Abstract
Purpose
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in childhood. The alveolar subtype of rhabdomyosarcoma (ARMS) is a paradigm for refractory and incurable solid tumors because more than half of the children at diagnosis have either regional lymph node or distant metastases. These studies follow our previous observation that Interleukin-4 receptor α (IL-4R) is upregulated in both human and murine ARMS, and that the IL-4R signaling pathway may be a target for abrogating tumor progression.
Experimental Design
By in vitro biochemical and cell biology studies as well as preclinical studies using a genetically-engineered mouse model, we evaluated the role of IL-4 and IL-13 in IL-4R mediated mitogenesis, myodifferentiation and tumor progression.
Results
IL-4 and IL-13 ligands accelerated tumor cell growth and activated STAT6, Akt or MAPK signaling pathways in the human RMS cell lines, RD and Rh30, as well as in mouse primary ARMS cell cultures. IL-4 and IL-13 treatment also decreased protein expression of myogenic differentiation factors MyoD and Myogenin, indicating a loss of muscle differentiation. Using a genetically-engineered mouse model of ARMS, we have demonstrated that inhibition of IL-4R signaling pathway with a neutralizing antibody has a profound effect on the frequency of lymph node and pulmonary metastases, resulting insignificant survival extension in vivo.
Conclusions
Our results indicate that an IL-4R-dependent signaling pathway regulates tumor cell progression in RMS, and inhibition of this pathway could be a promising adjuvant therapeutic approach.
Keywords: interleukin-4, interleukin-13, IL-4R, rhabdomyosarcoma, tumor progression, muscle
Introduction
Rhabdomyosarcoma (RMS) is a histologically distinct type of soft tissue sarcoma with a myogenic phenotype that is characterized by rapid growth, local invasiveness and high propensity to metastasize. The two major histotypes of RMS are embryonal RMS (ERMS) and alveolar RMS (ARMS)(1). While both diseases are capable of metastasizing, the alveolar subtype portends the poorest survival rate due to frequent metastases to sites such as bone marrow and lung. The 5 year progression free survival of metastatic ARMS is 20% at best despite aggressive multimodal regimens of chemotherapy, surgery and radiation (2, 3). Therefore, investigation into the mechanisms of disease progression is crucial to advancing therapeutic options for children with this cancer.
IL-4 receptor (IL-4R) signaling has diverse roles in physiology and disease. During mammalian muscle growth, the cytokine interleukin-4 (IL-4) acts as a myoblast recruitment factor to form mature myotubes (4). However, IL-4R signaling is better known for its role in T and B cell development. In murine breast cancer models, IL-4 and IL-13 secretion by CD4+ TH2 cells activate tumor associated macrophages (TAMs) to facilitate pulmonary metastasis mediated by secretion of EGF (5). With regard to rhabdomyosarcoma, Nanni et al recently reported that IL-4 treatment of a human ERMS cell line impairs the differentiation ability of these cells and increases cell growth and migration ability (6). Our laboratory has demonstrated by gene expression analysis that ARMS/ERMS in both humans and in a unique mouse model (3, 7)show a significantly higher expression of IL-4R when compared to normal skeletal muscle in the respective species. Taking all of these findings into account, we were intrigued that RMS seemed to rest at the center of multiple paradigms whereby IL-4 and IL-13 could potentially be seen as growth factors acting on tumor cells directly, or indirectly through the action of TAMs.
Materials and Methods
Mice
All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio. The conditional Pax3:Fkhr, p53 mouse model of alveolar rhabdomyosarcoma has been previously described (3, 8).
Human RNA Samples
De-identified human samples were obtained from the Pediatric Cooperative Human Tissue Network (Columbus, OH) with approval from the UTHSCSA institutional review board.
Cell Lines and Primary Tumor Cell Cultures
The human cell lines RD (embryonal rhabdomyosarcoma) and Rh30 (alveolar rhabdomyosarcoma) were graciously provided by Dr. Peter Houghton (St Jude Cancer Research Hospital, TN). The mouse myoblast cell line C2C12 was purchased from the American Type Culture Collection (Manassas, VA). The murine primary cell culture designated 21459 (alveolar rhabdomyosarcoma) was established by incubating tumor samples in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin (100 U/ml)/streptomycin (100 μg/ml) with Collagenase treatment (0.5%) overnight in 5% CO2 at 37°C (9). For all cell lines, the medium was changed every 48–72 hours, and then cells were cultured in a humidified atmosphere with 5% CO2 at 37°C.
Gene Expression Analysis
Total RNA was isolated from tumors using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s specifications. RNA was then purified using the RNeasy miniprep kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Using the first strand cDNA synthesis kit (Fermentas, Glen Burnie, MD) single-stranded cDNA was generated from total RNA according to the manufacture’s instruction. Real time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on an ABI Prism 7500 HT sequence detection system as instructed by protocol. The level of mRNA expression for each gene was normalized to phosphoglycerate kinase (Pgk) mRNA expression in human samples and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression in mouse samples. The primer sequences for each gene are described in Table S1 (see supplementary information).
In vitro Growth Assays
The Cell Titer-Glo Luminescent Cell Viability Assay (Promega, Fitchburg, WI) was utilized according to the manufacturer’s specifications. Mouse and human rhabdomyosarcoma cell lines or primary cell cultures were plated at 5 × 103 cells per well in 96-well plates. After 24 hours, the cells were treated with varying concentrations of IL-4 ligand, IL-13 ligand, or IL-4RαAb. After the cells were incubated for 72 hours, effects on cell viability were assessed using the Cell Titer-Glo Luminescent Cell Viability Assay and the Spectra Max M5 luminometer machine was used (Molecular Devices, Sunnyvale, CA).
