1. Introduction
1.1. A brief history of cellular imaging
The illustrious history of cellular imaging began in 1595 when spectacle makers Hans Lippershey and Hans Janssen in Middelburg, The Netherlands, developed a prototype microscope with lenses capable of threefold magnification. Using this revolutionary apparatus, Robert Hooke coined the word “cell” in 1665 upon witnessing the structure of non-living plant tissue (cork) that, to him, resembled monks’ chambers called “cellula”. Nearly a decade later, in 1674, Anton Van Leeuwenhoek became the first to describe live algae, bacteria, spermatozoa, and even blood flow in capillaries using a device equipped with hand ground lenses that were capable of greater than 200-fold magnification. Almost two centuries would pass before the seminal observations of Hooke, Leeuwenhoek, and contemporaries in botany were expanded to include animal cells by Theodor Schwann and Matthias Schleiden and cell division by Rudolf Virchow; ultimately giving rise to the universal Cell Theory stating that cells are the basic unit of life [1].
1.2. Non-invasive cellular imaging in the 21st century
Presently, a microscope can be found in nearly every biomedical laboratory and innumerable images of cells are published each month in research journals. Along with the omnipresent microscope, technological developments in histology, immunohistochemistry, mammalian cell culture, and molecular biology over the past century have provided enormous contributions to our understanding of cell development, physiology, and pathology. Analyses of cultured cells and postmortem tissue rapidly encouraged deeper enquiries into cell physiology in situ and the pathogenesis of disease. This flourishing interest in live cellular processes stimulated the development of multi-photon confocal microscopy and highly sensitive charge coupled devices for bioluminescent imaging (BLI) as well as the adaptation of non-invasive medical imaging devices, including ultrasound, magnetic resonance (MR), computerized tomography (CT), positron emission tomography (PET), and single photon emission computerized tomography (SPECT) scanners for imaging studies in animals and humans. Much like the dawn of the microscope, the advances in biomedical imaging achieved in recent decades have generated new possibilities for non-invasive detection of the progression of pathology at the cellular level and methods for monitoring the efficacy of cell therapy in animal models of disease.
The forté of non-invasive imaging is that cells and tissues within living animals can be visualized at regular time intervals without disturbing their anatomical context. Even more remarkable is the fact that this feat can generally be performed within 10–20 min; including the total time required to initialize the scanner, position the subject, apply contrast if necessary, and acquire an image. Though non-invasive imaging devices bear significant instrument costs and require dedicated facilities, support staff, and skilled operator training, they are nevertheless increasingly commonplace as shared resources in biomedical institutions. In this review, we discuss recent advances in non-invasive cell detection that have significantly expanded the researcher’s repertoire for monitoring the basic unit of life in its native or diseased context. In order to emphasize novelty, we chose to limit our discussion to major developments reported within the past five years.
2. Imaging modalities for non-invasive cellular detection
2.1. Multi-photon confocal microscopy as a starting point for non-invasive cellular imaging
Each imaging modality has well known relative advantages and disadvantages associated with detection method and imaging probe characteristics. For anatomical purposes, X-ray, ultrasound, CT, and MRI each provide excellent detail and are widely used for direct detection of gross tissue anomalies. Delving beyond anatomical features, however, often requires indirect means to reveal the biodistribution of specific cell populations and expose the cellular processes that underlie disease. Classically, cell labeling is achieved by either introduction of enzymatic or fluorescent molecules into a well defined cell population, binding of enzyme- or fluorophore-conjugated antibodies directed against cell-specific protein markers, or detection of the expression non-mammalian proteins from either constitutive or cell-specific promoters. Though potentially immunogenic in humans, the expression of non-mammalian proteins is well tolerated in mice, as indicated by the widespread usage of this cell labeling method in transgenic animals. For example, transgenic mice designed to express genes encoding non-mammalian proteins such as green fluorescent protein (GFP, Aequorea victoria) and red fluorescent protein (RFP, Discosoma sp.) are increasingly applied in organotypic cultures and in vivo to discern cellular responses to experimental manipulation and to probe cell–cell connectivity in highly complex systems such as the nervous system [2–5]. In particular, multi-photon confocal microscopy has been used to reveal dynamic changes in the position and morphology of fluorescently labeled neural cells (Thy1-promoter) within the brain [6,7] and the regenerating spinal cord [8,9] in remarkable detail. However, unlike medical imaging modalities, multi-photon laser scanning microscopy is semi-invasive, typically requiring skin incision, tissue retraction, or a cranial window. In addition the dispersion of light excitation and emission limits multi-photon confocal analyses to superficial tissue preparations (maximum depth of 2 mm) and a small (200–500 μm) field of view (FOV) [10–12]. This imaging modality is also best suited for the study of dispersed cells because large aggregates of fluorescently labeled cells saturate the field of view and are difficult to distinguish (Fig. 1). Also, for serial analyses, multi-photon confocal microscopy is best undertaken for short-term studies in motile cells or cells anchored within tissue [13]. In comparison to multi-photon confocal microscopy, ultrasound, BLI, micro-CT, micro-PET, micro-SPECT, and MR imaging are less limited by cell dispersion and cell depth, but at the expense of (sub)cellular detail and real-time visualization. Thus, with in vivo microscopy serving as an initial reference point for cellular detection, one can better appreciate what can and cannot be accomplished using non-invasive cellular imaging.
Fig. 1.
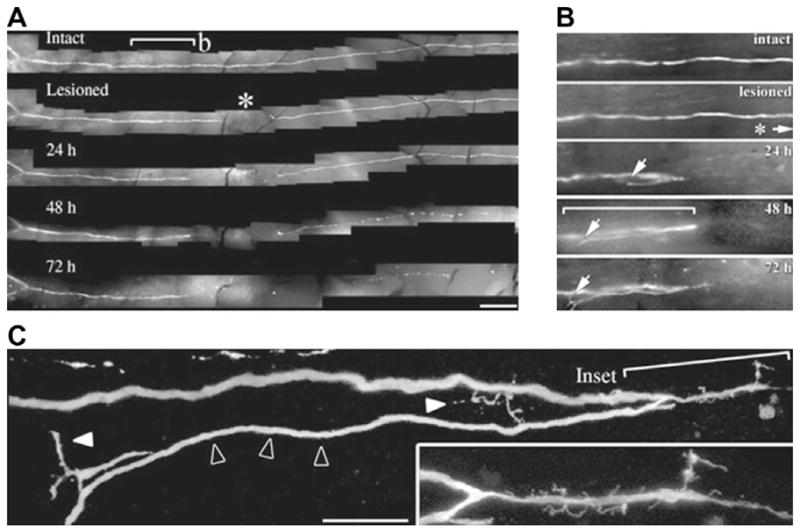
Serial multi-photon confocal fluorescent microscopy of a regenerating sensory axon in the central nervous system of a transgenic mouse engineered to express green fluorescent protein from the Thy1 promoter. This figure highlights the level of fine detail that is characteristic of multi-photon confocal microscopy along with the requirement of a limited cell population in a small field of view for semi-invasive detection. (A) Imaging of temporal differences in axon morphology following surgical transection demonstrates retraction and regeneration of the proximal axon within the course of 24 h and Wallerian degeneration of the distal axon stump within 48 h. Scale bar, 250 μm. (B) Closer inspection of the proximal axon reveals that the nerve regeneration is aberrant, proceeding in a direction opposite to that of denervated target tissue. (C) The long growing axon tip (open arrowheads) is of normal caliber and gives rise to smaller caliber, irregularly shaped, side branches (solid white arrowheads) and filopodia (inset). Scale bar, 25 μm. Reproduced, with permission, from Ref. [9].
2.2. Relative sensitivities of non-invasive imaging modalities for cellular imaging
Like GFP and related molecules in microscopy, exogenous bioengineered and/or non-mammalian factors have greatly facilitated non-invasive detection of cells in mammals. The most widely applied factors for non-invasive imaging studies consist of (super)paramagnetic metal ions, positron emitters, gamma radiation-emitting radionuclides, and low-energy photons generated by luciferase activity for MR, PET, SPECT, and BLI, respectively. In general, labeled cells in number greater than 104 can readily be detected by nearly all non-invasive medical imaging modalities, providing many options for general verification of cell transplant placement and routine monitoring of cell accumulation or cell attrition. However, non-invasive imaging of disease progression and cell therapy often demands high resolution (less than 0.5 mm) for longitudinal tracking of less than 104 cells within specific anatomical sites (e.g., lesions, tumors, and perivascular regions). While BLI, PET, and SPECT demonstrate very high sensitivity for molecular detection (less than 10−8 mol/L), spatial resolution for these modalities remains in the low millimeter range (1–3 mm), precluding early detection and localization of tumor metastases, immune cell infiltrates, and therapeutic cell engraftment in coronary infarcts and brain lesions (Fig. 2). Nevertheless, PET/SPECT detection of 18F-, 111In- and 99mTc-labeled radiotracers is increasingly applied in short-term studies to detect, en masse, cell proliferation [14], transplant viability [15,16], and the extent to which therapeutic factors are recruited to myocardial infarcts [17–19] or targeted to tumors [20–23]. Similarly, BLI is superior to MR imaging for detection of cell viability [24–26] and teratoma formation [27,28].
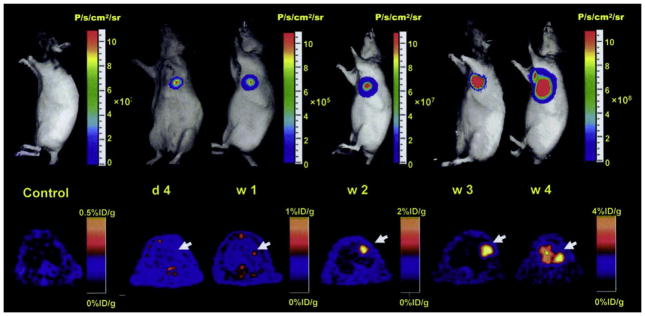
Fig. 2.
Cellular imaging of cardiomyocyte-differentiated murine ES cells that were transduced with a triple-fusion reporter gene construct prior to intramyocardial transplant into athymic nude rats. This figure highlights the ability of bioluminescent and PET imaging to reveal the general anatomical position, survival, and proliferation of transplanted cells. A representative animal showed significant bioluminescence (top) and PET (bottom) signals at day 4, week 1, week 2, week 3, and week 4. Firefly luciferase and truncated herpes simplex virus type 1 thymidine kinase (cytoplasmic form) activities increased significantly from week 2 to week 4 and extracardiac signals were observed during subsequent weeks. In this same study, the prodrug, ganciclovir, was later applied as a substrate for viral thymidine kinase phosphorylation to eliminate aberrant cells. Reduced, with permission, from Ref. [26].
2.3. Advantages of MR imaging for cell tracking
Unlike CT, PET, and SPECT scanners, which use potentially harmful radiation emission, MR scanners measure the harmless response of the proton in water, fat, and other endogenous biomolecules to applied radio waves in a strong homogenous magnetic field. Contrast in MR imaging originates from local variations in tissue water concentration and the chemically bound state of protons [29]. Two relaxation time constants, T1 and T2, which are associated with the time decay of proton magnetization back to equilibrium following a radio-frequency (RF) pulse, are applied to measure intrinsic tissue variations that generate anatomical contrast. T1, or spin–lattice relaxation, characterizes the energy transition of the nuclear spin to equilibrium following a RF pulse whereas T2, or spin–spin relaxation, corresponds to the loss of coherency among adjacent nuclear spins. T1 and T2 time constants are affected by the local microenvironment of water molecules, which may be influenced by temperature, viscosity, diffusion, bulk flow, and proximity to inhomogeneities induced by macromolecules or paramagnetic and superparamagnetic ions (also referred to as relaxation) [30,31]. Paramagnetic or superparamagnetic iron oxide (SPIO) nanoparticles induce local variations in proton relaxation times and can be introduced to generate specific contrast in labeled cells, tissues, or body fluids. Though particulate MR contrast agents are larger in size than highly soluble radioisotope conjugated PET contrast agents and require special means for cell incorporation, MR imaging permits safe, periodic, high resolution (~50 μm in live animals) detection of contrast-labeled cells within an living organism with reasonable scan duration. In addition to identifying transplanted cells in their anatomical context, MR imaging provides information about the surrounding milieu (i.e., edema, inflammation, and lesion size).
3. In vivo MR microscopy and microimaging
3.1. Testing the limits of MR imaging
A brief departure towards an area of MR research often referred to as MR microscopy or microimaging (voxel size <50 μm) is beneficial for an appreciation of the resolving power of MR imaging and the advantages conferred to cellular imaging by contrast agent labeling. MR microscopy is most commonly performed on cultured cells [32] or preserved tissue samples [33] in the absence of time constraints associated with MR imaging of living subjects. For example, in the most extreme case, an entire mouse brain was imaged at 15 × 15 × 35 μm resolution in 29 h (8 averages) using a 21.1 T magnet [34]. Using specially fabricated micro coils and customized pulse sequences, investigators achieved 3.3 × 3.3 × 3.7 μm volume resolution [35] and applied this methodology to living Paramecium and Spirogyra alga [36]. Similarly, an in plane resolution of 2 μm and a 50 μm slice thickness was used to image cells within the stem of a geranium leaf at 14.1 T [37].
