Abstract
Introduction
Sry is a transcription factor and our research group has shown that there are multiple copies of Sry in WKY and SHR rats and they have novel functions separate from testes determination. We hypothesized that exogenously delivered Sry3 to the normotensive WKY male kidney would activate the renin-angiotensin system (RAS) and raise blood pressure based on previous in vitro studies.
Methods
Sry3 or control vector was electroporated to the left kidney of male WKY rats and blood pressure measured by telemetry, renin angiotensin measures by RIA, plasma and tissue catecholamines by HPLC with electrochemical detection, Na by flame photometry and inulin by Elisa.
Results
Sry3 raised BP 10–20 mmHg compared to controls (p<0.01) and produced a significant 40% decrease in urine sodium compared to controls (p<0.05). Sry3 increased renal angiotensin II (Ang II) and plasma renin activity by more than 100% when compared to controls (p<0.01, p<0.05, respectively).
Conclusion
the findings presented here confirm and extend the argument for Sry3 as one of the genes responsible for the SHR hypertensive Y chromosome phenotype and are consistent with an increased tissue renin-angiotensin system activity due to Sry3 and increased Na reabsorption.
Keywords: renin-angiotensin, catecholamines, glomerular filtration, sodium reabsorption
Introduction
Most of the research concerning Sry has been with its role in testes determination and sex differentiation. There has been little focus on potential other functions of Sry until recently. Sry is the founding member of the Sox protein family with an HMG box DNA binding domain. Many of the Sox transcription factors (Sox 8, 9, 10) function in nervous system development.1 The target genes of Sry are not completely known but recent work suggests that Sry binds to a testes specific enhancer of Sox 9 and activates Sox 9 expression in cooperation with steroidogenic factor 1 during embryogenesis.2 The results of 2-D gel electrophoresis show that Sry overexpression causes a downregulation of many chaperone proteins and upregulates laminin and many zinc finger proteins.3 These Sox transcription factors appear to have diverse functions through protein-protein interactions and modulation of important signaling pathways.4
Sry is expressed in rodent and human adult brain which has stimulated a search for CNS function beyond what may be involved during brain development. Dewing et al. demonstrated that delivering Sry antisense oligonucleotides to the rat substantia nigra led to measurable motor defects in male rats 5, and suggests that the effect of Sry expression is partially responsible for sexual dimorphism in brain cells. They interpreted these results as a potential cellular sex determination in the brain not mediated by gonadal hormones. Arnold’s research group has introduced a powerful model system in mice for examining the separate and interactive effects of sex chromosomes and gonadal secretions on sexually dimorphic phenotypes.6 Also, Sry can interact in novel ways with other factors to repress or activate genes. For instance, Wu et al. (2009) showed that SRY activates monoamine oxidase (MAO)-promoter in a human male neuroblastoma cell line.7 Sry and Sp1 form a transcriptional complex and synergistically activate MAO-A transcription. This has implications for blood pressure and brain neurochemistry since MAO is a degradative enzyme for the catecholamines. Also Zhang et al. 8 showed that Sry may have blood pressure implications in that the chromogranin B promoter variants are responsive to Sry and influence blood pressure. This group 9 also showed that Sry effects the common variants of the human proximal promoter of tyrosine hydroxylase which is the rate limiting enzyme in the catecholamine pathway. Several other genes have also been reported to have specific promoter Sry binding sites that are involved in inhibiting ovarian development 10,11 and in regulating the mouse sulphamidase gene which produces a lysosomal enzyme. 12
Our laboratory originally focused on the contribution of the entire SHR Y chromosome on blood pressure (BP) and at that time (1991) the specific locus on the Y chromosome was unknown. The mechanism of gender differences in blood prwessure were being studied and androgens and androgen receptors appeared to be involved in SHR hypertension. 13 The SHR Y chromosome increased BP in an additive manner by 20–25 mmHg 14–16 and increased several indices of sympathetic nervous system (SNS) activity. 17 We spent several years and developed the Y chromosome backcross strains that allowed the SHR Y chromosome to be placed on the WKY autosomal background and the WKY Y chromosome on the SHR autosomal background. 18 We demonstrated that renal norepinephrine (NE) turnover rate was higher by 100% and renal NE content was 44% higher in males with the SHR Y chromosome as compared to males with a WKY Y chromosome. 19 Sry is expressed in many other tissues besides embryonic testis, such as, adult testis, brain, adrenal gland and kidney which suggest that it has other functions. 20–22 (For a current review of the Y chromosome, Sry and hypertension see Ely et al.23). Also we have reported endogenous expression of different Sry loci in the kidney of WKY males. 24 Therefore, a candidate for the hypertensive effect was the Sry gene complex on the rat Y chromosome. We describe this locus as the Sry gene complex because it is a single gene locus which has multiple functional Sry loci.25 One locus, Sry1, elevates BP when exogenously delivered to the kidney of WKY males while another copy, Sry2, when delivered to the kidney does not raise BP.26 To determine a potential mechanism for the Sry1 effect we transfected Sry1 in PC12 cells and showed that Sry1 increased tyrosine hydroxylase (Th) promoter activity. 27 Th is the rate limiting enzyme in the catecholamine biosynthesis pathway; thus regulation of Th by Sry1 is a potential pathway for an Sry locus to affect BP through elevated norepinephrine levels. The next locus we tested was Sry3 which has two amino acid differences compared to Sry1 and Sry3 is expressed in the kidney. In order to determine a potential mechanism for Sry3 we transfected Sry expression vectors into CHO cells with luciferase reporter constructs containing promoters of angiotensinogen (Agt −1430/+22), renin (Ren −1050/−1), ACE (ACE −1677/+21) and ACE2 (ACE2 −1091/+83). Sry1, Sry2 and Sry3 differentially upregulated activity of the promoters of angiotensinogen, renin and ACE genes, and down-regulated ACE2 promoter activity. 28 Based on this in vitro data, the next step in our research strategy was to test the hypothesis that Sry3 elevates BP when exogenously administered to the kidney through the renin angiotensin system.
Methods
Animals
This project was approved by the University of Akron IACUC. Care was taken to keep animal numbers to a minimum for statistical purposes and experiments were refined to reduce animal number. All protocols followed the NIH Guide for the Care and Use of Laboratory Animals. Adult WKY males (n = 38) were used in the following studies. The rationale for using WKY males is that they have normal blood pressure and if the exogenous Sry delivered would result in a BP elevation it would be better observed in the normotensive animal rather in one with borderline or fully developed hypertension. The experimental design consisted of : experiment 1- to study BP using WKY males with empty vehicle control plasmid (n=6), or Sry3 (n=6) delivered to the left kidney, n=6/group); experiment 2- studied the circulating levels of catecholamines and renin activity, urinary Na excretion and renal levels of tyrosine hydroxylase, catecholamines, ACE, angiotensin II and renin in animals after vehicle control or Sry3 was delivered to the left kidney (n=6/group); and experiment 3- the objective was to measure glomerular filtration rate by the inulin technique 18 days after Sry3 or control vehicle delivery to the left kidney of WKY males (n=6/group). The full length SHR Sry3 coding sequence (GenBank, AY157672), originally from an XbaI/Not l genomic fragment, was cloned into pcDNA3.1(−) to produce the expression construct, Sry3/pcDNA3.1(−).