Cell Differentiation Analysis
Mouse and human rhabdomyosarcoma cell lines were plated at 1.2 × 104 cells per chamber in an 8 chamber slide (BD Biosciences). After 24 hours, media was removed and cells were incubated in only low serum (2%)-DMEM supplemented with 1% penicillin/streptomycin, or the media with 50 ng/mL of IL-4 or IL-13. After 24 hours media was removed and cells were fixed in 4% paraformaldehyde (PFA) in PBS at room temperature for 20 minutes. Cells were carefully washed with PBS 3 times and incubated at room temperature in 5% normal goat serum (Invitrogen) and 0.01% Triton-X in PBS for 1 hour to inhibit nonspecific binding of antibodies. Cells were washed with PBS 3 times again and primary antibody diluted in 5% normal goat serum in PBS was added. The cells were subsequently incubated overnight at 4°C. The primary antibodies used were MyoD antibody at 1:200. Once cells were washed with PBS, Alexafluor594-conjugated anti-mouse IgG antibody (Invitrogen) at 1:200 was added and cells were incubated for 1 hour at room temperature. Slides were mounted using the VECTASHIELD Mounting Medium with DAPI (Vector Laboratories) and visualized on an Olympus IX81 confocal microscope equipped with Fluoroview™ 1.6A software(Olympus America, Center Valley, PA). Quantification of MyoD expression was determined by counting cells in randomly chosen-5 fields per chamber for each treatment group. Percentage of positive cells was determined by a ratio of positive cells to DAPI-positive total cells per field.
Preclinical Testing
Tumor size was measured using digital calipers, and IL-4R neutralizing antibody (BD Biosciences; monoclonal rat anti-mouse IL-4Rα antibody; # 552288) treatment was started when tumor size reached 0.2 cm3. For treatments, 125 μL of 1 μg/μL IL-4R blocking antibody per 45 gm mouse was administered intraperitoneally to Myf6ICNm/WT Pax3F3Fm/F3Fm Trp53F2-10/F2-10 mice twice weekly. When tumor size reached 1.5 cm3, mice were sacrificed and a necropsy was performed to diagnose tumor metastasis.
Results
IL-4 Receptor Signaling Axis Components and Ligands are Present in Human and Mouse Rhabdomyosarcoma
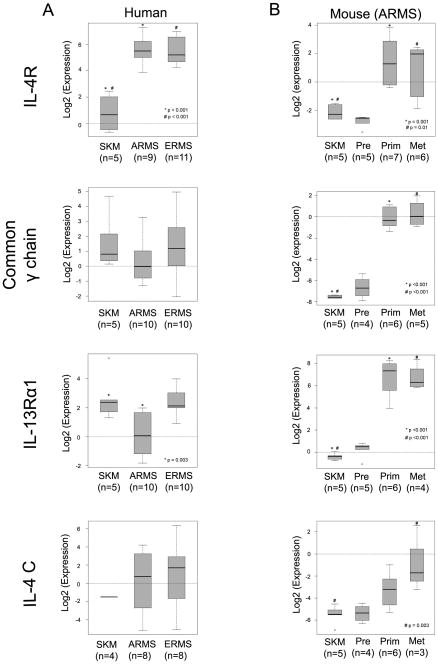
IL-4Rα forms heterodimeric receptor complexes with one of two distinct receptors, common γ chain or IL-13Rα1, and these different heterodimeric receptor complexes correspond to distinct ligand specificity, signaling pathways and functions (10). To compare gene expression of IL-4 receptor signaling components and ligands, we used human skeletal muscle (SKM, a control for the microenvironment from which tumors arise), human RMS (ARMS and ERMS) and mouse ARMS (Myf6ICNm/WT Pax3P3Fm/P3Fm Trp53F2-10/F2-10) tissue samples for quantitative RT-PCR. Mouse tissue included wild type SKM, preneoplastic muscle (Pre), primary tumor (Prim) and metastatic tumor (Met). In human tissue, IL-4 receptor (IL-4R) gene expression was increased in both ARMS and ERMS relative to SKM, although common γ chain expression showed no difference between skeletal muscle (SKM) and RMS. In addition, IL-13Rα1 expression was decreased in human ARMS, and IL-4c ligand expression was undetectable in all except one SKM sample but measurable in RMS tumor tissue (Fig. 1A). In mouse ARMS, Il-4r expression was increased not only in primary tumors but also in metastatic tumors (Fig. 1B). Interestingly, the expression of other IL-4 signaling components, common γ chain and Il-13rα1, were also increased in mouse RMS tumor (Fig. 1B). In addition, mouse ARMS exhibited increased Il-4c gene expression in tumor tissue, suggesting that the IL-4R signaling pathway is spontaneously accelerated in mouse ARMS and this pathway is associated with tumor progression in mouse ARMS (Fig. 1B). IL-13 gene expression was undetectable or low both in human and mouse RMS tissue (Supplementary Fig. S1).
Figure 1. IL-4R components are transcriptionally expressed in Rhabdomyosarcoma tumors.
(A) Quantitative RT-PCR (qPCR) analysis for human IL-4R, Common γ chain, IL-13Rα1 and IL-4C levels. SKM, skeletal muscle. ARMS, alveolar rhabdomyosarcoma. ERMS, embryonal rhabdomyosarcoma. (B) qPCR for mouse Il-4r, Common γ chain, Il-13rα1 and Il-4c levels. Pre, preneoplastic muscle. Prim, tumor primary. Met, tumor metastasis. Overall analysis performed by ANOVA, whereas pairwise contrasts were run with bootstrapped t-test.
