Abstract
Transmissible spongiform encephalopathies (TSEs, prion diseases) are a class of fatal neurodegenerative diseases affecting a variety of mammalian species including humans. A misfolded form of the prion protein (PrPTSE) is the major, if not sole, component of the infectious agent. Prions are highly resistant to degradation and to many disinfection procedures suggesting that, if prions enter wastewater treatment systems through sewers and/or septic systems (e.g., slaughterhouses, necropsy laboratories, rural meat processors, private game dressing) or through leachate from landfills that have received TSE-contaminated material, prions could survive conventional wastewater treatment. Here, we report the results of experiments examining the partitioning and persistence of PrPTSE during simulated wastewater treatment processes including activated and mesophilic anaerobic sludge digestion. Incubation with activated sludge did not result in significant PrPTSE degradation. PrPTSE and prion infectivity partitioned strongly to activated sludge solids and are expected to enter biosolids treatment processes. A large fraction of PrPTSE survived simulated mesophilic anaerobic sludge digestion. The small reduction in recoverable PrPTSE after 10-d anaerobic sludge digestion appeared attributable to a combination of declining extractability with time and microbial degradation. Our results suggest that if prions were to enter municipal wastewater treatment systems, most of the agent would partition to activated sludge solids, survive mesophilic anaerobic digestion, and be present in treated biosolids.
Introduction
Transmissible spongiform encephalopathies (TSEs) comprise a class of inevitably fatal neurodegenerative diseases afflicting a number of mammalian species including cattle (bovine spongiform encephalopathy [BSE] or “mad cow” disease), sheep and goats (scrapie), deer, elk and moose (chronic wasting disease [CWD]), ranched mink (transmissible mink encephalopathy [TME]), and humans (Creutzfeldt-Jakob disease, fatal familial insomnia, Gerstman-Stäussler-Scheinker disease, and kuru) (1). The novel etiological agent in these diseases is the prion, a pathogen apparently lacking nucleic acid and composed primarily, if not exclusively, of an abnormally folded isoform of the prion protein (designated PrPTSE) (2). PrPTSE is a widely accepted biomarker for prions and generally correlates with infectivity, although some uncertainty remains as to the precise nature of the prion. For example, prion infectivity varies with aggregate size (3). Prion infection appears to propagate in host tissue via PrPTSE-catalyzed conversion of the normal cellular prion protein (PrPC) (4-6).
The amino acid sequence of PrPTSE and PrPC are identical, and no differences in covalent posttranslational modification have been reported (7). The normal and abnormal forms of the protein differ only in conformation (7). Relative to PrPC, the disease-specific isoform has lower α-helix and substantially higher β-sheet content (8). The disease-associated conformation confers on the protein biophysical properties not exhibited by PrPC including insolubility, a propensity to form ordered aggregates, and remarkable resistance to chemical and thermal degradation (9). The prion protein can possess zero to two N-linked glycans (7). Well-resolved immunoblots yield three bands corresponding to the di-, mono- and unglycosylated protein. Mass spectrometry studies indicate that >160 PrP glycoforms exist in a single prion preparation (10).
Management of TSEs in livestock and wildlife populations often involves harvesting large numbers of animals creating the need to safely and economically dispose of large volumes of infected carcasses and other materials. Landfilling represents an economically attractive option for disposal of TSE-contaminated wastes; however, if prions migrate through the waste mass to the leachate collection system, they may enter municipal wastewater treatment (WWT) facilities. TSE agents could also potentially enter WWT systems through disposal of prion-contaminated waste by slaughterhouses, necropsy laboratories, rural meat processors and private game dressing (11,12).
The presence of prions in municipal WWT influent, effluent or biosolids has not been reported and remains insufficiently investigated. The possibility exists, however, that land application of biosolids derived from WWT facilities receiving TSE-contaminated wastes could result in the introduction of TSE agent into the environment (13). Prions are extraordinarily resistant to heat, UV irradiation, and many chemicals routinely used to inactivate bacteria and viruses (9). Little is known regarding the fate of prions in WWT facilities, and previous risk assessments on the transmission of prion disease via contaminated sewage sludge (12,14) have been hampered by lack of information on prion fate in WWT systems and the extent of degradation during sludge digestion. Most conventional WWT facilities rely on aerobic biological treatment processes to remove biodegradable organic matter and on anaerobic digestion to reduce the mass and volume of activated sludge biomass produced. Recent reports suggest that some bacteria may be able to degrade PrPTSE (15,16). Proteolytic enzymes produced by activated sludge and/or anaerobic digester sludge consortia may be capable of inactivating prions.