3.2. Development of frog and chick embryos
Recently, high resolution MR imaging has been applied to image cell development in situ. Larger, externally developing amphibian and avian embryos are preferred for high resolution MR imaging because of technical challenges arising from respiratory motion and uterine contractions in mammals. In chick, the motion artifacts and significant postural changes associated with advanced stages of development (day 12 to hatching at day 20) can be overcome by gentle cooling chicken eggs to 4 °C immediately prior to MR imaging at 7 T, providing new opportunities for MR imaging of brain, liver, and heart development in ovo [38]. First described in 1986 [39], MR imaging of frog (Xenopus laevis) embryos is yet more advanced. X. laevis embryos can be isolated in space and high resolution MR images (20 μm, 11.7 T) can be obtained at short intervals (5–10 min) because of a very small FOV (1 mm). Compared to complex mammalian embryos, X. laevis embryos are highly symmetric along the midline and are favorable for 2D imaging at high spatio-temporal resolution. Recognizing this advantage, investigators were able to directly image mitotic cell division in early blastomeres [40] as well as initial cell divisions, gastrulation, and the complete embryonic development of a X. laevis embryo in exquisite detail [41–43].
3.3. Zebrafish development
Along with chick and X. laevis, the zebrafish (Danio rerio) is a prime model organism for in vivo MR imaging studies of vertebrate development, because the zebrafish embryos develop into free-swimming larvae within 72 h from the moment of fertilization. Recently, zebrafish were non-invasively imaged at 7 T with 47 μm spatial resolution following Dotarem® injection at the one to four cell stage [44]. One major advantage of zebrafish embryos is that they have undergone extensive mutant screening and other genetic analysis, which allows the role of candidate molecules in cellular development to be tested in MR imaging experiments. For example, stable transgenic lines expressing a specific gene of interest can be created by raising fish to adulthood and screening founders for germline transmission of the transgene prior to MR phenotyping for gross differences in tissue development. Similarly, gene expression may be characterized in vivo upon cleavage of a MR-detectable substrate in beta galactosidase-expressing cells [45].
4. MR imaging of living cells
4.1. Methods for labeling cells with contrast agents
As discussed above, cellular-level resolution in the absence of contrast agent often involves very high field MR scanners, specialized coils, small FOV, and long acquisition times. These technical requirements are impractical for cell tracking in live mammalian tissues. Via shortening of T1 and T2 relaxation, paramagnetic and superparamagnetic contrast agents can be applied to indirectly distinguish transplanted cells from background tissue contrast by MR imaging. The initial challenges to in vivo cellular MR imaging consisted of: (1) the successful introduction of paramagnetic and superparamagnetic contrast agents into cells for subsequent MR detection and (2) assessing whether there existed any adverse cellular responses following intracellular uptake of the contrast agent. In recent years, significant advances have been attained in both of these critical steps. It is well established that cells with phagocytic capabilities, such as macrophages, readily ingest iron oxide nanoparticles [46,47], a feature that both facilitated their study and advanced the field of cell tracking. However, many cells lack substantial phagocytic function and require alternate means of contrast agent incorporation [48]. Recently developed methods for contrast agent labeling include particle bombardment [49,50], receptor mediated or fluid phase endocytosis [51], lipofection [52,53], polycation-based transfection agents (poly-L-lysine and polyarginyl rich protamine sulfate) [54–57], and magnetoelectroporation [58,59]. Direct methods, such as bombardment and magnetoelectroporation, circumvent potential cell survival and differentiation issues associated with overnight cell culture incubation for transfection- and endocytosis-based methods. Moreover, direct labeling methods allow therapeutic cell isolation, labeling, transplantation, and imaging to be performed on the same day, and avoid the necessary clinical approval issues associated with the use of secondary (transfection) agents. For each cell type, the parameters for the applied labeling approach must be systematically adjusted to ensure sufficient MR contrast agent uptake in the absence of intracellular aggregation and precipitation, adverse effects on cell proliferation, differentiation and migration, or cell toxicity [60–63].
4.2. Validation of cell labeling and MR findings
For serial MR imaging experiments in which labeled cells are regularly monitored in vivo for several days, it is necessary to verify contrast agent retention and characterize contrast agent dilution following cell division [63–65]. For example, the injection procedure can nonspecifically deposit SPIO-labeled cells along the injection path or damage blood vessels introducing iron-rich erythrocytes that are also hypointense upon deoxygenation of their heme moieties. Furthermore, should contrast label-bearing cells die and become phagocytosed, contrast agent may be deposited nonspecifically at sites of tissue necrosis or carried distantly within phagocytic cells, leading to detection of a false positive signal by MR (Fig. 3). Therefore, it is essential to validate the migration of labeled cells upon completion of the in vivo analysis using ex vivo high resolution 3D MR imaging followed by dual label immunohistochemistry for dextran [58] that comprises the shell of the SPIO nanoparticle and a cell-specific marker. In addition, visualization of cells expressing green fluorescent protein or other cellular label (i.e., BrdU [66]) can be performed in order to histochemically confirm the final location of transplanted cells ex vivo.
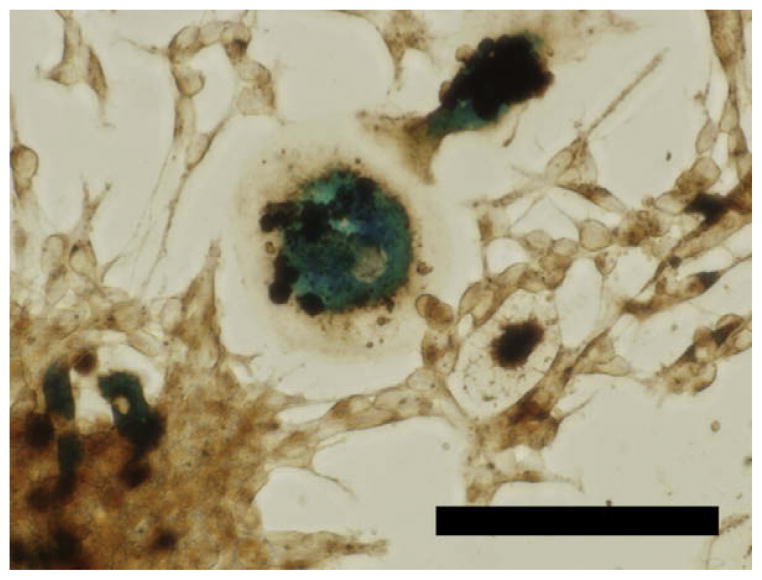
Fig. 3.
An example of the extensive phagocytic capacity of macrophages. Mouse neurospheres were labeled with Feridex/poly-L-lysine for 24 h using standard methods and allowed to adhere to the tissue culture dish. To confirm cell labeling, the culture was fixed with 4% paraformaldehyde and iron oxide nanoparticles were detected using a diaminobenzidine enhanced-Prussian blue protocol. In addition to numerous labeled neural precursors, this image contains two macrophages that have ingested more than six neural precursor cells that most likely did not survive the labeling procedure (unpublished data). Scale bar = 50 μm.
4.3. Negative MR contrast: iron oxide nanoparticles
Iron oxide nanoparticles are the most extensively applied contrast agent in cell imaging and cell tracking studies due to their strong negative contrast effect, biocompatibility, variety in core size and coating surface, and ease of microscopic detection by Prussian Blue staining and electron microscopy. Superparamagnetic iron oxide (SPIO) nanoparticles (50–200 nm diameter) and their ultra small (USPIO; ~35 nm diameter) variants [46,67] are composed of stabilized maghemite (Fe2O3) or magnetite (Fe3O4) and act to locally reduce T2 and relaxation via the induction of strong field inhomogeneities upon MR imaging with the appropriate pulse sequences to produce a hypointense or negative (black) signal [68]. Dextran, carboxydextran, or siloxane coating of the iron oxide core ensures iron oxide nanoparticle stability and solubility in biological media and is also thought to minimize any deleterious effects on cell function upon cellular uptake. One such SPIO contrast agent, Feridex® or Endorem®, is FDA-approved for clinical application, streamlining the transfer of experimental research findings to clinical settings [54,55,69–71] (Fig. 4).
Fig. 4.
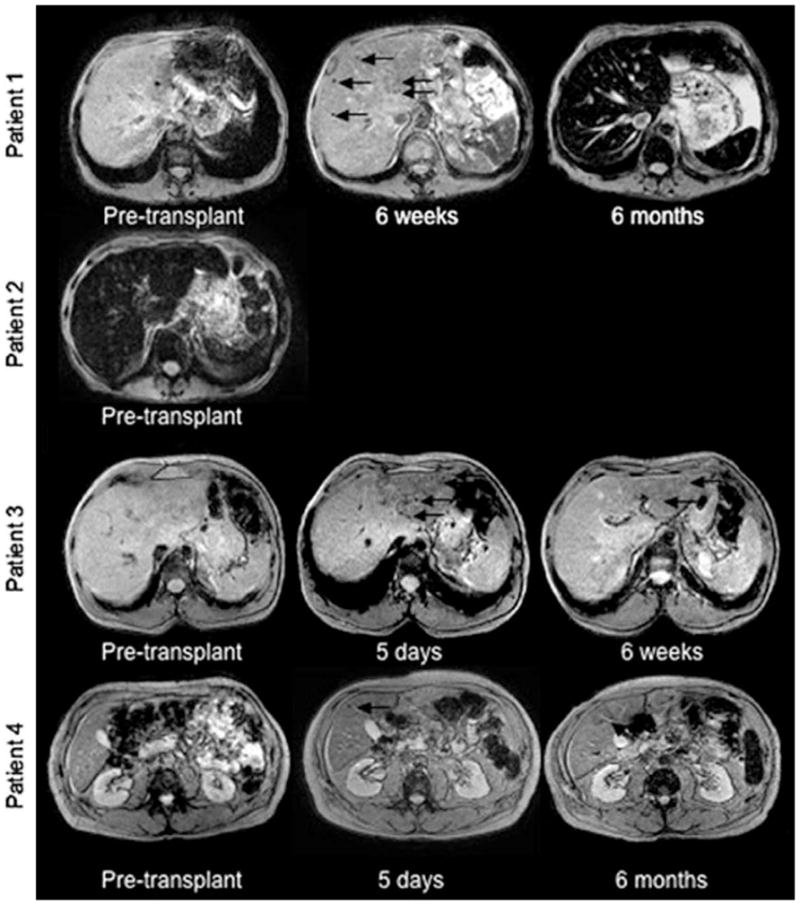
The safety and clinical application of cell tracking with SPIO was evaluated using human pancreatic islets which were transplanted into 4 patients after labeling with Endorem®, a clinical SPIO formulation. Compared to MR images obtained prior to transplantation, several hypointensities (black arrows) were detected within 5 days of transplantation and persisted for 6 weeks in Patient 1 and Patient 3; and as long as 6 months in Patient 4. This figure demonstrates the ability of MR imaging to detect variability in disease between patients as well as variability in graft survival and immunorejection. Reproduced, with permission, from Ref. [69].
Recent developments in engineered nanoparticles present further exciting possibilities for cell tracking. Using methods similar to SPIO synthesis, chemical cross-linking of monocrystalline iron oxide nanoparticles (MION) and free dextran yield an amine-terminated cross-linked iron oxide (CLIO) nanoparticle [72,73]. The incorporation of functional amine groups enables the coupling of a wide variety of linkers that increase the functionality and specificity of CLIO nanoparticles. In particular, molecules such as annexin V [74], oligonucleotides, fluorescent dyes [75,76], quantum dots [77], and TAT peptides [75,78,79] have been conjugated to CLIO nanoparticles.
Polystyrene microspheres containing iron oxides (MPIO) produced by Bangs Laboratories represent another format for the presentation of iron oxide to living cells. MPIO particles are noted for their use in single cell detection by ex vivo MR imaging [80–87]. Recently, MPIOs were used to monitor the migration of neural stem cells [83], macrophage delivery in the kidney [88], and macrophage recruitment to cardiac allografts [89] in vivo (Fig. 5). Though MPIOs are larger in particle diameter (0.35–1.6 μm) than SPIOs (50–200 nm), these particles are the most sensitive contrast agent available for cell tracking, due to their high iron content per particle (1 pg) and reduced partial volume effects. However, unlike (U)SPIOs, MPIO particles are not biodegradable by intracellular dextranases, are not supplied in sterile form, and the effects of long-term retention of MPIO on cell physiology are not known.
Fig. 5.
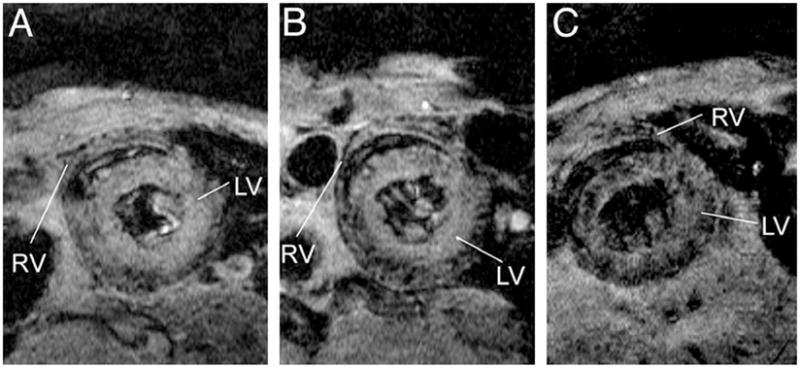
Serial MR detection of the accumulation of MPIO-labeled macrophages to a rejected cardiac allograft. A heart from a Dark-Agouti rat was transplanted in to Brown Norway rat, leading to acute tissue rejection based upon strain differences in immunological markers. MPIO was delivered intravenously 3.5 days after operation and macrophage recruitment to the transplanted heart was monitored by MR imaging at 3.5 days (A), 4.5 days (B) and, (C) 5.5 days. In (C), large numbers of punctate hypointensities are present in the parenchyma of the rejected heart. After comparing MPIO to USPIO for macrophage detection in rejected organs, this study found that single punctate hypointensities, presumably single macrophages, were more frequently detected using MPIO whereas USPIO resulted in larger, contiguous hypointensities. Reproduced, with permission, from Ref. [89].