Electroporation
All of the electroporation procedures used the following protocol. Based on previous research and our own pilot tests we performed electroporation in a pulsatile fashion immediately after DNA injection. Sry3/pcDNA3.1(−), or control vector, pcDNA3.1(−) without Sry3 sequence was injected (50ug, in 100μ4mm, 28g needle) into the left kidney medulla of 6 WKY adult male rats using pentothal as an anesthetic (50 mg/kg, ip) as previously described and Tylenol (0.5mg/ml) was given in the water 1 day before surgery and 3 days post surgery. 26 A mark was placed on the needle to determine the optimal depth to inject in the medulla (4mm determined using a dye marker in pilot studies). Results of this showed the dye marker limited to the medulla. Tweezer electrodes (BTX Tweezertrodes Model #522) connected to an electrostimulator (ElectroCellManipulator™, ECM® 830, BTX, a division of Harvard apparatus, Holliston, MA.), were placed on opposite sides of the kidney and 20 bipolar electrical pulses administered, 200 volts, 500msec/pulse after both injections. The animals were allowed to recover for a week. These animals were also implanted with telemetry devices and monitored as described below 2 weeks before electroporation procedures and on the figures, day 0 is the start of the 1st day after electroporation. In order to demonstrate Sry protein in tissues after delivery of the Sry plasmid we injected Sry 3 or control vehicle into the left kidney of WKY females and 14 days post delivery prepared the tissues for Western blot analysis.
Western Blot Analysis
Kidneys, testes, liver, lung, heart, adrenal glands were collectedon ice at 14 days following electroporation.The tissues were homogenized in a tissue lysis buffer consisting of 1X CellLytic B (Sigma), 1X protease inhibitor cocktail with no EDTA (Sigma), 1mMPMSF, 5 U DNAse I, 25 mM NaF, 25mM McCl, and phosphate buffered imidizole. Lysates were centrifuged at 13,000G for 5 minutes and the supernatant was be transferred to a 15mL tube containing 6mL PBI and incubated on a rotator for 1 hour at room temperature. The beads were collected by centrifugation at 500G for 5 minutes. Following 4 rinses with PBI, the his-tagged proteins were eluted with 1 mL of buffer EB containing 20mM Na2HPO4, 500mM NaCl, 500mM imidizole, pH 7.4. The his-tagged protein elutions were run on Precise® Protein Gels (Sigma) in Tris-Hepes-SDS running buffer and transferred onto PDVF membrane by semidry transfer at 15V for 1 hour. Membranes were stained using the SNAP I.D. system (Millipore, Billerica, MA) blocked for with 1% bovine serum albumin in PBS with 0.1% Tween-20 for 20 seconds under vacuum conditions at room temperature. Membranes were incubated with the blocking solution containing goat anti-cMyc (1:1000) for 10 minutes at room temperature and then actively driven through the membrane. The membranes were washed 4 times with PBS, 20 seconds under vacuum, and then incubated with Bethyl donkey anti-goat IgG, HRP conjugate (1:3000) in PBS for 10 minutes at room temperature. The 2° antibody was actively driven through the membrane and rinsed 4 times with PBS. The membranes were incubated with 2mL Luminol and 2mL hydrogen peroxide (Thermo Super Signal West Pico Chemiluminescent Substrate) for 5 minutes before capturing bands on the Kodak Gel Logic 2200 documentation system.
Blood Pressure
Systolic and diastolic and mean arterial BP and HR were measured using a telemetry system and the Dataquest IV data-acquisition system (Data Sciences; Roseville, MN). Animals were anesthetized with brevital sodium (50 mg/kg ip, Eli Lilly; Indianapolis, IN) and the transmitters were surgically implanted. Briefly, a midline abdominal incision was made and the descending aorta was exposed between the renal vessels and the bifurcation of the femoral vessels. The vena cava and aorta were separated and a ligature was placed under the aorta to restrict blood flow caudally. A 21-gauge needle was used to make a small hole in the aorta and guide the flexible catheter tip of the radio transmitter into the aorta. The catheter was secured in place with a bonded patch (Vetbond, 3M Animal Care Products; St. Paul MN). The transmitter was placed in the peritoneal cavity and sutured to the abdominal wall as the midline incision was closed. Penicillin was administered (2,500 IU im) immediately after the surgery. Animals were placed into individual recovery cages for 1 wk.
Blood pressure was measured continuously in conscious animals by telemetry (every 30 min) and plotted weekly (daily average used for figures) before and after electroporation for 28 days in (Data Sciences). 23,26
Catecholamine and Tyrosine Hydroxylase (Th) Assays
Blood was collected weekly under brevital anesthesia (50 mg/kg, ip) by retroorbital puncture into EDTA tubes and spun down for plasma separation, frozen at −80C and analyzed for plasma renin activity and at day 21 for plasma catecholamines.
Renal Th and catecholamines were measured 21 days after electroporation by HPLC with electrochemical detection as we previously reported (Waters 2465, Milford, MA). 23,26 Animals were terminated at the end of the experiment by pentothal overdose (200mg/kg,i.p.) and the heart removed. The left kidney was harvested for tyrosine hydroxylase activity and angiotensin II activity.
Renin/ Angiotensin Assays
Plasma renin activity (PRA) was determined by RIA with intra-run coefficient of variation 4.6–10% and the inter-run coefficient of variation was 5.6–7.6% (Dia Sorin, Stillwater, MN). For the ACE assay, kidney (1gm) was homogenized in 0.1M potassium phosphate buffer (pH 7.8). ACE was analyzed colorimetrically using a kit from Buhlmann Laboratories AG (Schonenbuch, Switzerland, distributed by ALPCO, Winham, NH). For Ang II analysis the kidney (0.2gm) was homogenized in 1ml of 0.1M potassium phosphate buffer with 100 uL of 0.5mM bestatin added to minimize the in vitro degradation of Ang II, and was assayed radioenzymatically (Buhlmann Laboratories AG (Schonenbuch, Switzerland, distributed by ALPCO, Winham, NH).
Urine Collection and Analysis
Rats were placed into metabolic cages for: 24 hour urine collection during baseline, before plasmid delivery, and post plasmid delivery. Previously the rats were acclimatized to the cages to reduce stress effects and given Pro-lab 3000 food and water ad libitum. Mineral oil was placed into the urine collection cup to prevent urine evaporation over the collection period for sodium and creatinine measures. Plasma and urine creatinine concentrations were assessed by a colorimetric method, using the Jaffe reaction in autoanalyzer methodology. The creatinine clearance was calculated by using standard formula: Ccr=UCr × V/PCr and was expressed as mL/min/100 g kidney weight. (Data Chem Analyzer, Thermo Electron; Pittsburgh, PA) and sodium by flame photometry (Instrumentation Laboratory, Bedford, MA.).