In the only previous study of prion stability in WWT processes, anaerobic digester sludge was spiked with PrPTSE and incubated under mesophilic (37°C) or thermophilic (55°C) conditions. Neither condition degraded all added PrPTSE (17) suggesting that some prion infectivity could remain in biosolids. In the previous study, upstream processes and infectivity were not assessed, and significant matrix interference with prion detection was reported (17). The goal of the present study was to provide a more complete analysis of the fate of prions during simulated WWT processes. Our objectives were to (1) optimize PrPTSE recovery from wastewater sludge matrices; (2) determine the partitioning of PrPTSE between activated sludge solids and supernatant; and (3) determine the degree to which PrPTSE survives activated sludge digestion and mesophilic anaerobic sludge digestion. To accomplish these objectives, the partitioning and survival of PrPTSE derived from TSE-infected hamster brain homogenate in simulated biological wastewater treatment processes was determined.
Materials and Methods
Source of wastewater sludges
Activated sludge mixed liquor and mesophilic anaerobic digester sludge were obtained from the Madison Metropolitan Sewerage District (MMSD) Nine Springs municipal WWT plant in Madison, Wisconsin, USA. The plant is operated as a modified University of Cape Town activated sludge process with mesophilic anaerobic digestion for sludge stabilization and an average daily flow of 150,000 m3·d−1 (40 million gallons per day, MGD). The sludge retention time was 9 d; the annual average total suspended solids concentration was °3.2 g·L−1. The secondary influent biochemical oxygen demand was °150 mg·L−1. The plant does not use chemical addition for phosphorus removal. Activated sludge mixed liquor was collected directly from the final aerobic tank in the aeration basin train. Anaerobic digester sludge was obtained from a pipe on the volute drain of the sludge recirculating pumps. The anaerobic digester sludge had an annual average total suspended solids of °22 g·L−1. Samples (1 L) of both activated and anaerobic sludge were collected at three times and used within 2 h of collection. No detectable PrP-immunoreactivity was present in the collected sludges (data not shown).
Source of PrPTSE
Infectious agent was prepared from brains of hamsters clinically affected with the Hyper (HY) strain of hamster TSE agent Hamster brains were homogenized with a Dounce homogenizer in phosphate buffered saline (PBS) to give a 10% (w/v) homogenate. The homogenate was stored at −20 °C. Proteinase K (PK)-treated brain homogenate was prepared by incubating the homogenized tissue with 50 μg/mL PK for 30 minutes. Activity of PK was blocked by adding PMSF to a final concentration of 3 mM.
Immunoblot analysis
Samples were denatured by 10-min heating in SDS-PAGE sample buffer (10% SDS, 100 mM Tris·HCl, pH 8.0, 7.5 mM EDTA, 100 mM dithiothreitol, 0.5% Bromophenol blue) and fractionated by SDS-PAGE on 4–15% precast gels (BioRad, Hercules, CA, USA) under reducing conditions. Proteins were electrophoretically transferred to polyvinyl difluoride (PVDF) membranes and immunoblotted with mAb 3F4 (1:40,000 dilution) as previously described (18). Detection was achieved with an HP-conjugated goat anti-mouse immunoglobulin G (1:10,000; BioRad) and Pierce Pico Western Blotting Detection (Pierce Biotechnology, Rockford, IL).
Extraction of spiked PrPTSE from activated and anaerobic digester sludges
The following detergents were tested for their ability to extract PrPTSE from activated sludge and anaerobic digester sludges: sodium dodecyl sulfate (SDS, 1% and 10% in distilled deionized water [ddH2O]), Triton® X-100 (1% and 10% in ddH2O), sodium undecyl sulfate (SUS, 1% and 10% in ddH2O), and sodium N-lauryl sarcosinate (Sarkosyl, 1% and 10% in ddH2O). Infected brain homogenate (BH; 100-200 μL of 10% BH) was added to 1 mL of fresh activated sludge or anaerobic digester sludge and vortexed for 10-min. The solid fraction was sedimented by 7-min centrifugation at 16,100g, the supernatant was removed, and the sedimented fraction resuspended in 1 mL of extraction solution. The samples were either heated at 100°C or vortexed for 10 min at room temperature and then centrifuged for 7-min at 16,100g. The resulting supernatants were aspirated, and 25 μL of each was analyzed by immunoblotting.