4.4. Positive MR contrast: paramagnetic metals
Paramagnetic metals with unpaired electrons such as manganese among the transition metals and gadolinium from the lanthanide family (elements 58–71) primarily decrease T1 relaxation, producing a hyperintense or positive (white) signal in the region where they distribute. Gadolinium ion stabilized by a shell of diethylenetriamine pentaacetic acid (Gd-DPTA) or tetraazacyclododecanetetraacetic acid (Gd-DOTA) and related Gd chelate derivatives are routinely applied as fluid phase (blood, cerebrospinal fluid, etc.) contrast agents at lower field strength. Acting via direct dipole–dipole interactions, the T1 relaxation effects of paramagnetic agents are, on a molar basis, weaker than iron oxide nanoparticles which dephase multiple proton spins via their effects on local magnetic field gradients. In addition, once internalized by cells, paramagnetic contrast agents may exhibit reduced T1 relaxivity compared to their unbound counterparts in solution. For example, a large intracellular aggregate of between 100 and 1000 Gd chelates, or 10–100 μM/cell, is estimated to be necessary for cell detection [90]. One approach to cell labeling with Gd involves pre-incubation of Gd-DPTA with albumin protein to form a complex that reduces the intracellular toxicity of Gd-DPTA while maintaining its T1 relaxation properties for non-invasive imaging [91]. However, because of relatively low T1 contrast effects, reduced relaxivity at higher field, and unknown intracellular biocompatibility, examples of cell labeling using Gd are limited [92–99] (Fig. 6). Some cell tracking studies had to rely on (negative contrast) effects with the reduced T1 relaxivity at higher field [100]. The most recently described advance is the development of Gadofluorine M, a novel amphiphilic gadolinium-based contrast agent that can be readily incorporated into mesenchymal stem cells [98], glioma cells [101], and macrophages [102] without the use of transfection reagents.
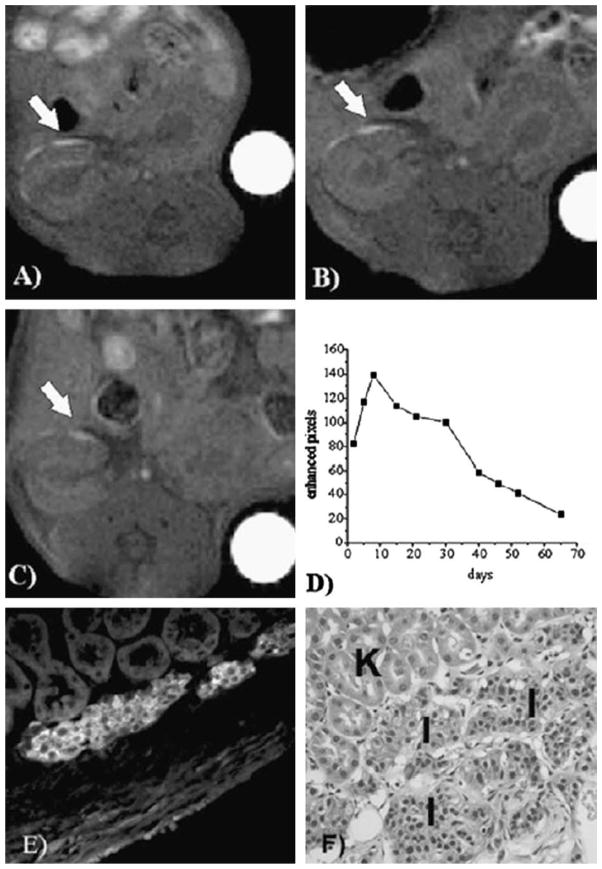
Fig. 6.
Serial MR detection of gadolinium-labeled pancreatic islets transplanted within the kidney capsule of immunodeficient mice. GdHDPO3A-labeled islets (white arrows) were detected at day 7 (A), day 30 (B), and day 65 (C) after transplantation into the kidney capsule. The number of enhanced pixels remained stable for one month and then declined steadily during the following month (D). Immunofluorescence (E) and histological (F) staining on day 65 demonstrate that the islet graft (I) was intact within the subcapsular space and distinct from kidney tubules (K). This study demonstrates that the neutral, hydrophilic contrast agent, GdHDPO3A, is well tolerated for long-term MR detection of nonproliferating cells following transplantation. In addition, this study shows how positive contrast using Gd can be applied to track cells in inherently T2 dark tissues, where iron oxides are less easily detected. Reproduced, with permission, from Ref. [94].
Manganese chloride (MnCl2) is another T1 enhancing contrast agent that is commonly used to label cardiac muscle cells or neurons for functional MR imaging following Ca2+ channel uptake. Along with indirect measures of electrical activity, MnCl2 is commonly applied to trace neuronal pathways following intraocular or intraparenchymal injections in the central nervous system (CNS) [103,104]. Based upon activity dependent axonal transport [105,106], MR imaging of MnCl2 distribution has revealed the presence of perturbed cortico–cortico fibers in a mouse model of multiple sclerosis [107], ventral forebrain defects in mutant mouse embryos in utero [108], and illuminated the connectivity of the entire mouse visual system from the retina to the secondary visual cortex [109]. MnCl2 has recently been applied to label T and B lymphocytes [110], which are notoriously difficult to label with iron oxides. Similarly, glioma cells were recently labeled with newly developed manganese oxide (MnO) nanoparticles using electroporation and imaged 24 and 72 h after transplantation into the mouse striatum [111]. Compared to SPIO-labeled cells in the contralateral striatum, the signal from MnO-labeled cells declined significantly after 3 days, indicating that MnO nanoparticles are not yet optimal for long-term cell tracking studies using positive contrast.
Further advances in chelation and particle formation yielding T1 contrast agents, such as Gadofluorine M and MnO, that are more amenable to cell labeling and detection would expand options for cell tracking. For example, when combined with MR imaging of cells labeled with superparamagnetic iron oxide nanoparticles, it may be possible to simultaneously follow two independently labeled cell populations [111]. However, in practice, a hyperintense signal and a hypointense signal colocalized in space could negate each other for detection under certain conditions. Moreover, in addition to T1-hyperintense signal, gadolinium chelates are known to induce signal loss in T2-weighted images [100].
4.5. Direct MR contrast: fluorine
Paramagnetic and superparamagnetic labeling agents cause local field inhomogeneities allowing for indirect MR detection. In the case of iron oxide contrast agents, cells containing nanoparticles create a signal decrease on weighted images, resulting in black pixel values in regions where cells are present. In some cases, iron oxide labeled cells can be difficult to distinguish from other hypointensities in the MR image that may arise from necrotic tissue, vascular hemorrhage or iron-rich tissues such as the liver and spleen. It is therefore difficult to assess the whole body distribution of iron oxide labeled cells following systemic administration. Developed shortly after the inception of MR technology, MR imaging of fluorine (19F) [112] has recently been revisited for direct cellular detection in the absence of false positive signals and very low background signal attributed to trace values within the body [113–117]. When conventional 1H images are acquired simultaneously, the superimposition of 19F images allows for the anatomical location of the 19F-labeled cells to be determined [118]. Moreover, the 19F signal can be spectroscopically quantified to estimate cell numbers. 19F imaging requires phagocytic intracellular labeling of cells with nanoparticles containing a tracer agent such as perfluoropolyether (PFPE) or perfluorocarbon (PFC) that contain 20 fluorine nuclei. Due to trace endogenous levels of intracellular 19F, cells must incorporate significant levels of PFPE (0.25 ng/cell) and exist in close proximity (>200 cells/voxel) for MR detection [113,114,116] (Fig. 7). Though cell viability, proliferation, differentiation and function following intracellular PFPE/PFC introduction has not been extensively characterized, related compounds such as LiquiVent® and Oxygent®, have been applied clinically as artificial blood substitutes [119].
Fig. 7.
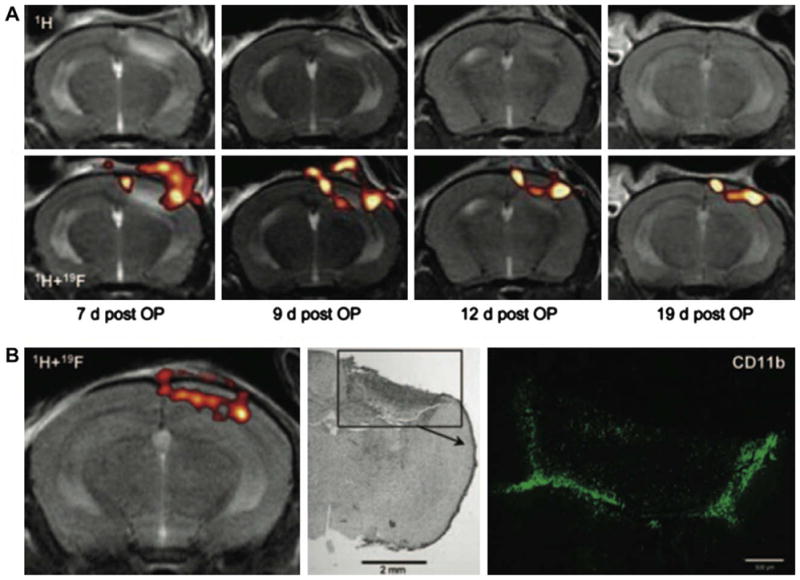
Serial MR detection of the accumulation of in situ-19F-labeled macrophages to a photothrombic CNS lesion. PFC was delivered intravenously on day 6 post-focal cerebral ischemia. MR imaging on day 7 reveals the presence of edema and inflammatory infiltrates in the right cerebral hemisphere (A, top left panel). MR imaging of 19F (A, bottom row) reveals the presence of labeled macrophages, indicated by “hot” pseudocoloring, within the same region. Edema decreased over time as detected by 1H MR imaging, whereas the presence of macrophages persisted for up to 19 days. In (B), the presence of 19F-labeled macrophages (left) within the lesion site (middle) was confirmed using immunohistochemistry for CD11b (green, right panel). Note the similarities in the distribution of 19F signal and CD11b immunoreactivity. This study accentuates the low background signal associated with MR imaging of 19F and the need to superimpose 1H and 19F images for anatomical correlation. In addition, panel B provides an idea of the number and degree of proximity of 19F-labeled cells that is necessary for MR detection. Reproduced, with permission, from Ref. [116].
4.6. Expression of iron binding proteins for MR detection
The two major limitations of labeling cells with exogenous contrast agent are (1) progressive or nonlinear dilution of finite quantities of intracellular contrast agent by cell division [65] and (2) the inability to distinguish between live and dead cells after contrast agent labeling. Both of these issues are critical for long-term non-invasive monitoring of cell therapy. One way to indelibly label viable cells is to either infect cells with viral DNA constructs that encode for markers designed to be specifically detected by MR when expressed [120], or create transgenic animals using similar genetic principles. Depending upon the type of promoter, genes of interest can be expressed constitutively, as in the case of cytomegalovirus (CMV) or elongation factor-1 (EF-1), in response to drug administration (e.g., tetracycline), or in a cell/tissue specific manner. Expression of non-mammalian proteins such as LacZ and green fluorescent protein have been used to mark cells for many purposes including: (i) detection of gene expression; (ii) study of protein–protein interactions; (iii) tracking cell fate in normal development or in response to genetic manipulation; and (iv) visualization of engrafted cells. Genetic markers, such as Herpes Simplex Virus type 1 thymidine kinase and firefly luciferase, have also proven effective for in vivo imaging with nuclear and optical methods [121] even though optical imaging methods are limited by tissue opacity and nuclear imaging lacks cellular resolution [122,123]. The successful application of genetic markers in MR imaging can overcome each of these limitations, providing three-dimensional anatomical detail and non-invasive detection of live cells.
Genetic markers for MR detection take advantage of the biology of endogenous proteins required for iron metabolism such as ferritin, transferrin, and the transferrin receptor (TfR). Iron serves as a regulatory cofactor in many metabolic pathways and is required for the proper function of many proteins [124]. In vertebrates, apotransferrin is converted into holotransferrin upon iron binding and is transported via the circulatory system for internalization by cells that express TfR [125]. Ectopic expression or overexpression of TfR was considered as a means of increasing intracellular iron and thus enabling MR detection [126]. Using this approach, a 50-fold overexpression of hTfR mRNA by a tumorigenic fibroblast cell line yielded a threefold increase in intracellular iron concentration and produced a significant reduction in signal in T2-weighted MR images. However, the high levels of hTfR expression required to generate relatively small changes in T2 relaxation are impractical for cell tracking in normal, less proliferative cells.
Increasing cellular iron storage by overexpression of ferritin is another route that was considered for cellular labeling. Ferritin is a globular protein comprised of 24 heavy (21 kDa) and light (19 kDa) chain subunits that are capable of binding up to 4500 iron atoms [127]. Ferritin exhibits a high transverse relaxivity (R2), even under conditions of low iron binding (apoferritin, <100 iron atoms), and R2 has been shown to increase linearly in proportion to the magnetic field [128–131]. The utility of ferritin as a means for intracellular iron loading was tested using tetracycline-inducible expression of a truncated ferritin heavy chain in a C6 glioma cell line [132] and in transgenic and double transgenic mice [133]. Similarly, adenoviral constructs injected stereotaxically into mice brains led to the expression of both heavy and light ferritin chains in neurons and glia [134]. Finally, ferritin expression in combination with TfR expression allowed for MR detection of mouse neural stem cells 10 days after CNS transplantation [135]. An interesting, different approach has been to transfect into cells the bacterial gene MagA that is responsible, along with a large set of other genes, for synthesis of biogenic magnetite in magnetotactic bacteria that reside in anaerobic sediments in ponds and oceans, and use the magnetic particles to steer towards the bottom milieu using the geomagnetic field. Fibroblasts transfected with MagA synthesize nanoparticles endogenously and appear hypointense on MR images following CNS transplantation [136].