Inulin Clearance
The single point inulin injection method described by Sturgeon et al. was used to evaluate GFR. 29 The rationale for using this method was threefold. First, conventional GFR methods that stipulate urine sample collection require complete bladder evacuation in order to be accurate; direct bladder puncture or urinary catheterization of anesthetized rats proved to be difficult in trial experiments. Secondly, the single-point injection method is quick, and was shown to be an accurate estimate of GFR. Thirdly, inulin is an attractive compound to be used in GFR analysis because it is safe, biologically inert, not metabolized in the body, and freely filtered at the glomerulus.
The animals were anesthetized with sodium pentothal (50mg/kg IP and titrated to effect. Femoral artery and vein were cannulated, 25 mg/kg of inulin was injected into the vein, and flushed with 1ml of saline. Separate blood samples were withdrawn from the arterial cannula at 3, 7, 10, 20, and 55 minutes post-injection. Approximately 0.2–0.3 ml (4–5 drops) of blood was withdrawn with each sampling, and the line was then flushed with excess (approximately 0.5 ml) heparin to prevent clotting. Following the 55 minute blood sample, the peritoneum was opened, and a urine sample was taken by direct bladder puncture. Both kidneys were removed for analysis, and then the animal was terminated while still under anesthesia. Samples were centrifuged at 12,000g for 10 minutes, and the plasma removed, refrigerated, and then tested for presence of inulin. The FITtm GFR assay (Functional Immunoassay Technologytm [FITtm] Glomerular Filtration Rate [GFR], BioPhysics Assay Laboratory [BioPALtm], Inc., Worcester, MA) was used, and the manufacturer’s assay protocol followed.
Statistical Analysis
Two-way ANOVA with repeated measures (treatment and time, Figures 1,2) was used to compare the groups across time with follow up appropriate tests. Student’s t-tests were used for comparison between two groups (Figures 3–5). Statistical significance was assumed if p<0.05.
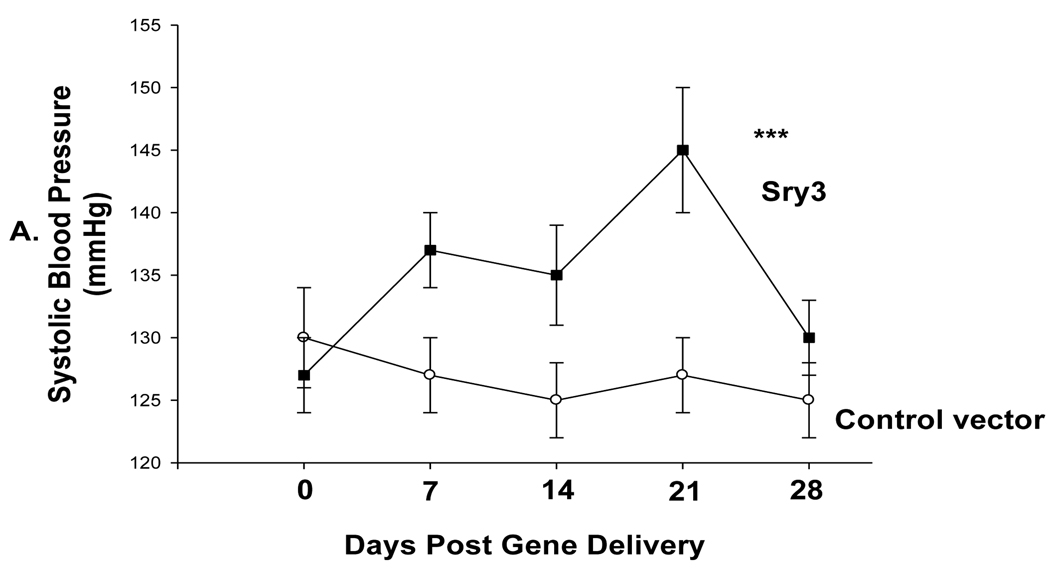
Figure 1.
A. Telemetered systolic blood pressure (SBP) was significantly elevated after Sry3 (solid squares, n=6) delivery to the kidney as compared to control vector (open circles, n=6) (means, +/−s.e.m., (two-way ANOVA with repeated measures, treatment: F=20.6, p<0.001, time: n.s., interaction of treatment and time: F=3.9,p<0.05); B. Telemetered mean arterial pressure )MAP) was significantly elevated after Sry3 (solid squares) delivery to the kidney as compared to control vector (open circles, means, +/−s.e.m., two-way ANOVA with repeated measures, treatment: F=18.6, ***=p<0.001, time: n.s., interaction not significant; C. Telemetered heart rate was not significantly different after Sry3 (solid squares) delivery to the kidney as compared to control vector (open circles, means, +/−s.e.m).
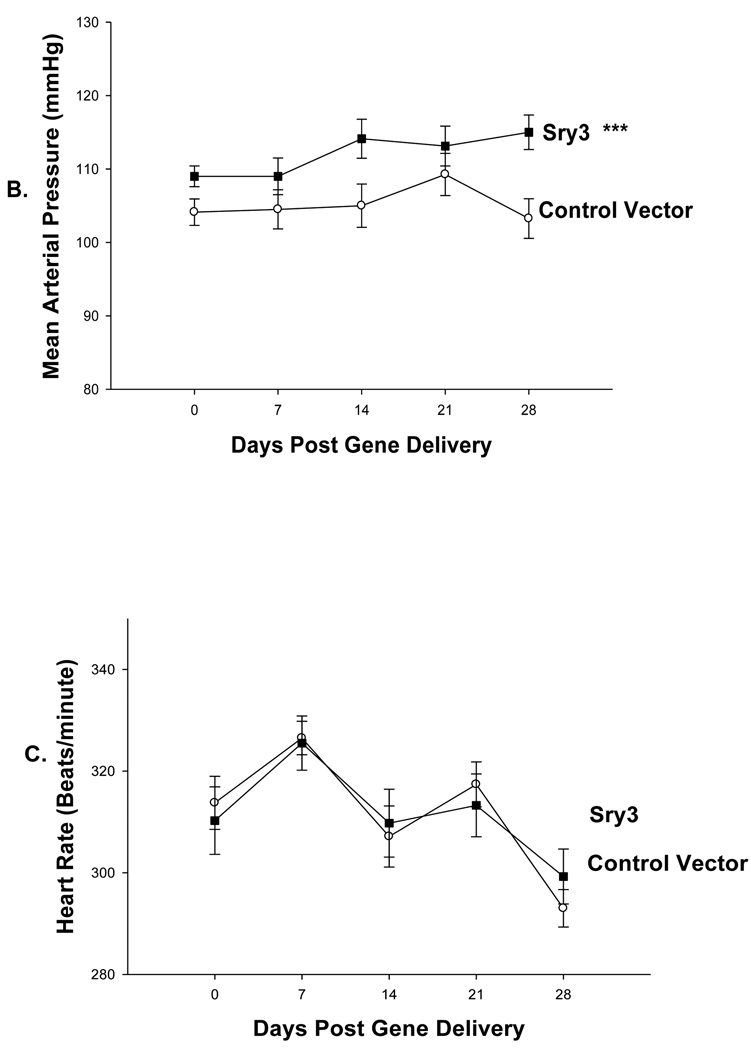
Figure 2.