We assessed the recovery of both PK-digested and full-length PrPTSE from each sludge by 1% SDS. The starting brain homogenate material and extracted protein was serially diluted (1:100) and analyzed by immunoblotting. The resultant immunoreactivity was quantified and compared using AlphaEase software (Alpha Innotech, San Leandro, CA). All extraction experiments were repeated at least three times.
PrPTSE partitioning between activated sludge solids and supernatants
To assess partitioning between activated sludge solids and liquid, 100 μL of 10% infected BH was added to 1 mL of fresh activated sludge. As a control, 100 μL of 10% infected BH homogenate was diluted into 1 mL of PBS and handled identically (sludge-free control). The samples were rotated for 7 h at 20 rpm at room temperature to simulate operating conditions at the full-scale Nine Springs WWT facility and then allowed to settle fully by gravity (∼10 min.). The supernatant was separated from the solid fraction by pipette. The solid fraction was extracted in 1% SDS with vortexing. The PrPTSE present in the sludge solids extracts and supernatants were examined by SDS-PAGE with immunoblotting. These experiments were conducted in triplicate.
Simulated mesophilic anaerobic sludge digestion
We examined the amount of PrPTSE recoverable after 10- and 20-d of simulated mesophilic anaerobic sludge digestion. In a WWT facility, any PrPTSE entering the anaerobic sludge digester would have undergone activated sludge digestion and would be embedded in activated sludge floc. We therefore incubated infected brain homogenate (150 μL of 10%) with 1 mL of activated sludge as described above. After mixing and settling, the supernatant was aspirated and stored at −20°C, and the BH in activated sludge solids was used for the anaerobic sludge digestion.
To initiate anaerobic sludge digestion, the BH/settled activated sludge solids mixture (67 μL) was mixed with 933 μL of anaerobic digester sludge, in triplicate, in microcentrifuge tubes. The remaining BH/settled activated sludge solid mixtures were snap frozen in liquid N2 and stored at −80°C. The anaerobic digester sludge + BH/settled activated sludge solids samples were placed in an N2-purged, pear-shaped glass flask (fitted with a pressure release valve), and simulated anaerobic sludge digestion was conducted for 10 or 20 d in the dark at 37°C. To determine the initial amount of PrPTSE in the simulated anaerobic digester sludge, a 200-μL aliquot was removed from each flask intended for 10-d digestion prior to incubation, snap frozen in liquid N2, and stored at −80°C (t = 0). At the conclusion of the 10- and 20-d incubations, the microcentrifuge tubes were removed from the flask, snap frozen in liquid N2, and stored at −80°C. Prior to immunoblot analysis, PrP was extracted from the samples as described above.
To provide insight into the mechanisms responsible for reduced recovery of PrPTSE after simulated anaerobic sludge digestion, mesophilic anaerobic digester sludge was inactivated prior to addition of infected BH by each of the following methods: autoclaving, γ-irradiation, and protease inhibition. Autoclaving (30 min at 121°C on a liquid load) inactivates microorganisms, denatures microbial proteases, and may alter the physical and chemical properties of sludge. γ-irradiation (16 h on a J.L. Shepherd Mark I irradiator equipped with a 137Cs source for a total dose of 7.5 kGy) effectively inactivates the majority of microorganisms (19) in complex environmental matrices without significantly altering the matrix (20). The dose employed was sufficient to effect > 4 log unit reduction in fecal coliform counts in wastewater sludges (21). This treatment was not expected to inactivate extracellular proteases. Anaerobic digester sludge microorganisms synthesize a variety of intra- and extracellular proteases, some of which could be capable of (partially) degrade PrPTSE. To inhibit these microbial proteases, a premixed protease inhibition cocktail designed to sufficiently inhibit 50 mL of cell extract (Roche, Basel, Switzerland) was used. The cocktail contained EDTA to inhibit metalloproteases and 4-(2-Aminoethyl)-benzenesulfonyl fluoride to inhibit both cysteine and serine proteases. The entire portion of the cocktail was added to 50 mL of anaerobic digester sludge. Sodium azide inhibits a number of respiratory enzymes in aerobic bacteria, resulting in slowed growth (22-24). NaN3 (0.5 g) was added to 50 mL of anaerobic digester sludge. These manipulated sludges were treated the same as the fresh anaerobic digester sludge, and served as biologically inactivated controls.