As with other contrast-labeling methods discussed above, there are technical challenges associated with metalloprotein-based genetic reporters in MR cell tracking. For example, cellular labeling in vivo with endogenous iron requires significant time for protein expression, binding, transport, and internalization for detectable iron accumulation to occur. This limitation may be overcome by incubating genetically engineered cells in an iron-rich medium prior to transfer. However, as for nanoparticles-based cell labeling, intracellular iron will become diluted as cells divide reducing the capacity for detection by MR at clinical field strengths. Finally, cellular iron homeostasis is critical for control of free radical damage via Fe3+. In particular, interfering with cellular iron metabolism affects CNS development [137] and can induce neuropathology as shown in a transgenic model of iron overload [138]. Therefore, the identification of genetic labeling approaches that are detectable by MR and yet independent of endogenous iron signaling and homeostasis may provide greater specificity.
4.7. Expression of artificial macromolecular contrast within cells
The temporal resolution of inducible genetic reporters is dependent upon the time required for protein synthesis and the accumulation of sufficient molecules for detection. The ability to rapidly activate and deactivate cellular markers using selective RF pulses would provide better temporal resolution and also permit non-invasive MR imaging of the underlying anatomy which can be obscured by (super)paramagnetic substances operating constitutively on local 1H relaxation. Such rapid and selective activation of cell label has recently been demonstrated in vivo using chemical exchange saturation transfer (CEST) to detect the expression of an artificial polypeptide reporter [120]. CEST imaging is based upon the transfer of magnetization from macromolecular 1H to bulk 1H in water following the delivery of a saturation RF pulse at a predetermined frequency that is selective for the macromolecule of interest. This phenomena was first demonstrated with poly-L-lysine polypeptides containing a high density of amide protons for rapid magnetization exchange with 1H in solution [139]. Gilad and coworkers applied this concept to cellular MR imaging by bioengineering an genetically encoded CEST agent comprised of 200 positively charged lysine moieties termed lysine-rich protein (LRP, 32 kDa) [120]. Protein extracts from cells that express LRP at micromolar concentrations demonstrated a significant increase in CEST MR contrast relative to protein extract from control cells. LRP protein expressed by 9 L glioma cells also produced a significant CEST signal in vivo following cell delivery into the striatum of the mouse CNS. Since different CEST MR reporters can be designed by manipulating exchangeable groups and charge characteristics, such that each reporter will be responsive to unique radiofrequencies encoding for their own specific “color” [140], this new technology opens the door for simultaneous non-invasive imaging of multiple cell types and/or the expression pattern of multiple genes.
4.8. Multimodality imaging and multifunctional probes
Imaging modalities can be divided roughly into anatomical (i.e., X-ray, CT, MRI, and ultrasound) and functional or molecular (i.e., PET, SPECT, and optical imaging) categories. Efforts directed towards combining imaging modalities from each of these groups, such as PET/CT [141], PET/MRI [14], and optical/MRI [21] have enabled coregistration of cellular function and anatomy (Fig. 8). Similarly, multifunctional probes provide flexibility in the selection of imaging modalities for specific purposes in the same experimental group. For the most part, the subject is simply transferred between imaging devices for data acquisition. However, efforts are also underway to integrate the hardware for two imaging modalities into a single unit [14,141,142]. Though merging imaging modalities can overcome the limitations of a singular modality, new concerns related to contrast agent synthesis and specificity, coregistration of organ position, hardware compatibility, and clinical applicability are raised, the latter issue arising particularly for mergers with optical methods. For example, while BLI effectively reveals dynamic processes such as hematopoiesis, infection, the spread of tumor metastases, tissue rejection, and cell signaling, BLI depends upon expression of a non-mammalian gene (luciferase) followed by systemic delivery of a foreign substrate (D-luciferin) for photon emission, which has a limited depth of tissue penetration and therefore confines its union with complementary modalities to animal studies. Similar issues arise for quantum dot nanoparticle probes that contain colloidal semiconductor cores and a cadmium–selenium surface coating. Quantum dots exhibit promising characteristics for multimodality imaging including broad excitation and narrow emission spectra, negligible autofluorescence, and long-term photostability. However, the absence of cellular toxicity following the introduction of quantum dot within the cytoplasm must be confirmed for cell tracking purposes [143] and the long-term fate and biocompatibility are unknown.
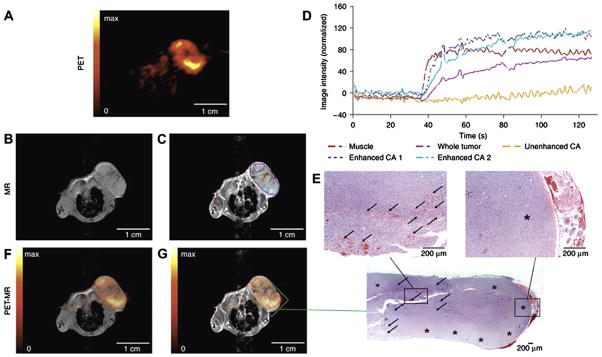
Fig. 8.
Fully integrated dual modality PET/MR imaging of a subcutaneous tumor. (A) PET images of (18F)FLT tracer uptake indicate regions of high cell proliferation (yellow) and necrosis (black). (B and C) Pre- and post-contrast MR images obtained using the same device reveal the underlying anatomy and further define sites of tissue necrosis (C, yellow region of interest in center of tumor). (D) Dynamic measurement of contrast enhancement within regions of interest highlighted in (C). (F and G) Coregistration of PET and MR images reveals areas of inflammation and necrosis that were confirmed by histology (E). Note that integration of the hardware for PET and MRI permits direct image coregistration as the anatomy of experimental subject was not shifted; as is often the case when moving patients between two imaging devices. Reproduced, with permission, from Ref. [14].
5. Non-invasive imaging of pathological progression at the cellular level
5.1. Detection of macrophage recruitment to normal and malignant lymph nodes
Macrophages and related phagocytic cells comprise the early endogenous response to tissue damage or infection. Because of their capacity to ingest iron oxide nanoparticles upon intravascular delivery, MR imaging of phagocytic cells has provided valuable insights into disease progression and offered options for clinical diagnosis. Kupffer cells, resident macrophages of the liver, were the first cell to be labeled for cellular MR imaging upon intravenous administration of SPIO [47,144]. In light of this finding, smaller iron oxide formulations with reduced body clearance (i.e., longer blood half-life), such as ultra small particles of iron oxides (USPIOs), were developed in an effort to increase blood half-life and contrast agent bioavailability to macrophages residing in smaller tissue compartments such as lymph nodes [46,145,146]. Recently, MR detection of USPIO was shown to rival PET/CT [147] in specificity and sensitivity of detection of lymph node metastasis in pelvic, head and neck, chest, and breast malignancies [146,148–151]. Compared to MR images obtained prior to intravenous USPIO delivery, normal lymph nodes appear hypointense upon MR imaging on the following day due to trafficking and/or residing macrophages that have ingested USPIO whereas malignant lymph nodes are distinguished by endogenous positive contrast attributed to a predominance of cancerous cells that have replaced the normal (phagocyte) tissue composition.
5.2. Imaging macrophages within atherosclerotic plaques
Large numbers of macrophages are associated with atherosclerotic plaques, also called atheromas, consisting of thin (<65 μm) fibrous caps overlying large, necrotic, lipid cores that are at high risk for rupture [152]. Therefore, the ability to non-invasively detect the presence of macrophages in proximity to atheromas may predict infarct risk [153–155]. Animal studies of atherosclerosis have shown that SPIO particles accumulate within macrophages associated with atheromas and induce areas of signal loss within the vessel wall in -weighted MR images [156–159]. More recently, human studies using the USPIO agent, Sinerem (Ferumoxtran-10), have confirmed the presence of macrophages in atherosclerotic regions and refined MR imaging parameters for atheroma detection [160–162].
5.2.1. Monitoring macrophage infiltration in diseases of the central nervous system
CNS lesions in multiple sclerosis are characterized by the presence of phagocytic macrophages. The accumulation of macrophages within the CNS of rats and mice with experimental autoimmune encephalomyelitis (EAE) was monitored non-invasively using MRI following intravenous delivery of iron oxides [163–167]. Once in the circulatory pool, the iron oxides are phagocytosed by circulating monocytes en route to or in transit through sites of blood–brain or blood–spinal cord barrier disruption. Similarly, macrophage infiltration in the CNS after EAE induction was recently detected indirectly using a Gd-coupled substrate for myeloperoxidase (MPO), an enzyme expressed by macrophages [168–170]. Compared to Gd-DPTA, imaging of MPO activity yielded improved detection of smaller inflammatory brain lesions, permitting detection of macrophage infiltration at earlier stages of disease progression. MRI detection of macrophage infiltration may provide a means for the correlation of macrophage cell number with the extent of inflammatory demyelination or with patient responses to immunosuppressive therapy [165,171,172].
Similarly, ischemic brain injury is characterized by an ongoing recruitment of mononuclear phagocytic cells from the circulatory pool [173]. The progression of this inflammatory response has been demonstrated non-invasively using MR in animal models of ischemic stroke [174–177]. The success of experimental stroke lesions in visualizing macrophages by USPIO-enhanced MRI has led to clinical pilot studies where USPIO-enhanced MRI has proven capable of monitoring macrophage infiltration in human ischemic stroke [178]. As with multiple sclerosis, MR cellular imaging of macrophage infiltration into early ischemic stroke lesions may help to more specifically target anti-inflammatory therapies in humans.
5.3. Imaging the host response to organ transplantation
MR imaging of macrophage infiltration into rejected organ grafts has been performed to characterize acute and chronic kidney allograft rejection [179,180] as well as immune rejection of lung [181] and heart transplants [182] in rodents. This approach was also applied clinically by Hauger et al., who used USPIO-enhanced MR imaging to compare the extent of macrophage infiltration between native and transplanted kidneys [183]. Recently, both SPIO and larger MPIO particles have been used to monitor the recruitment of macrophages during the early phases of myocardial rejection [89]. These studies form the foundation for the development and optimization of immunomodulatory agents to improve transplant survival.
5.4. Validation of targeted immunotherapy
Immunotherapy, based on the delivery of antigen-primed immune cells, can be used to ablate cells within solid tumors and hematopoietic cancers such as leukemia, lymphoma, and myeloma. Dendritic cells are phagocytic antigen-presenting cells that reside within the skin, lungs, and intestines and often underlie allergic and autoimmune reactions. Intracellular labeling of dendritic cells (DCs) for MR cell tracking was first performed using receptor mediated endocytosis of SPIO particles conjugated with monoclonal anti-CD11c [184]. As an alternative to antibody-mediated labeling, DC recruitment can also be detected in lymph nodes proximal to sites of subcutaneous SPIO injection. [185]. For anti-tumor cell therapy in humans, autologous DCs were treated with tumor-derived antigenic peptides and then labeled with clinically approved SPIOs prior to intranodal injection under ultrasound guidance in patients with advanced melanoma [71]. 111In-labeled DCs, were co-injected for nuclear scintigraphy. Compared to the latter, the greater spatial resolution of MR imaging revealed DC recruitment to larger numbers of lymph nodes. In addition, MR imaging (but not nuclear scinigraphy) was able to demonstrate that the physicians failed to inject the antigen-primed DCs correctly into the draining lymph nodes. Likewise, the trafficking of (U)SPIO-labeled macrophages and T lymphocytes was detected at tumor metastases [33,186–188] (Fig. 9).
Fig. 9.
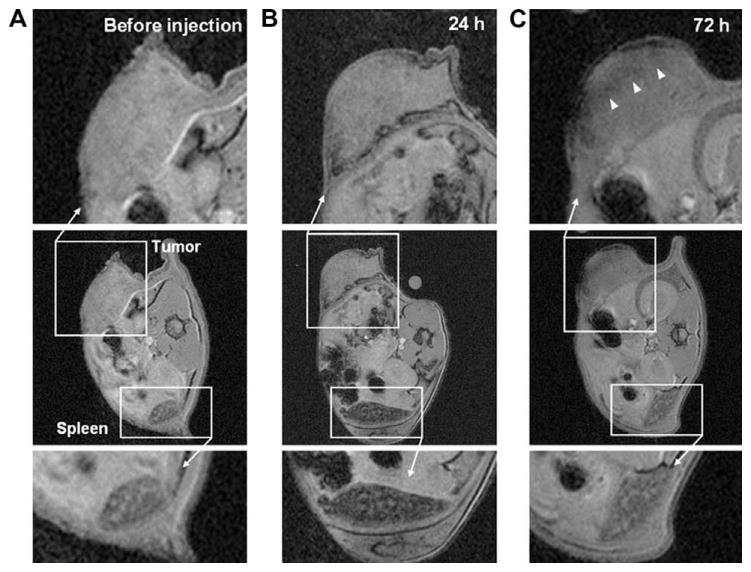
MR cell tracking of the progression of anticancer cell therapy. Ovalbumin-specific splenocytes (OT-1, 3 × 106 cells, 1.3 pg iron/cell) were labeled with SPIO and adoptively transferred to mice harboring ovalbumin-expressing lymphoma cells. Prior to cell transfer, the tumor and spleen appear homogenous by MR imaging (A). One day following cell transfer (B), the spleen showed significant negative contrast and volume enhancement whereas the tumor exhibited little change. After 72 h (C), the spleen showed slight positive contrast whereas the tumor became hypointense (white arrowheads). Reproduced, with permission, from Ref. [33].