IL-4 and IL-13 Signal through STAT6 and Akt in Rhabdomyosarcoma
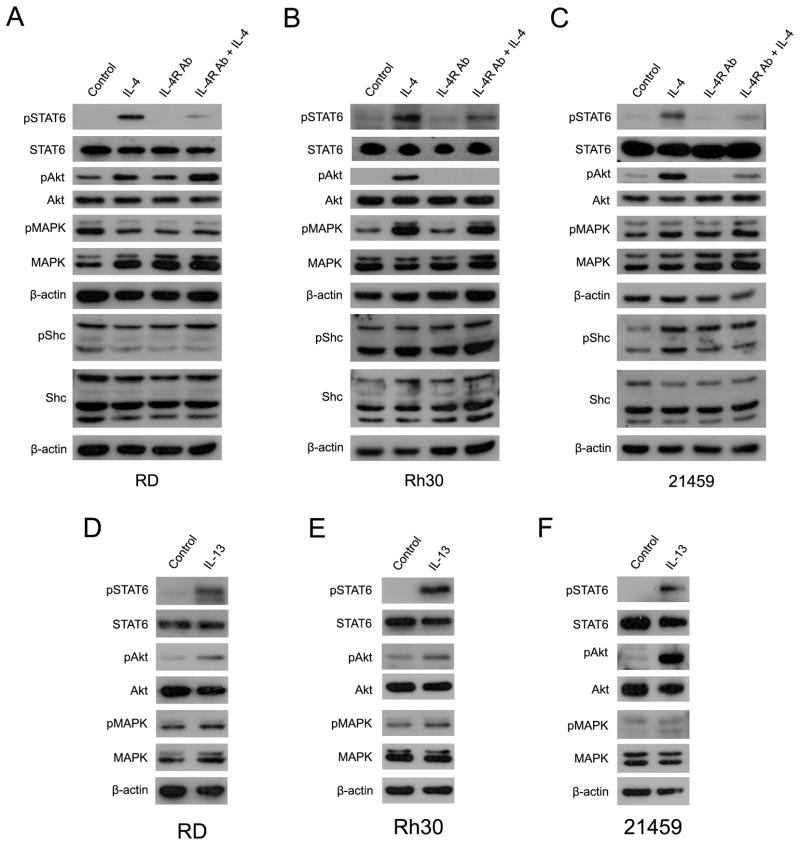
Both IL-4R and IL-13R intracellular signaling are mediated by JAK/STAT, PI3-kinase (PI3K)/Akt, and Shc/ERK1/2 (MAPK)-dependent pathways(11). To clarify whether over-expressed IL-4 and IL-13 receptors in RMS cells are functional and biologically significant in tumor cells, we stimulated this signaling pathway in the three different RMS cell lines RD (human ERMS), Rh30 (human ARMS) and 21459 (a mouse primary ARMS cell culture) by exogenous IL-4 treatment (validation of IL-4R signaling components for these cell lines is given in Supplementary Fig. S2A). Cells were serum-starved for 24 hours before IL-4 treatment, then recombinant IL-4 protein was added into the media for 20 or 60 minutes. IL-4 receptor signaling mediator, STAT6, was activated (phosphorylated) in all three RMS cell lines treated with IL-4 (Fig. 2A-C; Supplementary Fig. S2B), and phospho-STAT6 (pSTAT6) was decreased for cells pre-incubated with an IL-4 receptor neutralizing antibody (Fig. 2A-C), thus indicating that IL-4 receptor signaling in RMS cells is functional. In addition, another IL-4 receptor signal transducer, Akt, was also activated in ARMS cells by IL-4 treatment (Fig. 2A-C; Supplementary Fig. S2B). The literature suggests that IL-4 receptor signaling is less dependent upon the Shc-MAPK pathway and mainly mediated by the JAK/STAT and PI3K/Akt pathways (10). As expected, phospho-MAPK (pMAPK) and phospho-Shc (pShc) were not altered when IL-4 was added to RD cells. MAPK phosphorylation was, however, seen in Rh30 and 21459 ARMS cells at 60 minutes (Fig. 2B) but presumed to be mostly an indirect effect since the same degree of MAPK phosphorylation was not seen at 20 minutes (Supplementary Fig. S2B).
Figure 2. IL-4R signaling pathway is mediated by JAK/STAT6 and PI3K/Akt axis in RMS.
(A) RD cell cultures were starved in serum free media overnight and stimulated with IL-4 (20 ng/mL) for 60 min (IL-4). Cells were incubated in 1 μg/mL IL-4R Ab (IL-4R Ab) for 2h before adding IL-4 (20 ng/mL, for 60 min)(IL-4R Ab+IL-4). Cells were harvested at 60 min after IL-4 treatment and 20 μg of protein was applied in each well. (B) Rh30 cell cultures were treated as described in (A).(C) Mouse ARMS-derived 21459 cell cultures were also treated as described in (A). Similar results of 20 minutes treatment are shown in Supplementary Fig. S2B. (D) RD cell cultures were starved in serum free media for 24 hour before adding IL-13 (20 ng/mL)(IL-13) for 60 minutes. 20 μg of protein was applied, and Western blotting was performed. (E)Rh30 cell cultures were treated as described in (A). (F) Mouse ARMS-derived 21459 cell cultures were also treated as described in (A). Similar results for 20 minutes treatment are given in Supplementary Fig. S2C.
As noted above, IL-4 signaling is mediated by IL-4R heterodimeric complexes, whereas IL-13 signals through either an IL-4R complex or through IL-13Rα2. IL-4 or IL-13 signaling functionally overlaps because they share IL-4Rα as a common receptor component. IL-4Rα can heterodimerize with IL-13Rα1 or common γC chain receptor, which mediates IL-4/IL-13 or IL-4 (only) signaling, respectively (12–16). In ARMS cell lines, exogenous IL-13 administration activated STAT6 and Akt as seen for IL-4 stimulation, indicating that the effect of both IL-4 and IL-13 ligands can be mediated by the cell surface IL-4 receptor (Fig. 2 and Supplementary Fig. S2C). IL-13 increased Akt phosphorylation in both ARMS cells at 60 minutes (Fig. 2D-F), which is felt to be indirect because this pronounced effect was not seen at 20 minutes (Supplementary Fig. S2C). Interestingly, IL-13 also increased phospho-MAPK level at 20 minutes (Fig. 2D-F and Supplementary Fig. S2B). In related studies, Fujisawa et al., (2009) recently reported that IL-13 signaling through IL-13 receptor alpha 2 and MAPK-dependent signaling pathway is associated with cancer invasion and metastasis in pancreatic cancer (17). Thus, MAPK activation by IL-13 stimulation suggests the presence of IL-13Rα2 in RMS cell lines.