After thawing the samples, 200-μL aliquots of the anaerobic digester sludge was dewatered by 10-min centrifugation at 1800g to attain ∼5% solids by mass (approximate dry weight achieved by MMSD in belt filtration of biosolids). The supernatant was removed, and a 40-μL aliquot was analyzed by immunoblotting. The pellet was resuspended by 10-min vortexing in 200 μL of 1% SDS, followed by 7-min centrifugation at 16,100g; 40-μL aliquot of the resultant extract was analyzed by immunoblotting.
Oral infectivity assay
Animal bioassays were used to examine the partitioning of prion infectivity between activated sludge solids and liquid. Infected brain homogenate (0.1% w/v) was incubated in 1 mL of activated sludge, and Syrian hamsters were orally dosed with activated sludge solids or supernatant after gravity settling. As a positive control, 0.1% infected brain homogenate was orally administered to hamsters. Following oral dosing, hamsters were observed twice weekly for the onset of clinical symptoms (25) for 435 d.
Results and Discussion
Extraction of Spiked PrPTSE from Activated and Anaerobic Digester Sludge Solids
Prior to investigating PrPTSE survival during simulated wastewater treatment processes, we evaluated the ability of several detergents to extract PrPTSE from activated and anaerobic digester sludge solids. Two concentrations (1% & 10%, w/v) of each of four detergents were investigated: SDS, SUS, Triton® X-100 and Sarkosyl. Sodium dodecyl sulfate is an anionic surfactant commonly used for protein denaturation and solubilization. Sodium undecyl sulfate differs from SDS by one methylene group and facilitates disaggregation of prion rods (3). Triton® X-100 is a non-ionic surfactant that has been employed for bulk extraction of proteins from sludge (26). Sarkosyl (N-dodecyl-N-methyl glycine) is an anionic surfactant commonly used in the preparation of PrPTSE-enriched brain fractions (27,28).
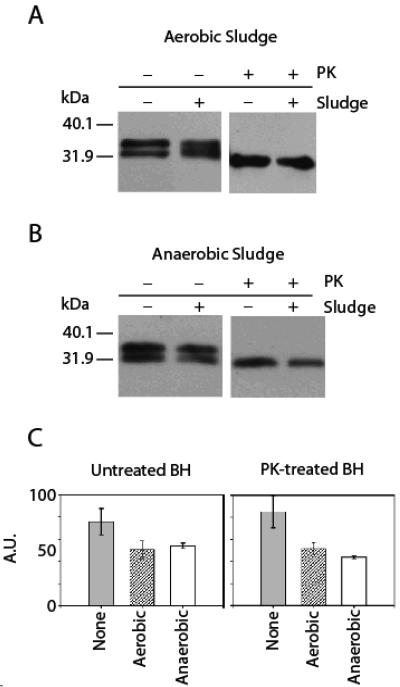
For both activated and anaerobic digester sludge, PrPTSE was efficiently recovered using 1% SDS (Figure 1A and B). PrPTSE was also efficiently extracted by 10% SUS and 1% Sarkosyl (anaerobic digester sludge only). Extractions with 1% SDS at 100°C were less efficient for both the activated and anaerobic digester sludge compared to 10-min vortexing. Because of the low concentration needed and compatibility with SDS-PAGE, we used 1% SDS and vortexing for all subsequent extractions. The recovery of full-length and PK-treated PrPTSE from both aerobic and anaerobic sludges by 1% SDS extraction was determined by comparing densitometric measurements of the starting material and the extracted protein on the immunoblots. For the aerobic sludge, recovery of full-length PrP was approximately 67% and PK-truncated PrP was approximately 61% (Figure 1). Extraction from the anaerobic sludge was approximately 71% and 52% for untreated and PK-treated PrP, respectively (Figure 1).
