is expected to both disrupt this interaction and be cation independent. In addition, our data are consistent with a model for this rate/pH behavior suggesting a general base role for scissile phosphate pro-Rp non-bridging phosphate oxygen (54).
More recently, molecular dynamic simulations and detailed free energy calculations indicate the apparent pKa of 6.1 to be attributed to the amine group of GlcN6P (55). Since both GlcN6P and GlcN retain an amine group at position 2, it is plausible that the apparent pKa of ~6.5 is attributable not to the phosphate group, but to the amine. To explore this idea further, we determined the pH reactivity profile of the glmS ribozyme utilizing saturating concentrations of GlcN in Mg2+ or 3 M NaCl background. Prefolding in MgCl2 followed by GlcN-initiation resulted in an 10-fold decrease in catalytic rate over the pH range tested, pH 5.5 – 8, fitting to a single ionization event with an apparent pKa of ~6.5. We did not observe a pH-dependent cleavage rate when reactions were initiated with 3 M Na+. It is possible that under these conditions a different, pH independent, step in the reaction is rate-limiting, however, the rate of GlcN-mediated catalysis is 10-fold faster in the presence of 3 M NaCl than in MgCl2 at pH 5.5. Another possible reason GlcN is less effective at promoting reaction rates closer to those produced by GlcN6P is due to the presence of unfilled space within the ligand binding pocket in the absence of the phosphate group. Without the phosphate moiety to form the fixed network of Mg2+ coordinated interactions with the various functional groups of the ligand binding pocket, it is possible that the hydrogen bonds coordinating the glucosamine ring are not sufficient to effectively position GlcN for reaction chemistry. In Na+ the binding pocket may be better able to tolerate protonation of specific residues.
The ligand-initiated site specific RNA cleavage catalysed by the glmS ribozyme is a very interesting and novel riboswitch mechanism. Ligand recognition is clearly central to the function of all riboswitches and our data on this topic provide new insight for the glmS ribozyme. We find that both the ligand phosphate moiety and the presence of Mg2+ ions make significant contributions to ligand binding and catalysis by the ribozyme. These effects are largely non-additive, suggesting that they affect the same molecular event. This new insight may be useful in designing chemical agonists or antagonists of the glmS ribozyme for antibiotic use against Gram positive bacteria of biomedical importance which employ this rcatalyst.
Supplementary Material
Acknowledgments
This work was supported by NIH GM065552.
The authors thank Dr. John M. Burke and the Department of Microbiology and Molecular Genetics, University of Vermont, for generously supporting this independent line of research, and Joyce Heckman for providing helpful discussions.
ABBREVIATIONS AND TEXTUAL FOOTNOTES
- ATP
adenosine triphosphate
- EDTA
ethylenediaminetetraacetic acid
- FMN
flavin mononucleotide
- GlcN
glucosamine
- GlcN6P
glucosamine 6-phosphate
- HDV
Hepatitis delta virus
- HEPES
N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- HPLC
high performance liquid chromatography
- mRNA
messenger ribonucleic acid
- PAGE
polyacrylamide gel electrophoresis
- PCR
polymerase chain reaction
- RNase
ribonuclease
- TAPS
N-tris[Hydroxymethyl]methyl-3-aminopropanesulfonic acid
- TBE
Tris borate EDTA
- Tris
tris(hydroxymethyl)aminomethane
- tRNA
transfer ribonucleic acid
- UV
ultraviolet
- VS
Varkud satellite
Footnotes
Figure SI1 demonstrates that hydroxyl-radical footprinting of the glmS ribozyme 3′product reveals identical protections in 15 mM MgCl2 and molar concentrations of NaCl. The data presented as Supporting Information Figures SI2 – SI4 show that under all solution conditions employed in these experiments the cation and/or ligand concentrations used were at the level beyond which no increase in catalytic rate is observed. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 2.Winkler W, Nahvi A, Breaker RR. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- 3.Mironov AS, Gusarov I, Rafikov R, Lopez LE, Shatalin K, Kreneva RA, Perumov DA, Nudler E. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. Cell. 2002;111:747–756. doi: 10.1016/s0092-8674(02)01134-0. [DOI] [PubMed] [Google Scholar]