IL-4 and IL-13 are Mitogens in Rhabdomyosarcoma
Recently, Nanni et al demonstrated that IL-4 stimulates cell proliferation of an ERMS cell line, RD/12, through the JAK/STAT signaling pathway (6). However, it is still unclear if IL-4 signaling plays the same functional role in ERMS and ARMS, and thus we performed experiments to clarify the role of IL-4 signaling in the cellular pathobiology of RMS. First, we performed cell proliferation assays using receptor blocking antibody and interleukin ligand treatments to RMS cells. Three RMS cell lines were cultured in 10% FBS/DMEM media with or without IL-4R neutralizing antibody for 72 hours. Although these cells lines showed different levels of sensitivity to the blocking antibody, cell proliferation of all RMS cell lines was significantly decreased by blocking IL-4 signaling (Supplementary Fig. S3A). Apoptosis as measured by cleaved Caspase 3 was unaffected at this dose of blocking antibody (Supplementary Fig. S2D). Conversely, cell proliferation of both ERMS and ARMS was accelerated by activation of IL-4 receptor signaling by IL-4 or IL-13 under low serum conditions (Supplementary Fig. S3B and S3C). Dose-dependence for human tumor cell lines was only linear at the lower doses (0–10 ng/ml), suggesting that receptors may have been saturated at the higher doses (>50 ng/ml). Taken together, IL-4R-dependent signaling in RMS is associated with mitogenesis, suggesting that blocking this pathway might be useful for RMS therapy.
IL-4 and IL-13 Lead to a Less Differentiated State in Rhabdomyosarcoma
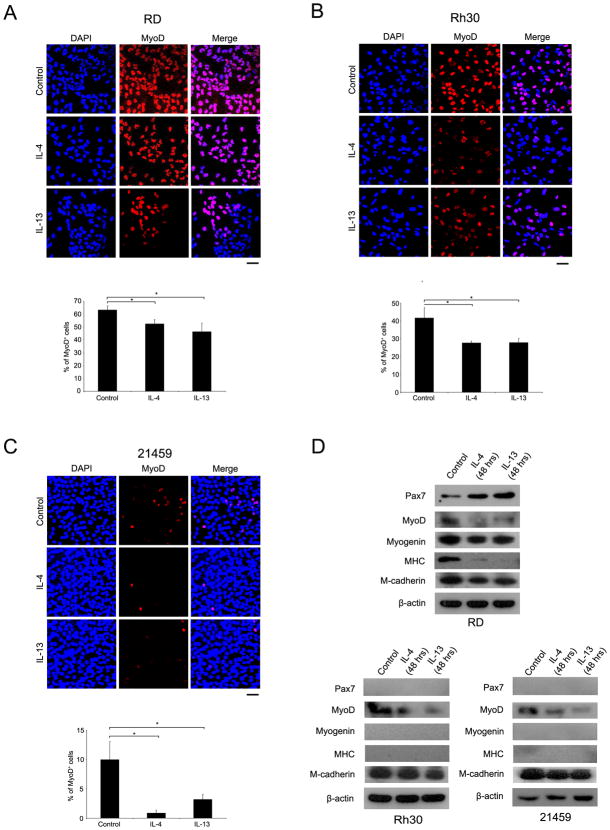
In postnatal skeletal muscle, satellite cells expressing the homeobox transcriptional factor Pax7 act as a reservoir of muscle stem cells and are associated with postnatal muscle growth (8, 18, 19). Activated satellite cells are committed to the myogenic lineage by the transcriptional regulation of MyoD, after which point committed myoblasts fuse to one other to form multinucleated myotubes (20). Although RMS cells are characterized by the expression of myogenic commitment factors such as MyoD, most cells within a tumor never advance to the terminal differentiation stage (21). As mentioned above, however, the interaction between these myogenic determination factors and RMS tumorigenesis is still unclear. A recent study suggested that decreased Myogenin expression could result in human ERMS tumor cell growth, and that Myogenin expression might be inhibited by IL-4 dependent signaling (6). We hypothesized that an acceleration of the IL-4R signaling pathway would decrease myogenic differentiation in both ARMS and ERMS and cause RMS cells to have a more satellite cell like phenotype. To test our hypothesis, we performed immunocytochemistry and western blot analysis for satellite cell, myoblast and myotube markers after IL-4 and IL-13 treatment of RMS cells. First, human RMS cell lines (RD and Rh30) and mouse primary tumor cells (21459) were cultured under IL-4 or IL-13 supplementation for 24 hours then immunocytochemistry for MyoD was performed. Interleukin ligand treatment reduced the MyoD-positive cell population in all RMS cells. This result is consistent with protein expression by immunoblotting for IL-4 or IL-13 treated cells under the same differentiation media conditions (Fig. 3D, Supplementary Fig. S2E). In ERMS, interestingly, expression of the muscle stem cell marker, Pax7, was increased by interleukin treatment while muscle differentiation markers, Myosin Heavy Chain (MHC), Myogenin and MyoD were decreased (Fig. 3D, Supplementary Fig. 2E). Similar results were seen for a murine ERMS primary cell culture (Supplementary Fig. 2E). In ARMS, on the other hand, Pax7, Myogenin and MHC expression were not altered by the stimulation of IL-4R signaling pathway, although the decrease of MyoD expression seen in the ERMS cell line was also seen in these ARMS cell lines (Fig. 3D). Furthermore, the satellite cell marker M-cadherin remained unchanged in ARMS cells following IL-4 or IL-13 treatment.
Figure 3. IL-4 and IL-13 signaling effects tumor cell differentiation.