6. Non-invasive imaging of stem cell therapy
6.1. Stem cells as therapeutic entities
Stem cells have significant potential in therapy for many clinical conditions because they possess mechanisms for overcoming natural cell replication limits and are yet capable of differentiation in response to extrinsic cues in vitro or to factors in the transplant microenvironment. Stem cells can be isolated from diverse sources and are defined functionally as cells with the capacity for self-renewal and the ability to yield multiple, distinct, differentiated cell types. Embryonic stem cells are derived from the inner cell mass of the blastocyst and are pluripotent, that is they are able to give rise to all the cell types of the derivatives of all three embryonic germ layers [189]. Isolation of these cells from humans poses ethical concerns and, because of a lack of control of their unlimited potential for proliferation and differentiation, there are risks of tumor formation or inappropriate differentiation following transplantation [24,27,28]. However, recent progress in the definition of signaling factors that underlie pluripotency may soon circumvent ethical concerns and allow for greater control of stem cell function [190–193]. Organ or tissue-specific stem cells are multipotent or restricted progenitors that contribute to the formation of fetal tissues and organs. In adults, these cells continue to proliferate and provide regenerative capacity in certain tissues. Compared to fetal sources, adult stem cells can be isolated in greater quantity for autologous or allogeneic transplantation. In addition, stem cell lines can be established for indefinite replication in tissue culture to provide large numbers of cells for systematic analysis and transplantation. One key advantage of stem cell lines is that the cells may be genetically engineered to improve their detection, engraftment, or therapeutic support at sites of transplantation [194–198]. Equally possible, gene deletion experiments can be performed in order to remove or suppress tumorigenic signaling pathways in order to enhance engraftment and therapeutic potential.
6.2. MR imaging of mesenchymal stem cell therapy
6.2.1. Ischemic heart disease
Bone marrow is a source for both hematopoietic bone marrow stem cells and non-hematopoietic stem cells, i.e., bone marrow stromal cells or mesenchymal stem cells (MSCs). Adult MSCs possess the ability to differentiate into chondrocytes, osteocytes, adipocytes, and myocytes [199–201] and have been tracked by non-invasive MR following cell therapy for infarcted myocardium [202–204] and focal CNS ischemia [194,195,205,206]. Transplanted MSCs are thought to be attracted by factors secreted by injured or diseased cardiac tissue [207] where, upon recruitment, they themselves may produce factors that support angiogenesis, reduce cardiomyocyte apoptosis, and improve contractile activity [208,209]. However, unlike MSCs slowly and continuously mobilized from bone marrow, cultured MSCs appear to become trapped upon systemic delivery en masse while en route through the coronary vasculature [19,210]. Improved cardiac function was obtained via injection of MSCs directly into the anterior wall of the left ventricle [211,212]. Though MR tracking of SPIO-labeled MSC integration in cardiac tissue is quite challenging because of the rapid contraction rate of the rodent heart, MSCs were recently tracked using non-invasive MR for one month after injection [202,204,213]. Equivocal data on MR tracking of MSC in the heart along with limited clinical efficacy in randomized clinical trials [214–216] indicate that cell therapy using MSC for patients with ischemic heart disease is not yet optimal. Ongoing developments in non-invasive imaging of MSCs in heart will therefore play a prominent role in the optimization of MSC delivery, integration, and survival.
6.2.2. Cerebral ischemia/stroke
MSC transplants have also been reported to attenuate functional deficits in rats following CNS injury [217,218] or cerebral ischemia/stroke [205,219]. Cytokine and growth factor secretion by MSCs is hypothesized as a mechanism which supports the survival of cells in the lesion site rather than cellular differentiation and engraftment [220]. In support of this theory, MSCs modified to express and secrete factors such as glial cell line derived trophic factor [194], brain derived neurotrophic factor [196,198], and placental growth factor [197] exhibit improved neuroprotective capability following cerebral ischemia. It will be of interest to non-invasively follow the migration of these genetically engineered MSC to sites of ischemic damage by MR. For example, following photochemical lesion of a localized region in the cerebral cortex, SPIO-labeled MSCs injected into the contralateral cerebral ventricle migrated to the lesion site within 2 weeks of administration [205].
6.3. MR imaging of neural stem cell therapy
In multiple sclerosis (MS), subcortical oligodendrocytes are lost due to either attack by autoimmune T cells, axonal loss, or both phenomena [221–223]. Activated and possibly non-activated lymphocytes can traverse the endothelial cell layer between blood and brain, known as the blood–brain barrier (BBB), and make contact with CNS tissue even in the absence of inflammation. Following antigen recognition, as in the case of infection or autoimmune attack, T cells may initiate an inflammatory cascade that compromises the integrity of the BBB allowing entry of greater numbers of leukocytes and macrophages into the CNS parenchyma [224]. As mentioned previously, endogenous tissue specific stem cells are thought to contribute to the restoration of organ function following cell loss caused by apoptosis, injury, or disease. In the CNS, the adult subventricular zone of the lateral ventricles and the subgranular zone of the dentate gyrus are two niches that exhibit the capacity to generate new neurons [225,226]. However, as MS progresses, endogenous neural stem cells are either depleted or functionally inhibited. Access to the potential of neural stem cells presents exciting possibilities for non-invasive MR imaging of cell therapy following traumatic CNS injury and in models of CNS disease where cell attrition is implicated. Neural stem cells divide continuously when cultured in a serum-free medium containing basic fibroblast growth factor and epidermal growth factor [227–229] and form neurospheres that are able to give rise to neurons, astrocytes, and oligodendrocytes [66,230–234]. Multipotent neural stem cells provide an opportunity to replace lost neurons and oligodendrocytes and several recent reports suggest that these cells possess immunomodulatory characteristics that may be favorable for cell therapy in MS [231,232,235,236].
The endogenous CNS-immune and graft-recipient interactions (e.g., transplant-CNS and transplant-immune) that are characteristic of rodent and primate models of MS are increasingly addressed by non-invasive MR imaging [165,172,237]. Transfection agent (dendrimer)-based methodology for labeling and in vivo MR tracking of neural stem cells was established in 2001, allowing investigators to follow cells for at least 6 weeks in vivo [238]. This labeling approach was readily applied to label human neural precursors [239] and human embryonic stem cell derived neurospheres [66] for MR detection in mouse CNS for up to 4 weeks. Tracking of SPIO-labeled neural precursor cells following intracerebroventricular (ICV) injection in mice with EAE revealed that these cells migrated in response to inflammatory cues within one week of transplantation and that the migration distance correlated with the severity of the disease [66]. ICV injection of neurospheres has been shown to decrease the severity of experimental allergic encephalomyelitis (EAE, an experimental model of MS) in mice [232,240]. Current efforts are directed towards the identification of optimal periods of intervention [232,235] and MR tracking of neural stem cell delivery and migration towards sites of inflammation and demyelination in EAE [66,241]. Though SPIO label generally appears to be well retained over 4–6 weeks by neural stem cells, depending on the cell type detection of SPIO-labeled stem cells beyond two weeks may be impaired in vivo when asymmetric division is present, i.e., in the case of immortalized cells [63,65].
7. Non-invasive MR imaging of cell therapy in the year 2020
7.1. Improving in vivo cell detection
How might non-invasive cell tracking be applied by the end of the next decade? Options for labeling cells for non-invasive MR detection are rapidly evolving and the speed, sensitivity and resolution of MR imaging devices is continually increasing. Higher field strength, custom designed gradient and RF coils, and optimized pulse sequences (e.g., 3D FIESTA) have improved the sensitivity of MR microimaging of SPIO-labeled cells in vivo [86,242,243]. These and upcoming advancements will eventually enable facile, high resolution pursuits for optimization and validation of cell therapy. For example, refined non-invasive measurements of cell biodistribution following exposure to soluble factors or genetic manipulation are imminent. Likewise, clinical tracking of stem cell therapy and tumor metastasis require the ability to distinguish individual cells or small groups of cells. For non-clinical MR devices, in vivo thresholds of cell detection are currently in the range of 100–500 cells containing 10–60 pg of iron oxide nanoparticles per cell [53,64,79,244] down to the single cell level using Bangs particles [83,86] yielding 100 pg of iron per cell. MR imaging of SPIO labeled cells grown in vitro in collagen gels, gelatin, or agarose have recently been used to test the level of cell detection of clinical grade 1.5 T [32,245–247] and 3 T [248,249] scanners and commercially available gradients. However, whether clinical MR scanners can detect individual cells in the presence of motion artifacts and larger FOV that are characteristic of in vivo imaging has not been determined. Finally, a greater number of contrast agents that are deemed suitable for safe diagnostic application in humans are needed.
7.2. Monitoring the progression of cell therapy
Presently, periodic non-invasive surveillance of a specific cell population in a vast in vivo milieu poses significant technical challenges that must be overcome for cell therapy. MR cell tracking is critical for the definition of optimal cell populations and delivery methods for therapeutic purposes. For example, MR may be used to evaluate whether therapeutic cells arrive correctly at sites of disease. In addition, MR imaging can be applied to compare the therapeutic properties (e.g., survival, migration, differentiation) of one cell population to another. Individual cells are difficult to resolve using 2D multi-slice imaging due to the slice thickness that is necessary (>400 μm) to ensure the presence of sufficient nuclear spins in each voxel (signal to noise ratio). High resolution imaging of a 3D slab of tissue significantly increases imaging time, even at high magnetic field strength. Though not always possible for 3D imaging, it is helpful to know, a priori, the location of the cells of interest within the subject so that the FOV can be adjusted to complete MR imaging within 1 h for live subjects. Current challenges in the application of serial non-invasive MR cell imaging include: (1) cellular spatial resolution using shorter acquisition time; (2) cellular image registration between imaging sessions; (3) attenuation of subject motion artifacts; (4) digital normalization to correct for intersession variability in subject orientation and FOV and (5) non-biased methods for quantitative analysis of cell biodistribution. These challenges have recently begun to be addressed. For example, a robust automated detection algorithm has been developed for quantification and localization of iron in 3D MR datasets [250].
7.3. Imaging the efficacy of cell therapy
MR imaging is applied extensively for clinical diagnosis and is rapidly developing as a method for non-invasive imaging of cells in experimental models of disease [251]. One excellent example of the potential synergy between clinic and laboratory is the merger between advancements in MR detection of CNS lesion progression in animal models of multiple sclerosis [163,252–254] and experimental efforts to non-invasively monitor neural stem cell recruitment to these demyelinated lesions [66,241,255,256]. Methods for MR monitoring of the temporal progression of CNS lesions in human MS have recently been established [257,258] and, through careful translation of research advances to the clinical practice, it may one day be possible to use MR imaging to observe a cessation or reduction in lesion progression in response to cell therapy. Moreover, the combination of MR imaging with nuclear medicine (PET and SPECT) or optical and bioluminescent imaging modalities will permit further characterization of cell transplant viability, function, proliferation, and differentiation in the same experimental cohort. For example, myelin formation by oligodendrocytes and synapse formation by neurons are highly complex processes that may be regarded as stringent end-points for judging differentiation by neural stem cells. However, it is not known how efficiently cell transplants survive and execute these cellular differentiation processes for therapeutic effect. In future studies, it will be essential to non-invasively determine the percentage of surviving transplanted cells and the rate and extent to which they recapitulate a particular biological process. In sum, non-invasive imaging is critical for monitoring cell therapy as both the pattern of disease and the response to cell therapy will vary between individuals.
Acknowledgments
The authors are supported by NMSS RG3630, The TEDCO Maryland Stem Cell Fund ESC 06-29-01, RO1 NS045062, RO1 EB007825, EUREKA RO1 DA02699, and Roadmap R21 EB005252.