Plasma renin activity across time between controls vector (open circles, n=6) compared to Sry3 (solid squares,n=6), means, +/− s.e.m., (two-way ANOVA with repeated measure, treatment: n.s., time: F=5.2, p<0.01, interaction of treatment and time: F=17.1, p<0.001).
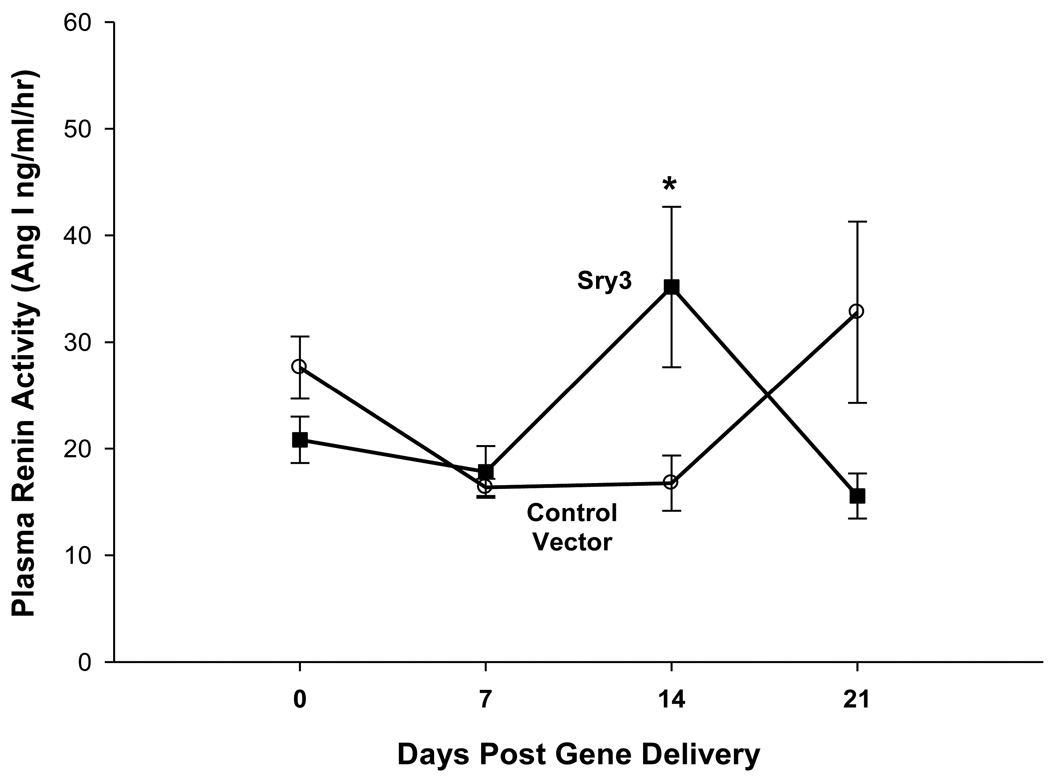
Figure 3.
Renal angiotensin II content of left kidney between control vector and Sry3 at 21 days after gene delivery (means, +/− s.e.m., **= p<0.01 compared to control vector, n=6/group).
Figure 5.
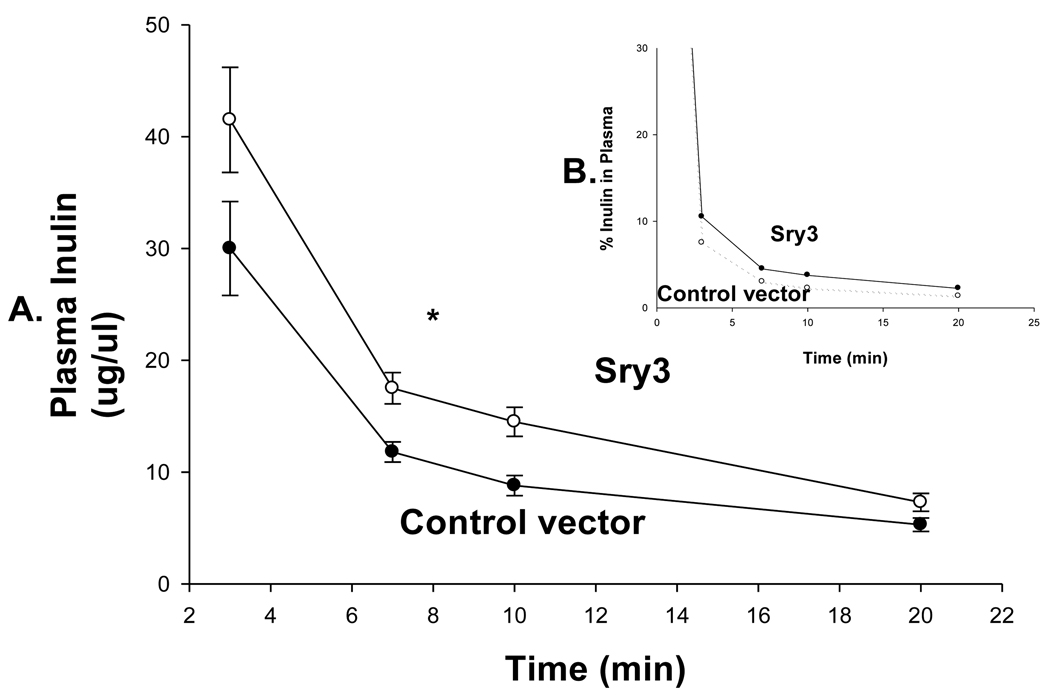
A. Inulin clearance measured at day 21 over 20 minutes in control vector (open squares, n=6) and Sry3 (solid squares, n=6) rats and was significantly (33%) reduced for the Sry3 group compared to controls. The slope for inulin concentration between 3 and 7 minutes was 6.0 for Sry3 and 4.5 for control vector, p<0.05. The graph only extends to the 20 min point to better illustrate the slope difference (data at 55 min was same for both groups). B. Insert plots inulin as a function of the % remaining in plasma, broken line control vector and solid line Sry3. Two way ANOVA with repeated measures: treatment-F=3.99, p<0.05, time-F=118, p<0.001 and interaction of time and treatment-n.s.