(A) RD cells were treated with IL-4 (50 ng/mL)or IL-13 (50 ng/mL) in low serum media for 24 hours, and Immunocytochemistry for MyoD was performed. MyoD-positive cell number was significantly decreased by IL-4 or IL-13 treatment. Results of quantitative scoring are shown in the bar graph. (B) Rh30 cell cultures were treated as described in (A).(C) 21459 cell cultures were also treated as described in (A).(D) Reduced MyoD positivity was confirmed by western blotting under the same differentiating conditions. Pax7, Myogenin, Myosin heavy chain (MHC), and M-cadherin were examined concurrently. Cell lysates was prepared after 48 hours Interleukin ligand treatment, and 20 μg of protein was applied to gel electrophoresis. MyoD protein expression was decreased by both IL-4 and IL-13 treatment as expected by both immunocytochemistry and immunoblotting. Conversely, Pax7 protein expression was increased in RD cells by the treatment. MHC and Myogenin expression were also decreased in RD cells by the treatment. In ARMS cells, Pax7, Myogenin and MHC were undetectable before or after treatment. Scale bar: 50 μm.*p<0.05, vs. control.
Inhibition of IL-4R signaling pathway in RMS mice extends survival
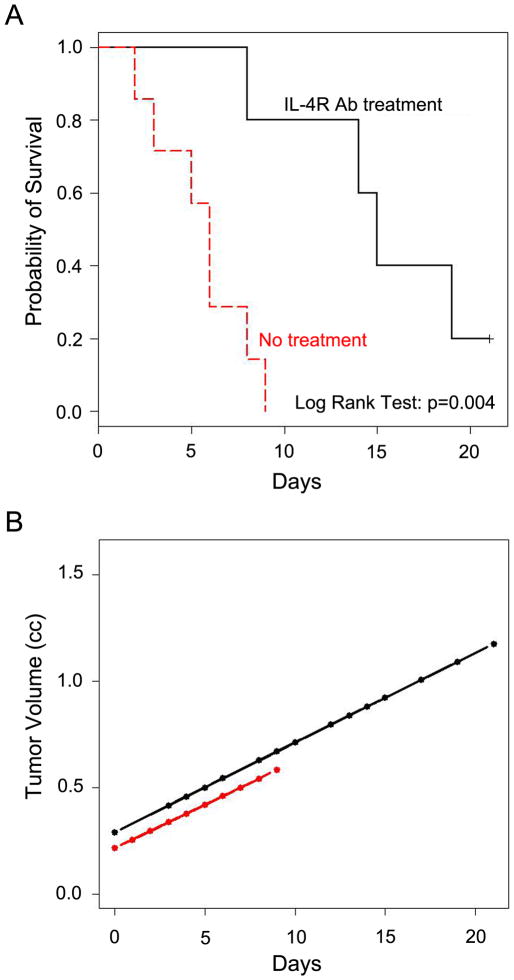
To test blockade of IL-4R signaling in vivo, we administered IL-4R neutralizing antibody (IL-4R Ab) to a preclinical ARMS mouse model and monitored tumor size and survival. Neutralizing antibody was intraperitoneally injected into Myf6ICNm/WT Pax3P3Fm/P3Fm Trp53F2-10/F2-10 mice (3, 8) twice weekly after tumor size reached at 0.2 cm3. Tumor size was monitored until reaching 1.5 cm3 or until mice met study criteria for termination (Fig. 4A, B). Tumor size gradually increased in both IL-4R Ab treated-and untreated-ARMS mice (Fig. 4B). This result suggests no effect of IL-4R blockade on growth of established tumors in vivo, which contrasts with in vitro studies. For survival, however, mice treated with IL-4R Ab exhibited longer overall survival than untreated-mice from the point at which tumors were 0.2 cm3 (5.7±2.3 vs. 13.4±5.3 days, p<0.01) (Fig. 4A). Scoring of extent of disease for tumor bearing mice was performed at necropsy according to the IRSG Presurgical Staging Classification(22). As shown in Table 1, only one instance out of 7 lymph node or pulmonary metastasis was (14%) observed in the IL-4R Ab-treated mice while 6 out of 7 (86%)and 4 out of 7 (43%) untreated mice harbored metastases to lymph node or lung, respectively. Taken together, blockade of IL-4R signaling inhibits tumor progression in RMS mice but not growth of large established tumors, and that inhibition of metastasis resulted in increased overall survival.
Figure 4. Blocking of IL-4R signaling pathway in vivo extends survival.
(A) Kaplan-Meier survival analysis indicating a significant survival advantage to mice treated with IL-4R blocking antibody (p=0.004). (B) Tumor volume is plotted by day for each cohort using least squares lines based on a repeated measures linear model of tumor volume in terms of day, treatment (IL-4R Ab treatment: black, no treatment: red), and the treatment by day interaction; there was no significant treatment effect (p=0.62) and treatment did not interact with day (p=0.94). Tumors grew larger in treated animals, but only because survival was extended.
Table 1.
Diagnosis of IL-4R neutralizing antibody treated-and untreated-tumor.
| Stage | I | II | III | IV | T | N | M |
|---|---|---|---|---|---|---|---|
| Untreated | n=1 | n=0 | n=2 | n=4 | 0a | 1-N0 | 3-M0 |
| 7b | 6-N1 | 4-M1 | |||||
| IL-4Rα Ab treated | n=6 | n=0 | n=1 | n=0 | 0a | 6-N0 | 6-M0 |
| 7b | 1-N1 | 1-M1 | |||||
Tumor diagnosis was performed according to the IRSG Presurgical Staging Classification (22). I, II, III, IV: tumor stage; T: Tumor size; a: ≤4 mm in diameter in size; b: >4 mm in diameter in size; N: Lymph node metastasis; N0: nodes not clinically involved; N1: nodes clinically involved by neoplasm; M: Hematogenous metastasis; M0: no distinct metastasis; M1: metastasis present. These are the same mice as for Figure 4.