Glossary
- BBB
blood–brain barrier
- BLI
bioluminescent imaging
- CEST
chemical exchange saturation transfer
- CLIO
cross-linked iron oxide
- CMV
cytomegalovirus
- CNS
central nervous system
- CT
computerized tomography
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- EF-1
elongation factor-1
- FOV
field of view
- Gd-DOTA
gadolinium tetraazacyclododecanetetraacetic acid
- Gd-DPTA
gadolinium diethylenetriamine pentaacetic acid
- GFP
green fluorescent protein
- ICV
intracerebroventricular
- LRP
lysine-rich protein
- MION
monocrystalline iron oxide nanoparticles
- MPIO
Micron-sized particles of iron oxide
- MPO
myeloperoxidase
- MR
magnetic resonance
- MS
multiple sclerosis
- MSC
mesenchymal stem cell
- PET
positron emission tomography
- PFC
perfluorocarbon
- PFPE
perfluoropolyether
- R2
transverse relaxivity
- RF
radio-frequency
- RFP
red fluorescent protein
- SPECT
single photon emission computerized tomography
- SPIO
superparamagnetic iron oxide
- TfR
transferrin receptor
- USPIO
ultrasmall superparamagnetic iron oxide
References
- 1.Mazzarello P. Nat Cell Biol. 1999;1:E13–E15. doi: 10.1038/8964. [DOI] [PubMed] [Google Scholar]
- 2.Dymecki SM, Kim JC. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjantonakis AK, Dickinson ME, Fraser SE, Papaioannou VE. Nat Rev Genet. 2003;4:613–625. doi: 10.1038/nrg1126. [DOI] [PubMed] [Google Scholar]
- 4.Shaner NC, Steinbach PA, Tsien RY. Nat Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 5.Giepmans BN, Adams SR, Ellisman MH, Tsien RY. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 6.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 7.De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 9.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 10.Sako Y, Sekihata A, Yanagisawa Y, Yamamoto M, Shimada Y, Ozaki K, Kusumi A. J Microsc. 1997;185:9–20. doi: 10.1046/j.1365-2818.1997.1480707.x. [DOI] [PubMed] [Google Scholar]
- 11.Kunwar PS, Siekhaus DE, Lehmann R. Annu Rev Cell Dev Biol. 2006;22:237–265. doi: 10.1146/annurev.cellbio.22.010305.103337. [DOI] [PubMed] [Google Scholar]
- 12.Halin C, Rodrigo Mora J, Sumen C, von Andrian UH. Annu Rev Cell Dev Biol. 2005;21:581–603. doi: 10.1146/annurev.cellbio.21.122303.133159. [DOI] [PubMed] [Google Scholar]
- 13.Hauser AE, Shlomchik MJ, Haberman AM. Nat Rev Immunol. 2007;7:499–504. doi: 10.1038/nri2120. [DOI] [PubMed] [Google Scholar]
- 14.Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Rocken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Doudet DJ, Studenov AR, Nian C, Ruth TJ, Gambhir SS, McIntosh CH. Nat Med. 2006;12:1423–1428. doi: 10.1038/nm1458. [DOI] [PubMed] [Google Scholar]
- 16.Furtado S, Sossi V, Hauser RA, Samii A, Schulzer M, Murphy CB, Freeman TB, Stoessl AJ. Ann Neurol. 2005;58:331–337. doi: 10.1002/ana.20564. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 18.Doyle B, Kemp BJ, Chareonthaitawee P, Reed C, Schmeckpeper J, Sorajja P, Russell S, Araoz P, Riederer SJ, Caplice NM. J Nucl Med. 2007;48:1708–1714. doi: 10.2967/jnumed.107.042838. [DOI] [PubMed] [Google Scholar]
- 19.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson MK, Doss M, Shaller C, Narayanan D, Marks JD, Adler LP, Gonzalez Trotter DE, Adams GP. Cancer Res. 2005;65:1471–1478. doi: 10.1158/0008-5472.CAN-04-2008. [DOI] [PubMed] [Google Scholar]
- 21.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. Nat Med. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 22.Dubey P, Su H, Adonai N, Du S, Rosato A, Braun J, Gambhir SS, Witte ON. Proc Natl Acad Sci USA. 2003;100:1232–1237. doi: 10.1073/pnas.0337418100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swijnenburg RJ, Schrepfer S, Cao F, Pearl JI, Xie X, Connolly AJ, Robbins RC, Wu J. Stem Cells Dev. 2008;17:1023–1030. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reumers V, Deroose CM, Krylyshkina O, Nuyts J, Geraerts M, Mortelmans L, Gijsbers R, Van Den Haute C, Debyser Z, Baekelandt V. Stem Cells. 2008:2382–2390. doi: 10.1634/stemcells.2007-1062. [DOI] [PubMed] [Google Scholar]
- 26.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Suzuki Y, Huang M, Cao F, Xie X, Connolly AJ, Yang PC, Wu JC. Stem Cells. 2008;26:864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao F, Drukker M, Lin S, Sheikh AY, Xie X, Li Z, Connolly AJ, Weissman IL, Wu JC. Cloning Stem Cells. 2007;9:107–117. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- 29.Lauterbur PC. Clin Orthop Relat Res. 1989:3–6. [PubMed] [Google Scholar]
- 30.Bulte JW, Vymazal J, Brooks RA, Pierpaoli C, Frank JA. J Magn Reson Imaging. 1993;3:641–648. doi: 10.1002/jmri.1880030414. [DOI] [PubMed] [Google Scholar]
- 31.Brooks RA, Vymazal J, Bulte JW, Baumgarner CD, Tran V. J Magn Reson Imaging. 1995;5:446–450. doi: 10.1002/jmri.1880050414. [DOI] [PubMed] [Google Scholar]
- 32.Bernas LM, Foster PJ, Rutt BK. J Neurosurg. 2007;106:306–313. doi: 10.3171/jns.2007.106.2.306. [DOI] [PubMed] [Google Scholar]
- 33.Smirnov P, Lavergne E, Gazeau F, Lewin M, Boissonnas A, Doan BT, Gillet B, Combadiere C, Combadiere B, Clement O. Magn Reson Med. 2006;56:498–508. doi: 10.1002/mrm.20996. [DOI] [PubMed] [Google Scholar]
- 34.Fu R, Brey WW, Shetty K, Gor’kov P, Saha S, Long JR, Grant SC, Chekmenev EY, Hu J, Gan Z, Sharma M, Zhang F, Logan TM, Bruschweller R, Edison A, Blue A, Dixon IR, Markiewicz WD, Cross TA. J Magn Reson. 2005;177:1–8. doi: 10.1016/j.jmr.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Ciobanu L, Seeber DA, Pennington CH. J Magn Reson. 2002;158:178–182. doi: 10.1016/s1090-7807(02)00071-x. [DOI] [PubMed] [Google Scholar]
- 36.Ciobanu L, Pennington CH. Solid State Nucl Magn Reson. 2004;25:138–141. doi: 10.1016/j.ssnmr.2003.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Lee SC, Kim K, Kim J, Lee S, Han Yi J, Kim SW, Ha KS, Cheong C. J Magn Reson. 2001;150:207–213. doi: 10.1006/jmre.2001.2319. [DOI] [PubMed] [Google Scholar]
- 38.Bain MM, Fagan AJ, Mullin JM, McNaught I, McLean J, Condon B. J Magn Reson Imaging. 2007;26:198–201. doi: 10.1002/jmri.20963. [DOI] [PubMed] [Google Scholar]
- 39.Aguayo JB, Blackband SJ, Schoeniger J, Mattingly MA, Hintermann M. Nature. 1986;322:190–191. doi: 10.1038/322190a0. [DOI] [PubMed] [Google Scholar]
- 40.Papan C, Boulat B, Velan SS, Fraser SE, Jacobs RE. Dev Dyn. 2006;235:3059–3062. doi: 10.1002/dvdy.20947. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs RE, Fraser SE. Science. 1994;263:681–684. doi: 10.1126/science.7508143. [DOI] [PubMed] [Google Scholar]
- 42.Lee SC, Mietchen D, Cho JH, Kim YS, Kim C, Hong KS, Lee C, Kang D, Lee W, Cheong C. Differentiation. 2007;75:84–92. doi: 10.1111/j.1432-0436.2006.00114.x. [DOI] [PubMed] [Google Scholar]
- 43.Papan C, Boulat B, Velan SS, Fraser SE, Jacobs RE. Dev Dyn. 2007;236:494–501. doi: 10.1002/dvdy.21045. [DOI] [PubMed] [Google Scholar]
- 44.Canaple L, Beuf O, Armenean M, Hasserodt J, Samarut J, Janier M. NMR Biomed. 2007:129–137. doi: 10.1002/nbm.1169. [DOI] [PubMed] [Google Scholar]
- 45.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 46.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Radiology. 1990;175:494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 47.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, Wittenberg J, Ferrucci JT. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 48.Bulte JW, Arbab AS, Douglas T, Frank JA. Methods Enzymol. 2004;386:275–299. doi: 10.1016/S0076-6879(04)86013-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang RL, Zhang L, Zhang ZG, Morris D, Jiang Q, Wang L, Zhang LJ, Chopp M. Neuroscience. 2003;116:373–382. doi: 10.1016/s0306-4522(02)00696-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Zhang L, Arniego P, Ho KL, Chopp M. Ann Neurol. 2003;53:259–263. doi: 10.1002/ana.10467. [DOI] [PubMed] [Google Scholar]
- 51.Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, Frank JA. Proc Natl Acad Sci USA. 1999;96:15256–15261. doi: 10.1073/pnas.96.26.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, Poremba C, Ebert W, Heindel W, Bremer C. Radiology. 2005;235:155–161. doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]
- 53.Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Focking M, Arnold H, Hescheler J, Fleischmann BK, Schwindt W, Buhrle C. Proc Natl Acad Sci USA. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank JA, Zywicke H, Jordan EK, Mitchell J, Lewis BK, Miller B, Bryant LH, Jr, Bulte JW. Acad Radiol. 2002;9(Suppl 2):S484–S487. doi: 10.1016/s1076-6332(03)80271-4. [DOI] [PubMed] [Google Scholar]
- 55.Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Jr, Bulte JW. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 56.Arbab AS, Yocum GT, Rad AM, Khakoo AY, Fellowes V, Read EJ, Frank JA. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 57.Reynolds F, Weissleder R, Josephson L. Bioconjug Chem. 2005;16:1240–1245. doi: 10.1021/bc0501451. [DOI] [PubMed] [Google Scholar]
- 58.Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW. Magn Reson Med. 2005;54:769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 59.Walczak P, Ruiz-Cabello J, Kedziorek DA, Gilad AA, Lin S, Barnett B, Qin L, Levitsky H, Bulte JW. Nanomedicine. 2006;2:89–94. doi: 10.1016/j.nano.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA. Radiology. 2003;229:838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- 61.Bulte JW, Kraitchman DL, Mackay AM, Pittenger MF. Blood. 2004;104:3410–3412. doi: 10.1182/blood-2004-06-2117. (author reply 3412–3413) [DOI] [PubMed] [Google Scholar]
- 62.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 63.Neri M, Maderna C, Cavazzin C, Deidda-Vigoriti V, Politi LS, Scotti G, Marzola P, Sbarbati A, Vescovi AL, Gritti A. Stem Cells. 2008;26:505–516. doi: 10.1634/stemcells.2007-0251. [DOI] [PubMed] [Google Scholar]
- 64.Magnitsky S, Watson DJ, Walton RM, Pickup S, Bulte JW, Wolfe JH, Poptani H. Neuroimage. 2005;26:744–754. doi: 10.1016/j.neuroimage.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 65.Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW. Magn Reson Med. 2007;58:261–269. doi: 10.1002/mrm.21280. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Hur T, van Heeswijk RB, Einstein O, Aharonowiz M, Xue R, Frost EE, Mori S, Reubinoff BE, Bulte JW. Magn Reson Med. 2007;57:164–171. doi: 10.1002/mrm.21116. [DOI] [PubMed] [Google Scholar]
- 67.Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Radiology. 1990;175:489–493. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- 68.Bulte JW, Kraitchman DL. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 69.Toso C, Vallee JP, Morel P, Ris F, Demuylder-Mischler S, Lepetit-Coiffe M, Marangon N, Saudek F, James Shapiro AM, Bosco D, Berney T. Am J Transplant. 2008;8:701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhu W, Li X, Tang Z, Zhu S, Qi J, Wei L, Lei H. J Huazhong Univ Sci Technol Med Sci. 2007;27:107–110. doi: 10.1007/s11596-007-0130-1. [DOI] [PubMed] [Google Scholar]
- 71.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, Bulte JW, Scheenen TW, Punt CJ, Heerschap A, Figdor CG. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 72.Nitin N, LaConte LE, Zurkiya O, Hu X, Bao G. J Biol Inorg Chem. 2004;9:706–712. doi: 10.1007/s00775-004-0560-1. [DOI] [PubMed] [Google Scholar]
- 73.Wunderbaldinger P, Josephson L, Weissleder R. Acad Radiol. 2002;9(Suppl 2):S304–S306. doi: 10.1016/s1076-6332(03)80210-6. [DOI] [PubMed] [Google Scholar]
- 74.Schellenberger EA, Hogemann D, Josephson L, Weissleder R. Acad Radiol. 2002;9(Suppl 2):S310–S311. doi: 10.1016/s1076-6332(03)80212-x. [DOI] [PubMed] [Google Scholar]
- 75.Kaufman CL, Williams M, Ryle LM, Smith TL, Tanner M, Ho C. Transplantation. 2003;76:1043–1046. doi: 10.1097/01.TP.0000090164.42732.47. [DOI] [PubMed] [Google Scholar]
- 76.Pittet MJ, Swirski FK, Reynolds F, Josephson L, Weissleder R. Nat Protoc. 2006;1:73–79. doi: 10.1038/nprot.2006.11. [DOI] [PubMed] [Google Scholar]
- 77.Santra S, Yang H, Holloway PH, Stanley JT, Mericle RA. J Am Chem Soc. 2005;127:1656–1657. doi: 10.1021/ja0464140. [DOI] [PubMed] [Google Scholar]
- 78.Bullok KE, Gammon ST, Violini S, Prantner AM, Villalobos VM, Sharma V, Piwnica-Worms D. Mol Imaging. 2006;5:1–15. [PubMed] [Google Scholar]