- 4.Nudler E, Mironov AS. The riboswitch control of bacterial metabolism. Trends Biochem Sci. 2004;29:11–17. doi: 10.1016/j.tibs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, Wickiser JK, Breaker RR. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci U S A. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, Boland F, Brignell SC, Bron S, Bunai K, Chapuis J, Christiansen LC, Danchin A, Debarbouille M, Dervyn E, Deuerling E, Devine K, Devine SK, Dreesen O, Errington J, Fillinger S, Foster SJ, Fujita Y, Galizzi A, Gardan R, Eschevins C, Fukushima T, Haga K, Harwood CR, Hecker M, Hosoya D, Hullo MF, Kakeshita H, Karamata D, Kasahara Y, Kawamura F, Koga K, Koski P, Kuwana R, Imamura D, Ishimaru M, Ishikawa S, Ishio I, Le Coq D, Masson A, Mauel C, Meima R, Mellado RP, Moir A, Moriya S, Nagakawa E, Nanamiya H, Nakai S, Nygaard P, Ogura M, Ohanan T, O’Reilly M, O’Rourke M, Pragai Z, Pooley HM, Rapoport G, Rawlins JP, Rivas LA, Rivolta C, Sadaie A, Sadaie Y, Sarvas M, Sato T, Saxild HH, Scanlan E, Schumann W, Seegers JF, Sekiguchi J, Sekowska A, Seror SJ, Simon M, Stragier P, Studer R, Takamatsu H, Tanaka T, Takeuchi M, Thomaides HB, Vagner V, van Dijl JM, Watabe K, Wipat A, Yamamoto H, Yamamoto M, Yamamoto Y, Yamane K, Yata K, Yoshida K, Yoshikawa H, Zuber U, Ogasawara N. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milewski S. Glucosamine-6-phosphate synthase--the multi-facets enzyme. Biochim Biophys Acta. 2002;1597:173–192. doi: 10.1016/s0167-4838(02)00318-7. [DOI] [PubMed] [Google Scholar]
- 9.Collins JA, Irnov I, Baker S, Winkler WC. Mechanism of mRNA destabilization by the glmS ribozyme. Genes Dev. 2007;21:3356–3368. doi: 10.1101/gad.1605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komatsuzawa H, Fujiwara T, Nishi H, Yamada S, Ohara M, McCallum N, Berger-Bachi B, Sugai M. The gate controlling cell wall synthesis in Staphylococcus aureus. Mol Microbiol. 2004;53:1221–1231. doi: 10.1111/j.1365-2958.2004.04200.x. [DOI] [PubMed] [Google Scholar]
- 11.Tinsley RA, Furchak JR, Walter NG. Trans-acting glmS catalytic riboswitch: locked and loaded. RNA. 2007;13:468–477. doi: 10.1261/rna.341807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochrane JC, Lipchock SV, Strobel SA. Structural investigation of the GlmS ribozyme bound to Its catalytic cofactor. Chem Biol. 2007;14:97–105. doi: 10.1016/j.chembiol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampel KJ, Tinsley MM. Evidence for preorganization of the glmS ribozyme ligand binding pocket. Biochemistry. 2006;45:7861–7871. doi: 10.1021/bi060337z. [DOI] [PubMed] [Google Scholar]
- 14.Klein DJ, Ferré-D’Amaré AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane JC, Lipchock SV, Smith KD, Strobel SA. Structural and chemical basis for glucosamine 6-phosphate binding and activation of the glmS ribozyme. Biochemistry. 2009;48:3239–3246. doi: 10.1021/bi802069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks KM, Hampel KJ. A rate-limiting conformational step in the catalytic pathway of the glmS ribozyme. Biochemistry. 2009;48:5669–5678. doi: 10.1021/bi900183r. [DOI] [PubMed] [Google Scholar]
- 17.Roth A, Nahvi A, Lee M, Jona I, Breaker RR. Characteristics of the glmS ribozyme suggest only structural roles for divalent metal ions. RNA. 2006;12:607–619. doi: 10.1261/rna.2266506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy TJ, Plog MA, Floy SA, Jansen JA, Soukup JK, Soukup GA. Ligand requirements for glmS ribozyme self-cleavage. Chem Biol. 2005;12:1221–1226. doi: 10.1016/j.chembiol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Cochrane JC, Strobel SA. Riboswitch effectors as protein enzyme cofactors. RNA. 2008;14:993–1002. doi: 10.1261/rna.908408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen JA, McCarthy TJ, Soukup GA, Soukup JK. Backbone and nucleobase contacts to glucosamine-6-phosphate in the glmS ribozyme. Nat Struct Mol Biol. 2006;13:517–523. doi: 10.1038/nsmb1094. [DOI] [PubMed] [Google Scholar]