Results
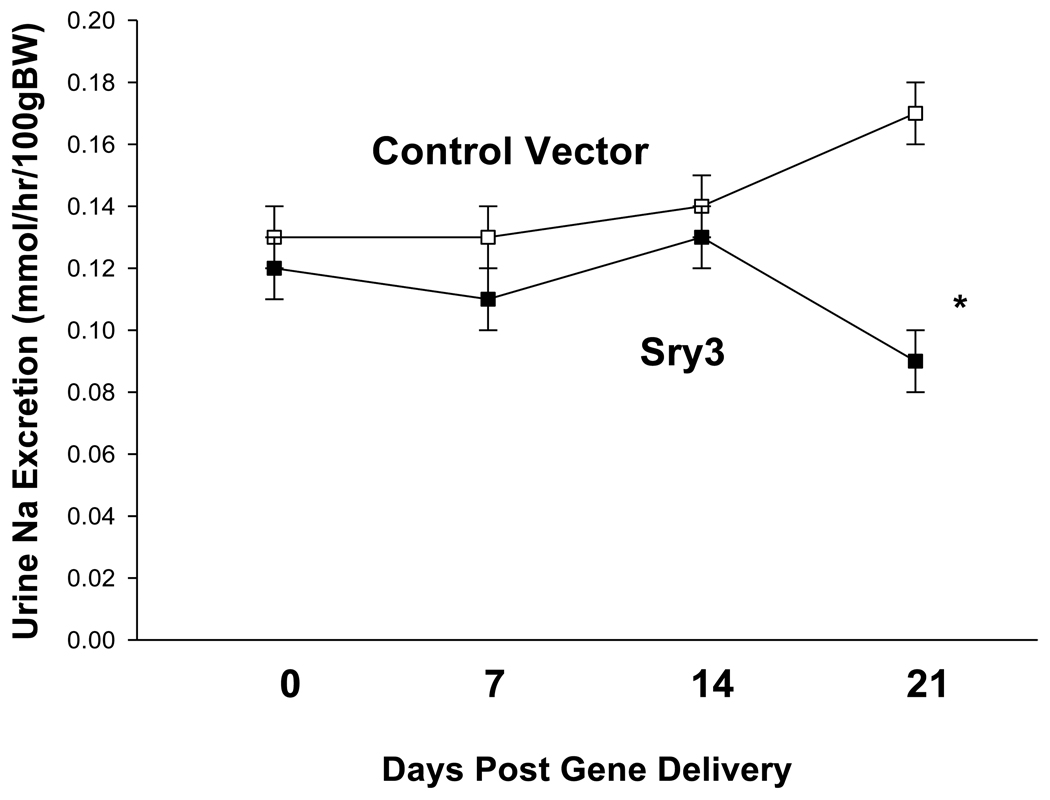
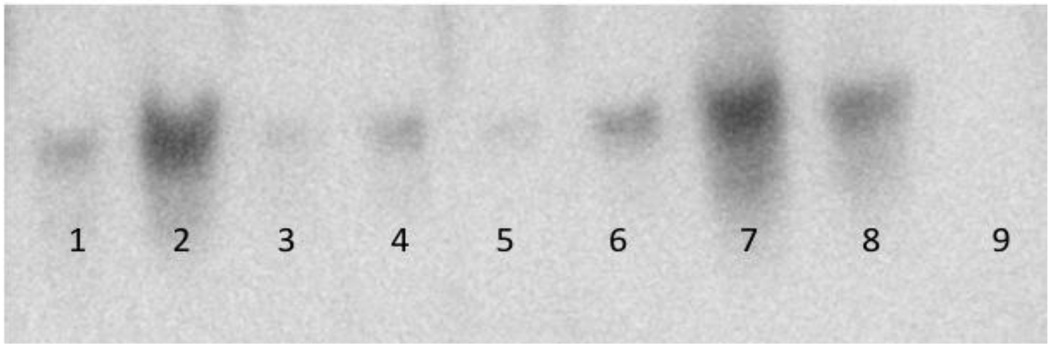
Figure 1 shows that Sry3 significantly raised SBP about 10mmHg compared to controls after 7 and 14 days and about 20mmHg after 21 days with normalization at 28 days (two-way ANOVA with repeated measures, treatment: F=20.6,p<0.001, time: n.s., interaction of treatment and time: F=3.9, p<0.05). Telemetered mean arterial pressure (MAP) was significantly elevated after Sry3 as compared to control vector (open circles, means, +/−s.e.m., two-way ANOVA with repeated measures, treatment: F=18.6, p<0.001, time: n.s., interaction not significant, but heart rate was not significantly different as compared to control vector. We measured plasma renin activity (PRA) once a week for 3 weeks following electroporation of Sry3 or the control vector (Fig.2). PRA remained between 10 and 25 ng/ml/hr for 2 weeks in the WKY rats treated with the control vector. While no differences in PRA were observed at the outset and after 7 days in the Sry3 treatment group, by 14 days, PRA had increased by 95%. By 21 days, PRA fell in the Sry3 group to basal levels whereas in the control vector group, the enzyme activity had markedly increased by 79% (two-way ANOVA with repeated measure, treatment: n.s., time: F=5.2, p<0.01, interaction of treatment and time: F=17.1, p<0.001). We measured Ang II renal content 3 weeks post electroporation and Sry3 significantly increased (140%) renal Ang II content compared to controls (p<0.01 at 21 days). SNS indices were not significantly elevated by Sry3 at 21 days as compared to controls (Table 1). The Na excretion was significantly reduced 21 days post Sry3 delivery (31% compared to baseline for Sry3 and the controls showed a 21% increase from their baseline (Fig. 4, two-way repeated measures ANOVA, treatment: F=3.4, p<0.05, time: n.s., interaction of treatment and time: n.s.). Figure 5 shows that the inulin clearance was significantly (33%) reduced for the Sry3 group compared to controls. The slope for inulin concentration between 3 and 7 minutes was 6.0 for Sry3 and 4.5 for control vector, p<0.05. The graph only extends to the 20 min point to better illustrate the slope difference (data at 55 min was basically the same for both groups, 2.8 for vehicle control and 3.3ug/ul for Sry3). Two way ANOVA with repeated measures: treatment-F=3.99, p<0.05, time-F=118, p<0.001 and interaction-not significant. Table1 shows that there were no significant differences between groups with regards to: plasma NE or epinephrine, renal catecholamines, renal tyrosine hydroxylase, ACE or urinary creatinine clearance. Figure 6 shows the Sry protein by Western blot 14 days after electroporation to the kidney in WKY females which do not have endogenous Sry but shows that the plasmid can travel and be expressed in other tissues.
Table 1.
Creatinine Clearance, Plasma and Renal Catecholamines, Renal Tyrosine Hydroxylase (TH) and Renal ACE
| Urinary Creatinine Clearance (ml/min/gm bw) Days 7,14,21 |
Plasma NE (pg/ml) Day 21 |
Plasma Epi (pg/ml) Day 21 |
Renal Dopamine (pg/g) Day 21 |
Renal NE (pg/g) Day 21 |
Renal TH (fmol/m in/mg) Day 21 |
Renal ACE (units/g) Day 21 |
|
|---|---|---|---|---|---|---|---|
| Control Vector (n=6) | 0.64,.065, 0.66 +/− .07,.06,.07 | 375 +/− 70 | 95 +/− 10 | 7,990 +/− 865 | 100,027 +/−8,365 | 15 +/−1 | 0.14 +/− 0.04 |
| Sry3 (n=6) | 0.64, 0.68, 0.66 +/− .07, .06, .06 | 360 +/− 60 | 125 +/− 20 | 6,853 +/− 561 | 91,985 +/− 7213 | 16 +/−1 | 0.11 +/−0.01 |
Means, +/− s.e.m., no significant differences between groups
Figure 4.