- 79.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 80.Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE. Blood. 2003;102:867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 81.Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, Pessanha BS, Guttman MA, Varney TR, Martin BJ, Dunbar CE, McVeigh ER, Lederman RJ. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. Proc Natl Acad Sci USA. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 84.Dunning MD, Kettunen MI, Ffrench Constant C, Franklin RJ, Brindle KM. Neuroimage. 2006;31:172–180. doi: 10.1016/j.neuroimage.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 85.Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, Mikulis DJ, Palmieri D, Bronder JL, Steeg PS, Yoneda T, MacDonald IC, Chambers AF, Rutt BK, Foster PJ. Magn Reson Med. 2006;56:1001–1010. doi: 10.1002/mrm.21029. [DOI] [PubMed] [Google Scholar]
- 86.Heyn C, Ronald JA, Mackenzie LT, MacDonald IC, Chambers AF, Rutt BK, Foster PJ. Magn Reson Med. 2006;55:23–29. doi: 10.1002/mrm.20747. [DOI] [PubMed] [Google Scholar]
- 87.Shapiro EM, Gonzalez-Perez O, Manuel Garcia-Verdugo J, Alvarez-Buylla A, Koretsky AP. Neuroimage. 2006;32:1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams JB, Ye Q, Hitchens TK, Kaufman CL, Ho C. J Magn Reson Imaging. 2007;25:1210–1218. doi: 10.1002/jmri.20930. [DOI] [PubMed] [Google Scholar]
- 89.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. Proc Natl Acad Sci USA. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arbab AS, Liu W, Frank JA. Expert Rev Med Devices. 2006;3:427–439. doi: 10.1586/17434440.3.4.427. [DOI] [PubMed] [Google Scholar]
- 91.Granot D, Kunz-Schughart LA, Neeman M. Magn Reson Med. 2005;54:789–797. doi: 10.1002/mrm.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aime S, Barge A, Cabella C, Crich SG, Gianolio E. Curr Pharm Biotechnol. 2004;5:509–518. doi: 10.2174/1389201043376580. [DOI] [PubMed] [Google Scholar]
- 93.Anderson SA, Lee KK, Frank JA. Invest Radiol. 2006;41:332–338. doi: 10.1097/01.rli.0000192420.94038.9e. [DOI] [PubMed] [Google Scholar]
- 94.Biancone L, Crich SG, Cantaluppi V, Romanazzi GM, Russo S, Scalabrino E, Esposito G, Figliolini F, Beltramo S, Perin PC, Segoloni GP, Aime S, Camussi G. NMR Biomed. 2007;20:40–48. doi: 10.1002/nbm.1088. [DOI] [PubMed] [Google Scholar]
- 95.Crich SG, Biancone L, Cantaluppi V, Duo D, Esposito G, Russo S, Camussi G, Aime S. Magn Reson Med. 2004;51:938–944. doi: 10.1002/mrm.20072. [DOI] [PubMed] [Google Scholar]
- 96.Crich SG, Lanzardo S, Alberti D, Belfiore S, Ciampa A, Giovenzana GB, Lovazzano C, Pagliarin R, Aime S. Neoplasia. 2007;9:1046–1056. doi: 10.1593/neo.07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daldrup-Link HE, Rudelius M, Metz S, Piontek G, Pichler B, Settles M, Heinzmann U, Schlegel J, Oostendorp RA, Rummeny EJ. Eur J Nucl Med Mol Imaging. 2004;31:1312–1321. doi: 10.1007/s00259-004-1484-2. [DOI] [PubMed] [Google Scholar]
- 98.Giesel FL, Stroick M, Griebe M, Troster H, von der Lieth CW, Requardt M, Rius M, Essig M, Kauczor HU, Hennerici MG, Fatar M. Invest Radiol. 2006;41:868–873. doi: 10.1097/01.rli.0000246147.44835.4c. [DOI] [PubMed] [Google Scholar]
- 99.Vuu K, Xie J, McDonald MA, Bernardo M, Hunter F, Zhang Y, Li K, Bednarski M, Guccione S. Bioconjug Chem. 2005;16:995–999. doi: 10.1021/bc050085z. [DOI] [PubMed] [Google Scholar]
- 100.Modo M, Cash D, Mellodew K, Williams SC, Fraser SE, Meade TJ, Price J, Hodges H. Neuroimage. 2002;17:803–811. [PubMed] [Google Scholar]
- 101.Nolte IS, Gungor S, Erber R, Plaxina E, Scharf J, Misselwitz B, Gerigk L, Przybilla H, Groden C, Brockmann MA. Magn Reson Med. 2008;59:1014–1020. doi: 10.1002/mrm.21503. [DOI] [PubMed] [Google Scholar]
- 102.Henning TD, Saborowski O, Golovko D, Boddington S, Bauer JS, Fu Y, Meier R, Pietsch H, Sennino B, McDonald DM, Daldrup-Link HE. Eur Radiol. 2007;17:1226–1234. doi: 10.1007/s00330-006-0522-9. [DOI] [PubMed] [Google Scholar]
- 103.Soria G, Wiedermann D, Justicia C, Ramos-Cabrer P, Hoehn M. Neuroimage. 2008;41:668–674. doi: 10.1016/j.neuroimage.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 104.Simmons JM, Saad ZS, Lizak MJ, Ortiz M, Koretsky AP, Richmond BJ. J Neurosci. 2008;28:7637–7647. doi: 10.1523/JNEUROSCI.1488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bearer EL, Falzone TL, Zhang X, Biris O, Rasin A, Jacobs RE. Neuroimage. 2007;37(Suppl 1):S37–S46. doi: 10.1016/j.neuroimage.2007.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Itoh K, Sakata M, Watanabe M, Aikawa Y, Fujii H. Neuroscience. 2008;154:732–740. doi: 10.1016/j.neuroscience.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 107.Chen CC, Zechariah A, Hsu YH, Chen HW, Yang LC, Chang C. Exp Neurol. 2008;210:322–330. doi: 10.1016/j.expneurol.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Deans AE, Wadghiri YZ, Berrios-Otero CA, Turnbull DH. Magn Reson Med. 2008;59:1320–1328. doi: 10.1002/mrm.21609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lindsey JD, Scadeng M, Dubowitz DJ, Crowston JG, Weinreb RN. Neuroimage. 2007;34:1619–1626. doi: 10.1016/j.neuroimage.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 110.Aoki I, Takahashi Y, Chuang KH, Silva AC, Igarashi T, Tanaka C, Childs RW, Koretsky AP. NMR Biomed. 2006;19:50–59. doi: 10.1002/nbm.1000. [DOI] [PubMed] [Google Scholar]
- 111.Gilad AA, Walczak P, McMahon MT, Na HB, Lee JH, An K, Hyeon T, van Zijl PC, Bulte JW. Magn Reson Med. 2008;60:1–7. doi: 10.1002/mrm.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holland GN, Bottomley PA, Hinshaw WS. J Magn Reson. 1977;28:133–136. [Google Scholar]
- 113.Ahrens ET, Flores R, Xu H, Morel PA. Nat Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 114.Partlow KC, Chen J, Brant JA, Neubauer AM, Meyerrose TE, Creer MH, Nolta JA, Caruthers SD, Lanza GM, Wickline SA. FASEB J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 115.Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Magn Reson Med. 2007;58:725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- 116.Flogel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masuda C, Maki Z, Morikawa S, Morita M, Inubushi T, Matsusue Y, Yamagata S, Taguchi H, Doi Y, Shirai N, Hirao K, Tooyama I. Neurosci Res. 2006;56:224–228. doi: 10.1016/j.neures.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 118.Bulte JW. Nat Biotechnol. 2005;23:945–946. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 119.Riess JG. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:567–580. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
- 120.Gilad AA, McMahon MT, Walczak P, Winnard PT, Jr, Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC. Nat Biotechnol. 2007;25:217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 121.Min JJ, Gambhir SS. Gene Ther. 2004;11:115–125. doi: 10.1038/sj.gt.3302191. [DOI] [PubMed] [Google Scholar]
- 122.Contag CH, Bachmann MH. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 123.Gambhir SS, Barrio JR, Phelps ME, Iyer M, Namavari M, Satyamurthy N, Wu L, Green LA, Bauer E, MacLaren DC, Nguyen K, Berk AJ, Cherry SR, Herschman HR. Proc Natl Acad Sci USA. 1999;96:2333–2338. doi: 10.1073/pnas.96.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arredondo M, Nunez MT. Mol Aspects Med. 2005;26:313–327. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 125.Egyed A. Br J Haematol. 1988;68:483–486. doi: 10.1111/j.1365-2141.1988.tb04241.x. [DOI] [PubMed] [Google Scholar]
- 126.Koretsky AP, Lin YJ, Schorle A, Jaenish H. Genetic control of MRI contrast by expression of the transferrin receptor. Presented at Proceedings of the International Society of Magnetic Resonance Medicine; 1996. p. 69. [Google Scholar]
- 127.Theil EC. J Nutr. 2003;133:1549S–1553S. doi: 10.1093/jn/133.5.1549S. [DOI] [PubMed] [Google Scholar]
- 128.Gottesfeld Z, Neeman M. Magn Reson Med. 1996;35:514–520. doi: 10.1002/mrm.1910350410. [DOI] [PubMed] [Google Scholar]
- 129.Vymazal J, Brooks RA, Bulte JW, Gordon D, Aisen P. J Inorg Biochem. 1998;71:153–157. doi: 10.1016/s0162-0134(98)10047-8. [DOI] [PubMed] [Google Scholar]
- 130.Vymazal J, Zak O, Bulte JW, Aisen P, Brooks RA. Magn Reson Med. 1996;36:61–65. doi: 10.1002/mrm.1910360111. [DOI] [PubMed] [Google Scholar]
- 131.Gossuin Y, Muller RN, Gillis P. NMR Biomed. 2004;17:427–432. doi: 10.1002/nbm.903. [DOI] [PubMed] [Google Scholar]
- 132.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Neoplasia. 2005;7:109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cohen B, Ziv K, Plaks V, Israely T, Kalchenko V, Harmelin A, Benjamin LE, Neeman M. Nat Med. 2007;13:498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 134.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. Nat Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 135.Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Magn Reson Med. 2006;56:51–59. doi: 10.1002/mrm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zurkiya O, Chan AW, Hu X. Magn Reson Med. 2008;59:1225–1231. doi: 10.1002/mrm.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sow A, Lamant M, Bonny JM, Larvaron P, Piaud O, Lecureuil C, Fontaine I, Saleh MC, Garcia Otin AL, Renou JP, Baron B, Zakin M, Guillou F. J Neurosci Res. 2006;83:403–414. doi: 10.1002/jnr.20741. [DOI] [PubMed] [Google Scholar]
- 138.Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK, Widel M, Bansal N, Delisle MB, Ghetti B. J Neurosci. 2008;28:60–67. doi: 10.1523/JNEUROSCI.3962-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goffeney N, Bulte JW, Duyn J, Bryant LH, Jr, van Zijl PC. J Am Chem Soc. 2001;123:8628–8629. doi: 10.1021/ja0158455. [DOI] [PubMed] [Google Scholar]
- 140.McMahon MT, Gilad AA, DeLiso MA, Cromer Berman S, Bulte JWM, van Zijl PCM. Magn Reson Med. 2008;60:803–812. doi: 10.1002/mrm.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johnson M, Sato M, Burton J, Gambhir SS, Carey M, Wu L. Mol Imaging. 2005;4:463–472. doi: 10.2310/7290.2005.05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cherry SR. Annu Rev Biomed Eng. 2006;8:35–62. doi: 10.1146/annurev.bioeng.8.061505.095728. [DOI] [PubMed] [Google Scholar]
- 143.Kirchner C, Liedl T, Kudera S, Pellegrino T, Munoz Javier A, Gaub HE, Stolzle S, Fertig N, Parak WJ. Nano Lett. 2005;5:331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 144.Saini S, Stark DD, Hahn PF, Wittenberg J, Brady TJ, Ferrucci JT., Jr Radiology. 1987;162:211–216. doi: 10.1148/radiology.162.1.3786765. [DOI] [PubMed] [Google Scholar]
- 145.Vassallo P, Matei C, Heston WD, McLachlan SJ, Koutcher JA, Castellino RA. Radiology. 1994;193:501–506. doi: 10.1148/radiology.193.2.7972768. [DOI] [PubMed] [Google Scholar]
- 146.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 147.Choi SH, Moon WK, Hong JH, Son KR, Cho N, Kwon BJ, Lee JJ, Chung JK, Min HS, Park SH. Radiology. 2007;242:137–143. doi: 10.1148/radiol.2421060093. [DOI] [PubMed] [Google Scholar]
- 148.Anzai Y, Piccoli CW, Outwater EK, Stanford W, Bluemke DA, Nurenberg P, Saini S, Maravilla KR, Feldman DE, Schmiedl UP, Brunberg JA, Francis IR, Harms SE, Som PM, Tempany CM. Radiology. 2003;228:777–788. doi: 10.1148/radiol.2283020872. [DOI] [PubMed] [Google Scholar]
- 149.Koh DM, Brown G, Temple L, Raja A, Toomey P, Bett N, Norman AR, Husband JE. Radiology. 2004;231:91–99. doi: 10.1148/radiol.2311030142. [DOI] [PubMed] [Google Scholar]
- 150.Harada T, Tanigawa N, Matsuki M, Nohara T, Narabayashi I. Eur J Radiol. 2007;63:401–407. doi: 10.1016/j.ejrad.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 151.Memarsadeghi M, Riedl CC, Kaneider A, Galid A, Rudas M, Matzek W, Helbich TH. Radiology. 2006;241:367–377. doi: 10.1148/radiol.2412050693. [DOI] [PubMed] [Google Scholar]
- 152.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 153.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 154.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 155.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. J Am Coll Cardiol. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 156.Schmitz SA, Taupitz M, Wagner S, Wolf KJ, Beyersdorff D, Hamm B. J Magn Reson Imaging. 2001;14:355–361. doi: 10.1002/jmri.1194. [DOI] [PubMed] [Google Scholar]
- 157.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 158.Schmitz SA, Coupland SE, Gust R, Winterhalter S, Wagner S, Kresse M, Semmler W, Wolf KJ. Invest Radiol. 2000;35:460–471. doi: 10.1097/00004424-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 159.Litovsky S, Madjid M, Zarrabi A, Casscells SW, Willerson JT, Naghavi M. Circulation. 2003;107:1545–1549. doi: 10.1161/01.cir.0000055323.57885.88. [DOI] [PubMed] [Google Scholar]