- 21.Klein DJ, Wilkinson SR, Been MD, Ferre-D’Amare AR. Requirement of helix P2.2 and nucleotide G1 for positioning the cleavage site and cofactor of the glmS ribozyme. J Mol Biol. 2007;373:178–189. doi: 10.1016/j.jmb.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeRose VJ. Metal ion binding to catalytic RNA molecules. Curr Opin Struct Biol. 2003;13:317–324. doi: 10.1016/s0959-440x(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 23.Draper DE. A guide to ions and RNA structure. RNA. 2004;10:335–343. doi: 10.1261/rna.5205404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray JB, Seyhan AA, Walter NG, Burke JM, Scott WG. The hammerhead, hairpin and VS ribozymes are catalytically proficient in monovalent cations alone. Chem Biol. 1998;5:587–595. doi: 10.1016/s1074-5521(98)90116-8. [DOI] [PubMed] [Google Scholar]
- 25.Nesbitt S, Hegg LA, Fedor MJ. An unusual pH-independent and metal-ion-independent mechanism for hairpin ribozyme catalysis. Chem Biol. 1997;4:619–630. doi: 10.1016/s1074-5521(97)90247-7. [DOI] [PubMed] [Google Scholar]
- 26.Serganov A, Huang L, Patel DJ. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature. 2008;455:1263–1267. doi: 10.1038/nature07326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boots JL, Canny MD, Azimi E, Pardi A. Metal ion specificities for folding and cleavage activity in the Schistosoma hammerhead ribozyme. RNA. 2008;14:2212–2222. doi: 10.1261/rna.1010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ke A, Ding F, Batchelor JD, Doudna JA. Structural roles of monovalent cations in the HDV ribozyme. Structure. 2007;15:281–287. doi: 10.1016/j.str.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Krasovska MV, Sefcikova J, Reblova K, Schneider B, Walter NG, Sponer J. Cations and hydration in catalytic RNA: molecular dynamics of the hepatitis delta virus ribozyme. Biophys J. 2006;91:626–638. doi: 10.1529/biophysj.105.079368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travers KJ, Boyd N, Herschlag D. Low specificity of metal ion binding in the metal ion core of a folded RNA. RNA. 2007;13:1205–1213. doi: 10.1261/rna.566007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon PM, Sontheimer EJ, Piccirilli JA. Kinetic characterization of the second step of group II intron splicing: role of metal ions and the cleavage site 2′-OH in catalysis. Biochemistry. 2000;39:12939–12952. doi: 10.1021/bi001089o. [DOI] [PubMed] [Google Scholar]
- 32.Shan S, Yoshida A, Sun S, Piccirilli JA, Herschlag D. Three metal ions at the active site of the Tetrahymena group I ribozyme. Proc Natl Acad Sci U S A. 1999;96:12299–12304. doi: 10.1073/pnas.96.22.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shan SO, Herschlag D. Probing the role of metal ions in RNA catalysis: kinetic and thermodynamic characterization of a metal ion interaction with the 2′-moiety of the guanosine nucleophile in the Tetrahymena group I ribozyme. Biochemistry. 1999;38:10958–10975. doi: 10.1021/bi990388e. [DOI] [PubMed] [Google Scholar]
- 34.Takamoto K, He Q, Morris S, Chance MR, Brenowitz M. Monovalent cations mediate formation of native tertiary structure of the Tetrahymena thermophila ribozyme. Nat Struct Biol. 2002;9:928–933. doi: 10.1038/nsb871. [DOI] [PubMed] [Google Scholar]
- 35.Klawuhn K, Jansen JA, Souchek J, Soukup GA, Soukup JK. Analysis of metal ion dependence in glmS ribozyme self-cleavage and coenzyme binding. Chembiochem. 2010;11:2567–2571. doi: 10.1002/cbic.201000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter NG, Yang N, Burke JM. Probing non-selective cation binding in the hairpin ribozyme with Tb(III) J Mol Biol. 2000;298:539–555. doi: 10.1006/jmbi.2000.3691. [DOI] [PubMed] [Google Scholar]