Discussion
IL-4R signaling plays important roles in diverse biological processes. In immunology, for example, IL-4R signaling pathway is involved in differentiation of naïve T cells to TH2 cells by IL-4 ligand binding to IL-4R α/common γ chain heterodimeric receptor as well as for the proliferation of TH2 cells, which are critical for the acceleration of immune responses (23). In muscle differentiation, however, IL-4R signaling pathway is important for the maturation of myotubes. Primary (nascent) myotubes, which are formed by myoblast-myoblast fusion, secrete IL-4 ligand through NFATc2-dependent regulation in order to recruit surrounding IL-4Rα+ myoblasts, inducing secondary (mature) myotube formation (myotube-myoblasts fusion) (4). Herein we demonstrated that in RMS cells, stimulation of the IL-4R signaling pathway enhanced tumor cell proliferation of both ERMS and ARMS cells. Conversely, inhibition of IL-4R signaling pathway decreased cell proliferation but did not lead to apoptosis. These results imply IL-4R signaling is mitogenic but not necessary for RMS cell survival. Our results for ARMS and ERMS are consistent with recent report showing IL-4 ligand accelerates tumor growth in a human ERMS cell line (6). In addition, we also demonstrated that expression of myotube marker MHC and myoblast markers MyoD and Myogenin were decreased by IL-4 and IL-13 treatment, while muscle stem cell marker Pax7 expression was increased in treated-ERMS cells. In related studies, Zammit et al (2003) reported that ectopic Pax7 expression in Pax7-null myoblasts prevents terminal differentiation but does not induce the quiescence of myoblasts (24). Similarly, MyoD−/− myoblasts derived from MyoD-null satellite cells have enhanced proliferation and less differentiation potential (25). Taken together with our study’s observations, enhancement of tumor cell growth by IL-4 and IL-13 might depend on the expression of these myogenic differentiation-associating factors and Pax7, and IL-4R blockade might be used to modulate transcriptional factors therapeutically in embryonal rhabdomyosarcoma. The potential of myogenic differentiation as a therapy for ERMS is supported by experimental evidence that forced expression of Myogenin in a human ERMS cell line inhibits tumor cell growth (6). In ARMS cells, however, the expression of myogenic markers at baseline and after IL-4 and IL-13 treatment was not dramatically different except for the effect of IL-4 and IL-13 on MyoD expression. Although elucidating the mechanisms for the discrepancy of pathobiology are the topics of many future studies, we speculate that the targets of the Pax3:Fkhroncogene (i.e., Rb1) may underlie the difference in response of ARMS vs. ERMS to IL-4R signaling.
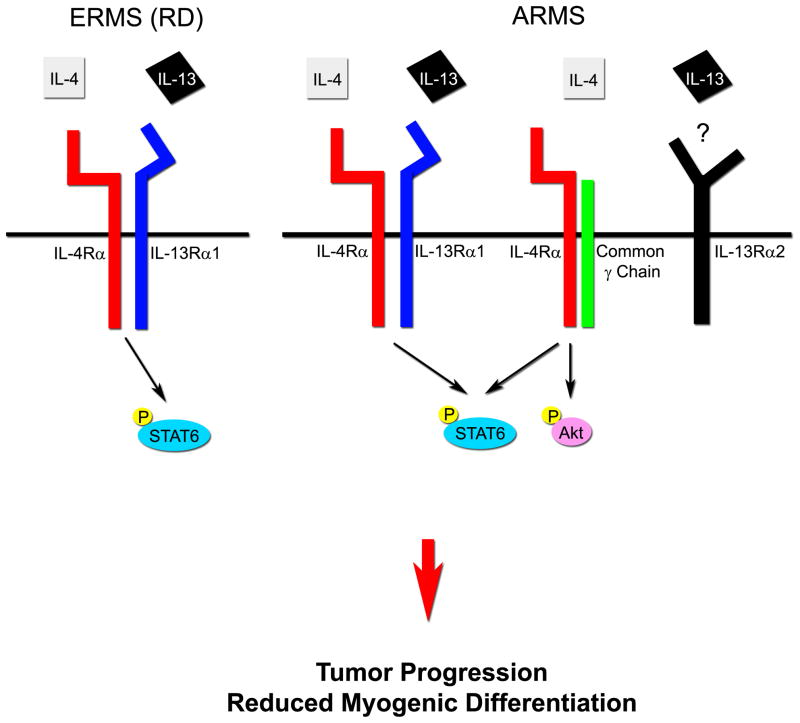
The IL-4R signaling pathway is mediated primarily by two types of heterodimeric receptors, the type I receptor composed of IL-4Rα and common γ chain, and the type II receptor composed of IL-4Rα and IL-13Rα1. IL-4 ligand binds to both type I and II receptors and activates an intracellular signaling cascade of STAT6 (as well as Akt in the case of type I receptor). On the other hand, IL-13 ligand binds to IL-13Rα1 when complexed to IL-4Rα and activates intracellular signaling via primarily STAT6 (26–28). In T cells, the type I receptor complex (IL-4Rα and common γ chain) are expressed but not IL-13Rα1, while myogenic cells express the type II receptor (IL-4Rα and IL-13Rα1) but not common γ chain (4, 23). These specific patterns of receptor expression are presumed to be the basis of cell type specific activity of IL-4R signaling. In our study, ARMS human and mouse tumors as well as cell cultures expressed IL-4Rα, IL-13α1 and common γ chain receptors at the RNA or protein levels. Furthermore, cell cultures were responsive to both IL-4 and IL-13 ligands, indicating that these ARMS cultures have dual intracellular signaling pathway mediated by the type I and type II IL-4R (Fig. 5). In these ARMS cultures, IL-4 and IL-13 converge on the same mitogenic phenotype which is effected by activation of STAT6, Akt and MAPK. Based on our observation of IL-13 stimulation of MAPK signaling, a role for IL-13Rα2 is also suggested (17). In some patient cases, however, ARMS signaling may be restricted to IL-4R and common γ chain type I receptor complexes, as reflected by the comparatively lower IL-13α1 levels by RT-PCR for certain human ARMS cases than ERMS cases. The situation for ERMS is also not necessarily generalizable: while the prototypic ERMS cell line that we used in our studies (RD) expressed only IL-4Rα and IL-13α1 but not common γ chain at the protein level, we found some cases of human ERMS that expressed common γ chain at levels comparable or higher than ARMS. The lesson, perhaps, is that while this signaling cascade is important in rhabdomyosarcomas generally, any therapy targeting ARMS or ERMS should be personalized to the biology of the individual patient’s cancer, or at a minimum the therapy should target the least common denominator for this signaling cascade, which is the IL-4Rα receptor subunit.