- 160.Trivedi RA, UK-I JM, Graves MJ, Cross JJ, Horsley J, Goddard MJ, Skepper JN, Quartey G, Warburton E, Joubert I, Wang L, Kirkpatrick PJ, Brown J, Gillard JH. Stroke. 2004;35:1631–1635. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 161.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 162.Trivedi RA, Mallawarachi C, UK-I JM, Graves MJ, Horsley J, Goddard MJ, Brown A, Wang L, Kirkpatrick PJ, Brown J, Gillard JH. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 163.Xu S, Jordan EK, Brocke S, Bulte JW, Quigley L, Tresser N, Ostuni JL, Yang Y, McFarland HF, Frank JA. J Neurosci Res. 1998;52:549–558. doi: 10.1002/(SICI)1097-4547(19980601)52:5<549::AID-JNR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 164.Dousset V, Delalande C, Ballarino L, Quesson B, Seilhan D, Coussemacq M, Thiaudiere E, Brochet B, Canioni P, Caille JM. Magn Reson Med. 1999;41:329–333. doi: 10.1002/(sici)1522-2594(199902)41:2<329::aid-mrm17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 165.Rausch M, Hiestand P, Baumann D, Cannet C, Rudin M. Magn Reson Med. 2003;50:309–314. doi: 10.1002/mrm.10541. [DOI] [PubMed] [Google Scholar]
- 166.Baeten K, Hendriks JJ, Hellings N, Theunissen E, Vanderlocht J, Ryck LD, Gelan J, Stinissen P, Adriaensens P. J Neuroimmunol. 2008;195:1–6. doi: 10.1016/j.jneuroim.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 167.Oweida AJ, Dunn EA, Karlik SJ, Dekaban GA, Foster PJ. J Magn Reson Imaging. 2007;26:144–151. doi: 10.1002/jmri.21005. [DOI] [PubMed] [Google Scholar]
- 168.Chen JW, Querol Sans M, Bogdanov A, Jr, Weissleder R. Radiology. 2006;240:473–481. doi: 10.1148/radiol.2402050994. [DOI] [PubMed] [Google Scholar]
- 169.Chen JW, Pham W, Weissleder R, Bogdanov A., Jr Magn Reson Med. 2004;52:1021–1028. doi: 10.1002/mrm.20270. [DOI] [PubMed] [Google Scholar]
- 170.Chen JW, Breckwoldt MO, Aikawa E, Chiang G, Weissleder R. Brain. 2008;131:1123–1133. doi: 10.1093/brain/awn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brochet B, Deloire MS, Touil T, Anne O, Caille JM, Dousset V, Petry KG. Neuroimage. 2006;32:266–274. doi: 10.1016/j.neuroimage.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 172.Dousset V, Brochet B, Deloire MS, Lagoarde L, Barroso B, Caille JM, Petry KG. AJNR Am J Neuroradiol. 2006;27:1000–1005. [PMC free article] [PubMed] [Google Scholar]
- 173.Stoll G, Jander S, Schroeter M. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 174.Wiart M, Davoust N, Pialat JB, Desestret V, Moucharaffie S, Cho TH, Mutin M, Langlois JB, Beuf O, Honnorat J, Nighoghossian N, Berthezene Y. Stroke. 2007;38:131–137. doi: 10.1161/01.STR.0000252159.05702.00. [DOI] [PubMed] [Google Scholar]
- 175.Rausch M, Sauter A, Frohlich J, Neubacher U, Radu EW, Rudin M. Magn Reson Med. 2001;46:1018–1022. doi: 10.1002/mrm.1290. [DOI] [PubMed] [Google Scholar]
- 176.Saleh A, Schroeter M, Jonkmanns C, Hartung HP, Modder U, Jander S. Brain. 2004;127:1670–1677. doi: 10.1093/brain/awh191. [DOI] [PubMed] [Google Scholar]
- 177.Kleinschnitz C, Bendszus M, Frank M, Solymosi L, Toyka KV, Stoll G. J Cereb Blood Flow Metab. 2003;23:1356–1361. doi: 10.1097/01.WCB.0000090505.76664.DB. [DOI] [PubMed] [Google Scholar]
- 178.Saleh A, Schroeter M, Ringelstein A, Hartung HP, Siebler M, Modder U, Jander S. Stroke. 2007;38:2733–2737. doi: 10.1161/STROKEAHA.107.481788. [DOI] [PubMed] [Google Scholar]
- 179.Beckmann N, Cannet C, Fringeli-Tanner M, Baumann D, Pally C, Bruns C, Zerwes HG, Andriambeloson E, Bigaud M. Magn Reson Med. 2003;49:459–467. doi: 10.1002/mrm.10387. [DOI] [PubMed] [Google Scholar]
- 180.Zhang Y, Dodd SJ, Hendrich KS, Williams M, Ho C. Kidney Int. 2000;58:1300–1310. doi: 10.1046/j.1523-1755.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 181.Kanno S, Lee PC, Dodd SJ, Williams M, Griffith BP, Ho C. J Thorac Cardiovasc Surg. 2000;120:923–934. doi: 10.1067/mtc.2000.110184. [DOI] [PubMed] [Google Scholar]
- 182.Kanno S, Wu YJ, Lee PC, Dodd SJ, Williams M, Griffith BP, Ho C. Circulation. 2001;104:934–938. doi: 10.1161/hc3401.093148. [DOI] [PubMed] [Google Scholar]
- 183.Hauger O, Grenier N, Deminere C, Lasseur C, Delmas Y, Merville P, Combe C. Eur Radiol. 2007;17:2898–2907. doi: 10.1007/s00330-007-0660-8. [DOI] [PubMed] [Google Scholar]
- 184.Ahrens ET, Feili-Hariri M, Xu H, Genove G, Morel PA. Magn Reson Med. 2003;49:1006–1013. doi: 10.1002/mrm.10465. [DOI] [PubMed] [Google Scholar]
- 185.Baumjohann D, Hess A, Budinsky L, Brune K, Schuler G, Lutz MB. Eur J Immunol. 2006;36:2544–2555. doi: 10.1002/eji.200535742. [DOI] [PubMed] [Google Scholar]
- 186.Rogers JM, Jung CW, Lewis J, Groman EV. Magn Reson Imaging. 1998;16:917–923. doi: 10.1016/s0730-725x(98)00090-3. [DOI] [PubMed] [Google Scholar]
- 187.Anzai Y, Blackwell KE, Hirschowitz SL, Rogers JW, Sato Y, Yuh WT, Runge VM, Morris MR, McLachlan SJ, Lufkin RB. Radiology. 1994;192:709–715. doi: 10.1148/radiology.192.3.7520182. [DOI] [PubMed] [Google Scholar]
- 188.Bellin MF, Roy C, Kinkel K, Thoumas D, Zaim S, Vanel D, Tuchmann C, Richard F, Jacqmin D, Delcourt A, Challier E, Lebret T, Cluzel P. Radiology. 1998;207:799–808. doi: 10.1148/radiology.207.3.9609907. [DOI] [PubMed] [Google Scholar]
- 189.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 190.Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Nat Protoc. 2008;3:1180–1186. doi: 10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]
- 191.Liu SV. Stem Cells Dev. 2008;17:391–397. doi: 10.1089/scd.2008.0062. [DOI] [PubMed] [Google Scholar]
- 192.Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Arauzo-Bravo MJ, Ruau D, Han DW, Zenke M, Scholer HR. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 193.Eminli S, Utikal JS, Arnold K, Jaenisch R, Hochedlinger K. Stem Cells. 2008:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 194.Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 196.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 197.Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Neuroscience. 2005;136:161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 200.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 201.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 202.Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, Ocherashvilli A, Holbova R, Yosef O, Barbash IM, Leor J. Circulation. 2007;116:I38–I45. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 203.He G, Zhang H, Wei H, Wang Y, Zhang X, Tang Y, Wei Y, Hu S. Int J Cardiol. 2007;114:4–10. doi: 10.1016/j.ijcard.2005.11.112. [DOI] [PubMed] [Google Scholar]
- 204.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 205.Jendelova P, Herynek V, Urdzikova L, Glogarova K, Kroupova J, Andersson B, Bryja V, Burian M, Hajek M, Sykova E. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 206.Shyu WC, Chen CP, Lin SZ, Lee YJ, Li H. Stroke. 2007;38:367–374. doi: 10.1161/01.STR.0000254463.24655.14. [DOI] [PubMed] [Google Scholar]
- 207.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 208.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 209.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 211.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 212.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 213.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 215.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 216.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 217.Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. J Neurotrauma. 2001;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 218.Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. J Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- 219.Li Y, Chen J, Wang L, Lu M, Chopp M. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 220.Jendelova P, Herynek V, DeCroos J, Glogarova K, Andersson B, Hajek M, Sykova E. Magn Reson Med. 2003;50:767–776. doi: 10.1002/mrm.10585. [DOI] [PubMed] [Google Scholar]
- 221.Bruck W. J Neurol. 2005;252(Suppl 5):v10–v15. doi: 10.1007/s00415-005-5003-6. [DOI] [PubMed] [Google Scholar]
- 222.Terajima K, Matsuzawa H, Tanaka K, Nishizawa M, Nakada T. Neuroimage. 2007;37:1278–1285. doi: 10.1016/j.neuroimage.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 223.Prat A, Antel J. Curr Opin Neurol. 2005;18:225–230. doi: 10.1097/01.wco.0000169737.99040.31. [DOI] [PubMed] [Google Scholar]
- 224.Hickey WF, Hsu BL, Kimura H. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 225.Ming GL, Song H. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 226.Sailor KA, Ming GL, Song H. Expert Opin Biol Ther. 2006;6:879–890. doi: 10.1517/14712598.6.9.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Reynolds BA, Tetzlaff W, Weiss S. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Reynolds BA, Weiss S. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 230.Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, Wahlberg LU. Exp Neurol. 1999;158:265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- 231.Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, Lavon I, Baniyash M, Lassmann H, Ben-Hur T. Ann Neurol. 2007;61:209–218. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- 232.Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, Milonas I, Karussis D, Abramsky O, Ben-Hur T. Exp Neurol. 2006;198:275–284. doi: 10.1016/j.expneurol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 233.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 234.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Mol Cell Neurosci. 2003;24:1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 236.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 237.Floris S, Blezer EL, Schreibelt G, Dopp E, van der Pol SM, Schadee-Eestermans IL, Nicolay K, Dijkstra CD, de Vries HE. Brain. 2004;127:616–627. doi: 10.1093/brain/awh068. [DOI] [PubMed] [Google Scholar]
- 238.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 239.Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, Capela A, Greve J, Malenka RC, Moseley ME, Palmer TD, Steinberg GK. Proc Natl Acad Sci USA. 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 241.Politi LS, Bacigaluppi M, Brambilla E, Cadioli M, Falini A, Comi G, Scotti G, Martino G, Pluchino S. Stem Cells. 2007;25:2583–2592. doi: 10.1634/stemcells.2007-0037. [DOI] [PubMed] [Google Scholar]
- 242.Smirnov P, Gazeau F, Beloeil JC, Doan BT, Wilhelm C, Gillet B. Contrast Media Mol Imaging. 2006;1:165–174. doi: 10.1002/cmmi.104. [DOI] [PubMed] [Google Scholar]
- 243.Heyn C, Bowen CV, Rutt BK, Foster PJ. Magn Reson Med. 2005;53:312–320. doi: 10.1002/mrm.20356. [DOI] [PubMed] [Google Scholar]
- 244.Stroh A, Faber C, Neuberger T, Lorenz P, Sieland K, Jakob PM, Webb A, Pilgrimm H, Schober R, Pohl EE, Zimmer C. Neuroimage. 2005;24:635–645. doi: 10.1016/j.neuroimage.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 245.Foster-Gareau P, Heyn C, Alejski A, Rutt BK. Magn Reson Med. 2003;49:968–971. doi: 10.1002/mrm.10417. [DOI] [PubMed] [Google Scholar]
- 246.Hsiao JK, Tai MF, Chu HH, Chen ST, Li H, Lai DM, Hsieh ST, Wang JL, Liu HM. Magn Reson Med. 2007;58:717–724. doi: 10.1002/mrm.21377. [DOI] [PubMed] [Google Scholar]
- 247.Zhang Z, van den Bos EJ, Wielopolski PA, de Jong-Popijus M, Duncker DJ, Krestin GP. MAGMA. 2004;17:201–209. doi: 10.1007/s10334-004-0054-8. [DOI] [PubMed] [Google Scholar]
- 248.Pinkernelle J, Teichgraber U, Neumann F, Lehmkuhl L, Ricke J, Scholz R, Jordan A, Bruhn H. Magn Reson Med. 2005;53:1187–1192. doi: 10.1002/mrm.20455. [DOI] [PubMed] [Google Scholar]
- 249.Zhang Z, van den Bos EJ, Wielopolski PA, de Jong-Popijus M, Bernsen MR, Duncker DJ, Krestin GP. MAGMA. 2005;18:175–185. doi: 10.1007/s10334-005-0108-6. [DOI] [PubMed] [Google Scholar]
- 250.Mills PH, Wu YJ, Ho C, Ahrens ET. Magn Reson Imaging. 2008;26:618–628. doi: 10.1016/j.mri.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Modo M, Hoehn M, Bulte JW. Mol Imaging. 2005;4:143–164. doi: 10.1162/15353500200505145. [DOI] [PubMed] [Google Scholar]
- 252.Boretius S, Schmelting B, Watanabe T, Merkler D, Tammer R, Czeh B, Michaelis T, Frahm J, Fuchs E. NMR Biomed. 2006;19:41–49. doi: 10.1002/nbm.999. [DOI] [PubMed] [Google Scholar]
- 253.Nessler S, Boretius S, Stadelmann C, Bittner A, Merkler D, Hartung HP, Michaelis T, Bruck W, Frahm J, Sommer N, Hemmer B. Brain. 2007;130:2186–2198. doi: 10.1093/brain/awm105. [DOI] [PubMed] [Google Scholar]
- 254.Zhang J, Hutton G. Annu Rev Med. 2005;56:273–302. doi: 10.1146/annurev.med.56.082103.104739. [DOI] [PubMed] [Google Scholar]
- 255.Ben-Hur T, Einstein O, Bulte JW. Curr Drug Targets. 2005;6:3–19. doi: 10.2174/1389450053345000. [DOI] [PubMed] [Google Scholar]
- 256.Politi LS. Neuroradiology. 2007;49:523–534. doi: 10.1007/s00234-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 257.Meier DS, Guttmann CR. Neuroimage. 2006;32:531–537. doi: 10.1016/j.neuroimage.2006.04.181. [DOI] [PubMed] [Google Scholar]
- 258.Meier DS, Weiner HL, Guttmann CR. Neurotherapeutics. 2007;4:485–498. doi: 10.1016/j.nurt.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]