Urinary Na excretion between control vector (open squares, n=6) and Sry3 (solid squares, n=6) from 0–21 days post gene delivery, means, +/− s.e.m., (two-way repeated measures ANOVA, treatment: F=3.4, p<0.05, time: n.s., interaction of treatment and time: n.s.).
Figure 6.
Western blot detection of Sry/Myc fusion proteins 14 days after vector delivery and electroporation. Lane 1) Heart. Lane 2) Testes. Lane 3) Lung. Lane 4) Liver. Lane 5) Right Adrenal Gland. Lane 6) Left Adrenal Gland. Lane 7) Right Kidney. Lane 8) Left Kidney. Lane 9) Empty Vector (−) Left Kidney.
Discussion
In this investigation we electroporated Sry3 into a normotensive WKY kidney which produced a significant rise in BP, whereas, Sry2 from a previous study did not. 26 Also the BP effect of 10–20 mmHg, is comparable to the 20 mmHg effect produced through breeding by replacing the entire SHR or WKY Y chromosome. 14 These increases are larger or equal to the effects of BP QTLs in other rat models of hypertension 30 which are also potentially composed of multiple genetic BP components. We are proposing that the mechanism for this effect is due to several Sry effects on the RAS. The first line of evidence is based on our in vitro studies showing that Sry binds to the promoter region of several RAS genes. When Sry expression vectors were co-transfected into CHO cells with luciferase reporter constructs containing promoters of angiotensinogen (Agt −1430/+22), renin (Ren −1050/−1), ACE (ACE −1677/+21) and ACE2 (ACE2 −1091/+83), that Sry1, Sry2 and Sry3 differentially upregulated activity of the promoters of angiotensinogen, renin and ACE genes, and down-regulated ACE2 promoter activity. 28 The largest effect was seen with Sry3, which increased activity of angiotensinogen promoter by 1.7 fold, renin promoter by 1.3 fold, ACE promoter by 2.6 fold, and decreased activity of ACE2 promoter by 0.5 fold. The effect of Sry1 on promoter activity was significantly less than Sry3. Sry2 activated promoters at a significantly lower level than Sry1. Based on the literature on plasmids delivered by electroporation, the peak physiological activity is often observed at 14–21 days before the protein and plasmid are either degraded or physiological compensations normalize the activity. Based on our Sry1 data we saw BP elevate rapidly at 7 days and maintain for 21 days. This may have been due to the more rapid effects of the SNS which Sry1 influenced, whereas with Sry3 the RAS may take more time to reach peak activity. However, BP in this study did elevate at 7 and 14 days it just rose more at 21 days.
The RAS has many important components but two of the more important ones are Ang II and Ang-(1–7). With regard to blood vessel contractile tone the two peptides have opposing actions: Ang II causes vasoconstriction and Ang-(1–7) causes vasorelaxation .31 The relative contribution of these two Ang peptides can produce vasoconstriction and increased BP with more Ang II or decreased BP with relatively more Ang-(1–7). Any regulator of RAS genes like Sry has the potential to alter the balance of Ang II to Ang-(1–7) which could ultimately influence BP. Also we acknowledge that that the measurement of Ang II could include Ang III since the antibody does not differentiate between the two but either can elevate blood pressure. Also tissue Ang II may not reflect plasma Ang II since different tissues have their own RAS and may be differentially regulated.
In the current study, using whole animals, we observed a 40% increase in renal ACE with Sry3. The result of either an additive effect of Sry regulation of multiple RAS genes or alterations in expression of a single gene could favor generation of vasoconstrictive Ang II and decreased levels of Ang-(1–7), which could result in increased BP. 28 We found that endogenous Sry is differentially expressed in the kidney depending upon age. For instance, in SHR/y males, Sry kidney expression increased 7.25+/−2.75 fold in 15 week vs. 4 week old animals. Also, in 15 week SHR/y males, Sry expression in the kidney was 31.6 +/− 1.78 fold higher than in adrenal glands. 32 So it seems reasonable that exogenous Sry could add to the endogenous amount of renal Sry.
In whole animal telemetry studies with blood collection and tissue assays for SNS and RAS indices, it is animal costly to collect multiple tissue samples at different times since it is a terminal procedure. However, this brings up an important point concerning the timing of the SNS and RAS markers with reference to Sry3 delivery. The timing of each component of the RAS could be informative for how Sry3 influences each of them and consequently blood pressure regulating systems. Therefore, since the tissue markers for both the SNS and RAS were obtained at 21 days we cannot determine the relative contributions of these indices across time. It is possible that Sry3 could alter renal NE or TH earlier and we missed the effect. Likewise, Sry3 could alter renal RAS indices at earlier times but plasma renin was not different at 0,7 or 21 days, only at 14 days. Also urine Na was not different at 0,7,14 days which suggests that tissue ACE or angiotensin II was not elevated. Therefore, future studies need to examine both SNS and RAS markers across time to determine their contribution due to Sry3 effects. As part of an ongoing study we administered an angiotensin II antagonist (olmesartan) to WKY males who had received Sry3 to the kidney when blood pressure was elevated at 14 days and blood pressure was normalized (145-110mmHg) in 3 days which further supports the idea that Sry3 alters some of the RAS components (unpublished results, Boehme et al. 2010).
With regard to what type of binding region Sry targets, we have in vitro data to show that Sry differentially upregulated activity of the promoters of angiotensinogen, renin and ACE genes, and down-regulated ACE2 promoter activity which would suggest that vasoconstrictor tone would be enhanced and vasodilator tone reduced thus contributing to increased blood pressure28
In other recent Sry studies, specific binding sites have been identified. Sry can interact in novel ways not fully understood with other factors to repress or activate genes. For instance, Wu et al.7 showed that SRY activates monoamine oxidase (MAO)-promoter in a human male neuroblastoma cell line. Sry and Sp1 form a transcriptional complex and synergistically activate MAO-A transcription. This has implications for blood pressure and brain neurochemistry since MAO is a degradative enzyme for the catecholamines. Also Zhang et al.8 showed that SRY may have blood pressure implications in that the chromogranin B promoter variants are responsive to SRY and influence blood pressure. This group 9 also showed that SRY effects the common variants of the human proximal promoter of tyrosine hydroxylase which is the rate limiting enzyme in the catecholamine pathway. Several other genes have also been reported to have specific promoter Sry binding sites that are involved in inhibiting ovarian development 10,11 and in regulating the mouse sulphamidase gene which produces a lysosomal enzyme. 12
The physiological effects observed do not seem to be due to kidney inflammation from the electroporation procedure because in a previous study we histologically compared control kidneys with no electroporation to those electroporated and we saw no differences in lymphocytes or morphological changes using H&E staining (Fig.6 in Ely et al26) and creatinine clearance was normal. In our preliminary studies we used an Sry antibody and also a B-gal plasmid to identify uptake areas in the kidney after electroporation. Both tubular and glomerular tissue revealed either Sry or B-gal. However, a complex and confounding variable is the fact that some of the injected plasmid can be picked up by the renal microcirculation and be transported to other organs such as we found with the Western blot. The liver, lungs and contralateral kidney would be expected to take up the plasmid which is then expressed and protein synthesized so would be expected to show up in the Western blot, but how this influences blood pressure or the RAS cannot be determined at this time. Further studies will document the tissue distribution and cellular localization of Sry protein.