Figure 5. The model of IL-4R signaling pathway in RMS.
In embryonal rhabdomyosarcoma (ERMS), IL-4R signaling pathway is mediated by the type II IL-4R complex (IL-4Rα/IL-13Rα1). Both IL-4 and IL-13 stimulate the type II heterodimeric receptor, then intracellular STAT6 protein is phosphorylated to induce tumor progression. In alveolar rhabdomyosarcoma (ARMS), both the type I (IL-4Rα/common γ chain) and type II receptor complex are highly expressed. IL-4 and/or IL-13 activate IL-4R signaling pathway in ARMS mediated by these receptor complexes, resulting in tumor progression including lymph node and pulmonary metastasis.
Previous studies demonstrated that IL-4 and IL-13 ligands lead to leukemic cell growth and dissemination mediated by STAT6 in myeloid cells (29, 30), whereas IL-13 has been shown to promote invasion and metastasis of pancreatic cancer through IL-13Rα2 (but not through IL-4Rα) (17). Our study shows a similar role of IL-4 and IL-13 in tumor progression. However, the cellular source of these cytokines has not been fully identified. While IL-4 and IL-13 were expressed in mouse and human RMS tumor tissue, whether IL-4 and IL-13 are autocrine, paracrine or endocrine cytokines has yet to be established. In an experiment to address this issue, cachectic muscle from colon-C26 tumor-bearing mice (34) showed higher IL-4 and IL-13 gene expression than normal skeletal muscle. However, expression levels of cachectic muscle were an order of magnitude lower than thymus of newborn mice (data not shown), suggesting that IL-4 and IL-13 production were not as heavily influenced by cachectic, paraneoplastic muscle as compared with the influence of tumor-infiltrating lymphocytes. Because IL-4-producing CD4+ T cells (TH2 cells) promote pulmonary metastasis of mammary tumors by activation of IL-4Rα+ M2-macrophages, also known as tumor-associated macrophages (TAMs) (5), CD4+ T cells might be one potential source of interleukins which affect RMS tumor metastasis. In ARMS mice, in vivo treatment of IL-4Rα neutralizing antibody inhibited lymph node and pulmonary metastasis but did not halt growth of established tumors (which may nonetheless be improved by pharmacokinetics or dosing). Nevertheless, IL-4R blockade resulted in prolonged overall survival. Together, these results indicate the possibility that TH2 cell-derived interleukin ligands may act on RMS and metastasis through the direct effects on early tumor cell growth and/or by indirect effects on tumor cells by TAMs, which can be induced by interleukin ligands to release angiogenic and tumor growth factors(5). Alternatively, IL-4 and/or IL-13 may also be acting simultaneously on tumor cells and macrophages to secrete cathepsin and metalloprotease enzymes that modify the tumor microenvironment, as has been shown recently for breast and pancreatic cancer model systems (17, 31).
An important aspect of our studies was that IL-4R blockade halted tumor cell growth but did not affect cell survival; therefore, one therapeutic strategy would be to combine IL-4R blockade with inhibition of another receptor implicated in cell survival. It is widely known that human RMS cell lines express Insulin growth factor-I receptor (IGF-IR), and antisense oligonucleotides or small molecule IGF-IR kinase inhibitors abrogate tumor cell proliferation and colony formation by inducing apoptosis in vitro and in vivo in a xenograft model (32–34). In addition, our recent studies affirm that IGF-IR is overexpressed in human and mouse ARMS tissues, and that inhibition of this signaling pathway using an IGF-IR specific kinase inhibitor decreases tumor cell growth and induces Caspase 3 cleavage(Abraham et al., in review). Because of the precedent that myeloid cells become sensitized to IL-4R signaling when IGF signaling is active (35), we speculate that concurrent inhibition of IL-4Rα and IGF-IR might exhibit synergistic antitumor activity in RMS.
How feasible of a target is IL-4R? Subcutaneously administered humanized IL-4R blocking antibody AMG-317 has been safely tolerated in clinical trials for asthma (36), as is also true of the IL-4 neutralizing antibody pascolizumab (37). In addition, clinical trials to evaluate safety and efficacy of the humanized IL-13 neutralizing antibody TNX-650 are underway, and endotoxin-tethered IL-4 (IL-4(38-37)-PE38KDEL) and IL-13 (cintredekin besudotox) have gone through clinical trials or already been reported to have been safely tolerated in brain tumor patients (38), respectively. Whereas the efficacy of IL-4R modulating agents has been modest for modulating immune-mediated diseases (36), we speculate that these same agents may have an important second life in molecularly-targeted cancer therapies.
In conclusion, our results provide important insights into the mechanism of IL-4R signaling in RMS and the possibility that inhibition of IL-4R signal may be a useful adjunct approach for RMS therapy. The most dramatic effect seen in our study was the impact on lymphatic and pulmonary metastasis, which may imply a role for IL-4R blockade (concurrent with another pro-apoptotic therapy as IGF-R1 inhibition) as part of consolidation or maintenance therapy for RMS. Further mechanistic and preclinical studies will be needed to test this approach.
Supplementary Material
Acknowledgments
This work was supported in part from a medical student research seed grant from the AMA Foundation to MIA, training awards to KN and JA from the Scott Carter Foundation, NCI award 5R01CA133229-03 to CK, and a research grant to the Preclinical Testing Initiative (PPTI) from the Joanna McAfee Childhood Cancer Foundation. Human tissue samples were provided by the Pediatric Cooperative Human Tissue Network which is funded by the National Cancer Institute. The Developmental Studies Hybridoma Bank is developed under the auspices of the NICHD and maintained by The University of Iowa, Iowa City, IA.