- 37.Chowrira BM, Berzal-Herranz A, Burke JM. Novel RNA polymerization reaction catalyzed by a group I ribozyme. EMBO J. 1993;12:3599–3605. doi: 10.1002/j.1460-2075.1993.tb06033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowan JA. Metallobiochemistry of RNA. Co(NH3)6(3+) as a probe for Mg2+(aq) binding sites. J Inorg Biochem. 1993;49:171–175. doi: 10.1016/0162-0134(93)80002-q. [DOI] [PubMed] [Google Scholar]
- 39.Schnabl J, Sigel RK. Controlling ribozyme activity by metal ions. Curr Opin Chem Biol. 2010;14:269–275. doi: 10.1016/j.cbpa.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Woodson SA. Metal ions and RNA folding: a highly charged topic with a dynamic future. Curr Opin Chem Biol. 2005;9:104–109. doi: 10.1016/j.cbpa.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Lambert D, Draper DE. Effects of osmolytes on RNA secondary and tertiary structure stabilities and RNA-Mg2+ interactions. J Mol Biol. 2007;370:993–1005. doi: 10.1016/j.jmb.2007.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S, Rambo RP, Strauss-Soukup J, Cate JH, Ferre-D’Amare AR, Strobel SA, Doudna JA. A specific monovalent metal ion integral to the AA platform of the RNA tetraloop receptor. Nat Struct Biol. 1998;5:986–992. doi: 10.1038/2960. [DOI] [PubMed] [Google Scholar]
- 43.Takamoto K, Das R, He Q, Doniach S, Brenowitz M, Herschlag D, Chance MR. Principles of RNA compaction: insights from the equilibrium folding pathway of the P4–P6 RNA domain in monovalent cations. J Mol Biol. 2004;343:1195–1206. doi: 10.1016/j.jmb.2004.08.080. [DOI] [PubMed] [Google Scholar]
- 44.Perrotta AT, Been MD. HDV ribozyme activity in monovalent cations. Biochemistry. 2006;45:11357–11365. doi: 10.1021/bi061215+. [DOI] [PubMed] [Google Scholar]
- 45.Celander DW, Cech TR. Visualizing the higher order folding of a catalytic RNA molecule. Science. 1991;251:401–407. doi: 10.1126/science.1989074. [DOI] [PubMed] [Google Scholar]
- 46.Grosshans CA, Cech TR. Metal ion requirements for sequence-specific endoribonuclease activity of the Tetrahymena ribozyme. Biochemistry. 1989;28:6888–6894. doi: 10.1021/bi00443a017. [DOI] [PubMed] [Google Scholar]
- 47.McConnell TS, Herschlag D, Cech TR. Effects of divalent metal ions on individual steps of the Tetrahymena ribozyme reaction. Biochemistry. 1997;36:8293–8303. doi: 10.1021/bi9700678. [DOI] [PubMed] [Google Scholar]
- 48.Cernak P, Madix RA, Kuo LY, Lehman N. Accommodation of Ca(II) ions for catalytic activity by a group I ribozyme. J Inorg Biochem. 2008;102:1495–1506. doi: 10.1016/j.jinorgbio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Perrotta AT, Been MD. A single nucleotide linked to a switch in metal ion reactivity preference in the HDV ribozymes. Biochemistry. 2007;46:5124–5130. doi: 10.1021/bi602569x. [DOI] [PubMed] [Google Scholar]
- 50.Moncrief MB, Maguire ME. Magnesium transport in prokaryotes. J Biol Inorg Chem. 1999;4:523–527. doi: 10.1007/s007750050374. [DOI] [PubMed] [Google Scholar]
- 51.Serganov A, Huang L, Patel DJ. Coenzyme recognition and gene regulation by a flavin mononucleotide riboswitch. Nature. 2009;458:233–237. doi: 10.1038/nature07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipfert J, Sim AY, Herschlag D, Doniach S. Dissecting electrostatic screening, specific ion binding, and ligand binding in an energetic model for glycine riboswitch folding. RNA. 2010;16:708–719. doi: 10.1261/rna.1985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang L, Serganov A, Patel DJ. Structural insights into ligand recognition by a sensing domain of the cooperative glycine riboswitch. Mol Cell. 2010;40:774–786. doi: 10.1016/j.molcel.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banas P, Walter NG, Sponer J, Otyepka M. Protonation states of the key active site residues and structural dynamics of the glmS riboswitch as revealed by molecular dynamics. J Phys Chem B. 2010;114:8701–8712. doi: 10.1021/jp9109699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin Y, Hamelberg D. Deciphering the role of glucosamine-6-phosphate in the riboswitch action of glmS ribozyme. RNA. 2010 doi: 10.1261/rna.2334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.