One of the more interesting functional Sry3 results was the significantly increased (50%) renal Na reabsorption. This may be explained by the elevation of renal Ang II and plasma renin. The timing for the Ang II and plasma renin were not simultaneous since the Ang II was elevated at 21 days and the plasma renin at 14 days. However, the relationship between plasma RAS indicators and tissue RAS indicators are complex and do not necessarily respond simultaneously. The RAS is very active in the kidney and the production of Ang II is highly complex. 33 In addition to the classical pathway Ang II is formed locally and angiotensinogen mRNA. 34–36 and ACE mRNA are present in proximal tubular brush border membranes. 37 Transgenic animal models also show that AngII is produced in the kidneys in high concentrations. 38,39 Ang II may play a role at the cellular level at many locations in the nephron but their regulation is not known. In addition, the reduced GFR as indicated by inulin clearance could have contributed to the increased Na reabsorption. However, the creatinine clearance values do not match the inulin clearance values. We are not sure why there is this discrepancy but we think the inulin values are more sensitive. However, they reflect the state of the kidneys at 18 days and just over a 50 min period during the day when these measures were taken rather than the 24 hr data at 7, 14 and 21 day collection periods for the creatinine clearance. The inulin data suggested a small but significant slope difference for inulin clearance between control and Sry3 animals, however, the kidneys may have compensated for this difference over a 24 hr period and normalized the creatinine clearance. Therefore, we are suggesting that this small difference in inulin clearance does not have significant biological renal function consequences in the long term.
We speculate that the differential function of Sry1 vs. Sry3 may be due to the substitution of a glutamine for a histidine at aa 37 in the HMG box in Sry3. Sry3 and all other rat Sry genes, as well as mouse and human Sry, have a glutamine at aa 37 which is within the HMG box of the protein. In addition, the change to threonine at amino acid 86 in Sry3, replacing the proline that is present in Sry1 and other Sry proteins, may affect protein folding and interactions with DNA. We are currently investigating the significance of this difference in Sry3 compared to the other proteins. The function of Sry in adult humans in other cells and organs besides the reproductive system is just beginning to be explored. For instance, Wu et al.7 using a human male neuroblastoma BE(2) cell line showed that SRY activates MAO-A promoter and catalytic activities which is the first study in human cells that suggests a novel mechanism for sexual dimorphism in neural function and neuropsychiatric disorders. Also Zhang et al. 8,9 using human SRY implicates regulation of catecholamine pathways which could modulate blood pressure.
In conclusion, the findings presented here confirm and extend the argument for Sry3 as one of the genes responsible for the SHR hypertensive Y chromosome phenotype. These results are consistent with an increased tissue renin-angiotensin system activity due to Sry3. The functions of the other copies of the Sry complex remain to be determined. Since the DNA binding portion of Sry is highly conserved in placental mammals, either mutations and/or expression of human SRY in the kidney could elevate BP with similar mechanisms.
Acknowledgements
We appreciate the help of Emily Njus for animal care and welfare. The authors are grateful for grant support from: NIH (RO1-HL71579-01A5), Department of Biology, University of Akron and the Ohio Board of Regents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None
Contributor Information
Daniel Ely, Department of Biology, University of Akron, Akron, OH 44325-3908.
Shannon Boehme, Department of Biology, University of Akron, Akron, OH 44325-3908.
Gail Dunphy, Department of Biology, University of Akron, Akron, OH 44325-3908.
Michael Hart, Department of Biology, University of Akron, Akron, OH 44325-3908.
Frank Chiarappa, Department of Biology, University of Akron, Akron, OH 44325-3908.
Brian Miller, Department of Biology, University of Akron, Akron, OH 44325-3908.
Almir Martins, Department of Physiology and Biophysics, Federal University of Minas Gerais, Belo Horizonte, Brazil.
Monte Turner, Department of Biology, University of Akron, Akron, OH 44325-3908.
Amy Milsted, Department of Biology, University of Akron, Akron, OH 44325-3908.
References
- 1.Stolt CC, Wegner M. SoxE function in vertebrate nervous system development. Internat J Biochem & Cell Biol. 2010;42:437–440. doi: 10.1016/j.biocel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Sekido R. SRY: A transcriptional activator of mammalian testis determination. Internatl J Biochem & Cell Bio. 2010;42:417–420. doi: 10.1016/j.biocel.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Sato Y, Shinka Y, Chen G, Yan H-T, Sakamoto K, Ewis AA, Aburatani H, Nakahori Y. Proteomics and transcriptome approaches to investigate the mechanism of human sex Determination. Cell Biol Internatl. 2009;33:839–847. doi: 10.1016/j.cellbi.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Bernard P, Harley VR. Acquisition of SOX transcription factor specificity through protein–protein interaction, modulation of Wnt signalling and post-translational modification. Internat J Biochem & Cell Biol. 2010;42:400–410. doi: 10.1016/j.biocel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut P-O, Kelly S, Chesselet M-F, Micevych PE, Albrecht KH, Harley VRa, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Current Biology. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 6.De Vries GJ, Rissman EF, Simerly RB, Yang L-Y, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. Model System for Study of Sex Chromosome Effects on Sexually Dimorphic Neural and Behavioral Traits. Neuroscience. 2002;15:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JB, Chen K, Li Y, Lau YFC, Shih JC. Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB Journal. 2009;23:4029–4038. doi: 10.1096/fj.09-139097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K, Rao F, Wang L, Rana BK, Ghosh S, Mahata M, Salem RM, Rodriguez-Flores JL, Fung MM, Waalen J, Tayo B, Taupenot L, Mahata SK, OConnor DT. Common functional genetic variants in catecholamine storage vesicle protein promoter motifs interact to trigger systemic hypertension. J Am Coll Cardiol. 2010;55:1463–1475. doi: 10.1016/j.jacc.