Footnotes
The authors have no conflicts of interest.
References
- 1.Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, et al. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003;63:7926–35. [PubMed] [Google Scholar]
- 2.Qualman SJ, Coffin CM, Newton WA, Hojo H, Triche TJ, Parham DM, et al. Intergroup Rhabdomyosarcoma Study: update for pathologists. Pediatr Dev Pathol. 1998;1:550–61. doi: 10.1007/s100249900076. [DOI] [PubMed] [Google Scholar]
- 3.Keller C, Arenkiel BR, Coffin CM, El-Bardeesy N, DePinho RA, Capecchi MR. Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes Dev. 2004;18:2614–26. doi: 10.1101/gad.1244004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–94. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 5.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanni P, Nicoletti G, Palladini A, Astolfi A, Rinella P, Croci S, et al. Opposing control of rhabdomyosarcoma growth and differentiation by myogenin and interleukin 4. Mol Cancer Ther. 2009;8:754–61. doi: 10.1158/1535-7163.MCT-08-0678. [DOI] [PubMed] [Google Scholar]
- 7.Keller C, Hansen MS, Coffin CM, Capecchi MR. Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev. 2004;18:2608–13. doi: 10.1101/gad.1243904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishijo K, Hosoyama T, Bjornson CR, Schaffer BS, Prajapati SI, Bahadur AN, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. Faseb J. 2009;23:2681–90. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi E, Nishijo K, McCleish AT, Michalek JE, Grayson MH, Infante AJ, et al. PDGFR-A is a therapeutic target in alveolar rhabdomyosarcoma. Oncogene. 2008;27:6550–60. doi: 10.1038/onc.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 11.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–9. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Letzelter F, Wang Y, Sebald W. The interleukin-4 site-2 epitope determining binding of the common receptor gamma chain. Eur J Biochem. 1998;257:11–20. doi: 10.1046/j.1432-1327.1998.2570011.x. [DOI] [PubMed] [Google Scholar]
- 13.Miloux B, Laurent P, Bonnin O, Lupker J, Caput D, Vita N, et al. Cloning of the human IL-13R alpha1 chain and reconstitution with the IL4R alpha of a functional IL-4/IL-13 receptor complex. FEBS Lett. 1997;401:163–6. doi: 10.1016/s0014-5793(96)01462-7. [DOI] [PubMed] [Google Scholar]
- 14.Murata T, Taguchi J, Puri RK. Interleukin-13 receptor alpha’ but not alpha chain: a functional component of interleukin-4 receptors. Blood. 1998;91:3884–91. [PubMed] [Google Scholar]
- 15.Obiri NI, Debinski W, Leonard WJ, Puri RK. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common gamma chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem. 1995;270:8797–804. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 16.Obiri NI, Leland P, Murata T, Debinski W, Puri RK. The IL-13 receptor structure differs on various cell types and may share more than one component with IL-4 receptor. J Immunol. 1997;158:756–64. [PubMed] [Google Scholar]
- 17.Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69:8678–85. doi: 10.1158/0008-5472.CAN-09-2100. [DOI] [PubMed] [Google Scholar]
- 18.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. Embo J. 2004;23:3430–9. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–86. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 20.Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–95. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Tapscott SJ, Thayer MJ, Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993;259:1450–3. doi: 10.1126/science.8383879. [DOI] [PubMed] [Google Scholar]
- 22.Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 23.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4-and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–32. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 25.Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J Cell Biol. 1999;144:631–43. doi: 10.1083/jcb.144.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keegan AD, Johnston JA, Tortolani PJ, McReynolds LJ, Kinzer C, O’Shea JJ, et al. Similarities and differences in signal transduction by interleukin 4 and interleukin 13: analysis of Janus kinase activation. Proc Natl Acad Sci U S A. 1995;92:7681–5. doi: 10.1073/pnas.92.17.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–8. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 28.Pernis A, Witthuhn B, Keegan AD, Nelms K, Garfein E, Ihle JN, et al. Interleukin 4 signals through two related pathways. Proc Natl Acad Sci U S A. 1995;92:7971–5. doi: 10.1073/pnas.92.17.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol. 2000;165:6015–9. doi: 10.4049/jimmunol.165.11.6015. [DOI] [PubMed] [Google Scholar]
- 30.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–20. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 31.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L, et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res. 16:530–40. doi: 10.1158/1078-0432.CCR-09-0816. [DOI] [PubMed] [Google Scholar]
- 33.Scotlandi K, Manara MC, Nicoletti G, Lollini PL, Lukas S, Benini S, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868–76. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro DN, Jones BG, Shapiro LH, Dias P, Houghton PJ. Antisense-mediated reduction in insulin-like growth factor-I receptor expression suppresses the malignant phenotype of a human alveolar rhabdomyosarcoma. J Clin Invest. 1994;94:1235–42. doi: 10.1172/JCI117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soon L, Flechner L, Gutkind JS, Wang LH, Baserga R, Pierce JH, et al. Insulin-like growth factor I synergizes with interleukin 4 for hematopoietic cell proliferation independent of insulin receptor substrate expression. Mol Cell Biol. 1999;19:3816–28. doi: 10.1128/mcb.19.5.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–96. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 37.Hart TK, Blackburn MN, Brigham-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol. 2002;130:93–100. doi: 10.1046/j.1365-2249.2002.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogelbaum MA, Sampson JH, Kunwar S, Chang SM, Shaffrey M, Asher AL, et al. Convection-Enhanced Delivery of Cintredekin Besudotox (Interleukin-13-Pe38qqr) Followed by Radiation Therapy with and without Temozolomide in Newly Diagnosed Malignant Gliomas: Phase 1 Study of Final Safety Results. Neurosurgery. 2008 doi: 10.1227/01.neu.0000303199.77370.9e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.