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang K, Zhang L, Rao F, Brar B, Rodriquez-Flores JL, Taupenot L, OConnor DT. Human tyrosine hydroxylase natural genetic variation: delineation of functional transcriptional control motifs disrupted in the proximal promoter. Circ Cardiovascular Gen. 2010 doi: 10.1161/CIRCGENETICS.109.904813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard P, Sim H, Knower K, Vilain E, Harley V. Human SRY inhibits B-catinin-mediated transcription. Internatl J Biochem Cell Bio. 2008;40:2889–2900. doi: 10.1016/j.biocel.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng H, Ivanov AV, Oh HJ, Lau YF, Rauscher FJ. Epigenetic gene silencing by the SRY protein is mediated by a KRAB_O protein that recruits the KAP1 co-repressor machinery. J Biol Chem. 2009;284:35670–35680. doi: 10.1074/jbc.M109.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costanzi E, Beccari T, Aisa MC, Tiribuzi R, Hopwood JJ, Orlacchio A. Mouse sulphamidase gene: characterization of the promoter region of the gene and expression in mouse tissues. Gene. 2003;310:143–149. doi: 10.1016/s0378-1119(03)00531-6. [DOI] [PubMed] [Google Scholar]
- 13.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hyper. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 14.Ely DL, Turner M. Hypertension in the spontaneously hypertensive rat is linked to the Y chromosome. Hypertension. 1990;16:270–281. doi: 10.1161/01.hyp.16.3.277. [DOI] [PubMed] [Google Scholar]
- 15.Ely D, Milsted A, Bertram J, Ciotti M, Dunphy G, Turner M. Sry delivery to the adrenal medulla increases blood pressure and adrenal medullary tyrosine hydroxylase of normotensive WKY rats. BMC Cardiovascular Disorders. 2007;7:6. doi: 10.1186/1471-2261-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ely DL, Daneshvar H, Turner ME, Johnson ML, Salisbury RL. The hypertensive Y chromosome elevates blood pressure in F11 normotensive rats. Hyper. 1993;21:1071–1075. doi: 10.1161/01.hyp.21.6.1071. [DOI] [PubMed] [Google Scholar]
- 17.Ely D, Caplea A, Dunphy G, Daneshvar H, Turner M, Milsted A, Takiyyuddin M. Spontaneously hypertensive rat Y chromosome increases indices of sympathetic nervous system activity. Hyper. 1997;29:613–618. doi: 10.1161/01.hyp.29.2.613. [DOI] [PubMed] [Google Scholar]
- 18.Turner M, Johnson M, Ely D. Separate sex-influenced and genetic components In SHR hypertension. Hyper. 1991;17:1097–1103. doi: 10.1161/01.hyp.17.6.1097. [DOI] [PubMed] [Google Scholar]
- 19.Caplea A, Seachrist D, Daneshvar H, Dunphy G, Ely D. Noradrenergic content and turnover rate in kidney and heart shows gender and strain differences. J Appl Physio. 2002;92:567–571. doi: 10.1152/japplphysiol.00557.2001. [DOI] [PubMed] [Google Scholar]
- 20.Cao QP, Gaudette MF, Robinson DH, Crain WR. Expression of the mouse testis-determining gene Sry in male preimplantation embryos. Mol Reprod Develop. 1995;40:196–204. doi: 10.1002/mrd.1080400208. [DOI] [PubMed] [Google Scholar]
- 21.Lahr G, Maxson SC, Mayer A, Just W, Pilgrim C, Reisert I. Transcription of the Y chromosomal gene, Sry, in the adult mouse brain. Mol Brain Res. 1995;33:179–182. doi: 10.1016/0169-328x(95)00136-g. [DOI] [PubMed] [Google Scholar]
- 22.Zwingman T, Fujimoyo H, Lai L-W, Boyer T, Ao S, Stalvey JRD, Blecher SR, Erickson RP. Transcription of circular and noncircular forms of Sry in mouse testes. Mol Reprod Develop. 1994;37:370–381. doi: 10.1002/mrd.1080370403. [DOI] [PubMed] [Google Scholar]
- 23.Ely D, Underwood A, Dunphy G, Boehme S, Turner M, Milsted A. Review of the Y chromosome, Sry and hypertension. Steroids. 2009 doi: 10.1016/j.steroids.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunmire J, Farkas J, Ely D, Turner M, Milsted A. Tissue specific expression of transcripts from multiple Sry loci in male rats. FASEB J. 2007;21:A476. 584.2. [Google Scholar]
- 25.Turner ME, Martin C, Martins AS, Dunmire J, Farkas F, Ely DL, Milsted A. Genomic and expression analysis of multiple Sry loci from a single Rattus norvegicus Y chromosome. BMC Genetics. 2007;8:11. doi: 10.1186/1471-2156-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ely DL, Milsted A, Dunphy G, Boehme S, Dunmire J, Hart M, Toot J, Martins A, Turner M. Delivery of sry1, but not sry2, to the kidney increases blood pressure and sns indices in normotensive wky rats. BMC Physiology. 2009;9:10. doi: 10.1186/1472-6793-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milsted A, Serova L, Sabban E, Dunphy G, Turner M, Ely D. Regulation of tyrosine hydroxylase gene transcription by Sry. Neuroscience Letters. 2004;369:203–207. doi: 10.1016/j.neulet.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 28.Milsted A, Underwood AC, Dunmire J, DelPuerto HL, Martins AS, Ely DL, Turner ME. Regulation of multiple renin-angiotensin system genes by Sry (2010) J Hyper. 2009;28:59–64. doi: 10.1097/HJH.0b013e328332b88d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturgeon C, Sam A, II, Law R. Rapid determination of glomerular filtration rate by single-bolus inulin: a comparison of estimation analyses. J App Physiol. 1998;84:6. doi: 10.1152/jappl.1998.84.6.2154. [DOI] [PubMed] [Google Scholar]
- 30.Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiolog Reviews. 2000;80:135–172. doi: 10.1152/physrev.2000.80.1.135. [DOI] [PubMed] [Google Scholar]
- 31.Santos RA, Frézard F, Ferreira AJ. Angiotensin-(1–7): blood, heart, and blood vessels (2005) Curr Med Chem Cardiovasc Hematol Agents. 3:383–391. doi: 10.2174/156801605774322373. [DOI] [PubMed] [Google Scholar]
- 32.Turner M, Farkas J, Dunmire J, Ely DL, Milsted A. Which Sry locus is the hypertensive Y chromosome locus? Hyper. 2009;53(part 2):430–435. doi: 10.1161/HYPERTENSIONAHA.108.124131. [DOI] [PubMed] [Google Scholar]
- 33.Bader M, Peters J, Baltatu O, Müller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med. 2001;79:76–102. doi: 10.1007/s001090100210. [DOI] [PubMed] [Google Scholar]
- 34.Darby I, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 35.Gomez R, Lynch K, Chevalier R, Everett A, Johns D, Wilfong N, Peach M, Carey R. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol. 1988;254:900–906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 36.Ingelfinger J, Zuo W, Fon E, Ellison K, Dzau V. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz W, Hagler H, Buja L, Erdos E. Ultrastructural localization of angiotensin I-converting enzyme (EC 3.4.15.1) and neutral metalloendopeptidase (EC 3.4.24.11) in the proximal tubule of the human kidney. Lab Invest. 1988;59:789–797. [PubMed] [Google Scholar]
- 38.Mullins J, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 39.Navar L, Harrison-Bernard LM, Imig J, Wang C, Cervenka L, Mitchell K. Intrarenal angiotensin II generation and renal effects of AT (1) receptor blockade. J Am Soc Nephrol. 1999;10:S266–S272. [PubMed] [Google Scholar]