FIGURE 1.
PrPTSE extraction efficiency from aerobic and anaerobic sludge using 1% SDS. Brain homogenate (BH) or proteinase K (PK)-treated BH was incubated with each sludge and extracted using 1% SDS and vortexing. Extracted protein was compared to starting material by immunoblot analysis and densitometry. A minimum of three independent replicates were used for each experiment. (A) Extraction from aerobic sludge, (B) Extraction from anaerobic sludge, (C) Densitometric measurements of extraction of full-length and truncated PrP from each sludge relative to the highest densitometric value observed. Error bars represent the standard deviation of these densitometric measurements. A.U. = absorbance units
Partitioning of PrPTSE between Activated Sludge Solids and Liquid
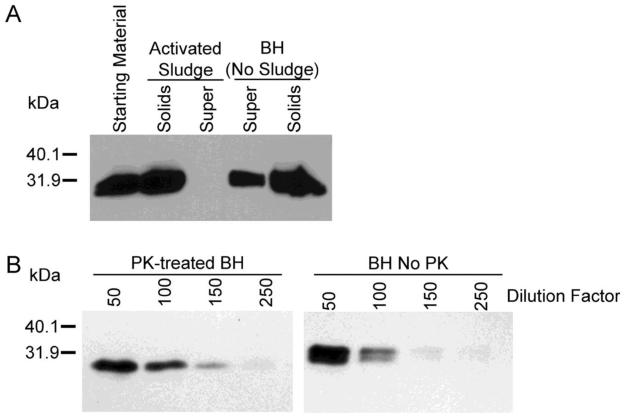
As an initial step toward understanding the fate of prions if they were to enter a WWT facility, we examined the partitioning of PrPTSE between activated sludge solids and liquid. To the limits of immunoblotting detection, PrPTSE partitioned to the gravity-settled activated sludge solids (Figure 2A). Based on the detection limits for our immunoblots (Figure 2B), >99% of the PrPTSE is expected to partition to activated sludge floc during contact with the sludge. Thus, a large fraction of PrPTSE entering an activated sludge system would be removed from suspension and routed to anaerobic sludge digestion.
FIGURE 2.
(A) Partitioning of PrPTSE between supernatant and activated sludge solids. (B) Immunoblot sensitivity. PrPTSE was semiquantitatively assessed by serial dilution to the limit of immunoblotting detection. The dilution at which no detectable immunoreactivity remained provided a basis for comparison with samples lacking immunoreactivity before dilution. Abbreviations: PK, proteinase K; Solids, settled activated sludge solids; Sup, supernatant (PrPTSE not associated with settled activated sludge solids).
Oral bioassay of the supernatant and settled solids fractions from the simulated contact with activated sludge demonstrated that all detectable infectivity was associated with the sludge solids (Table 1). Disease penetrance and incubation period (measures of prion infectivity in bioassay) were approximately the same for the sludge solids and the positive (equal volume of 0.1% w/v infected brain homogenate) controls. These data suggest that, under the secondary clarification conditions simulated, that prion infectivity partitioned in a similar manner as did PrPTSE and that infectivity was not diminished by contact with settled activated sludge solids. We present these data with the caveat that the amount of infectivity measured in the settled activated sludge solids and in the control were near the limit of detection for oral bioassay. Based on the immunoblotting and infectivity assay results, we focused the subsequent investigations on prion survival during downstream biosolids processing.
TABLE 1.
Partitioning of Oral Infectivity Following Settling of Activated Sludgea
| inoculum | positive animals/ total animals |
onset of clinical symptoms (dpi)b |
|---|---|---|
| HY BH (0.1% weight/volume) | 4/8 | 150, 185, 192, 209 |
| Supernatant from activated sludge spiked with 0.1% HY BH |
0/8 | not applicable |
| Settled fraction from activated sludge spiked with 0.1% HY BH |
6/8 | 192, 192, 209, 209, 223, 353 |
Abbreviations: BH, brain homogenate; dpi, days post inoculation; HY, Hyper strain of hamster-adapted transmissible mink encephalopathy.
Animals showing no clinical signs of TSE infection were sacrificed 435 dpi.
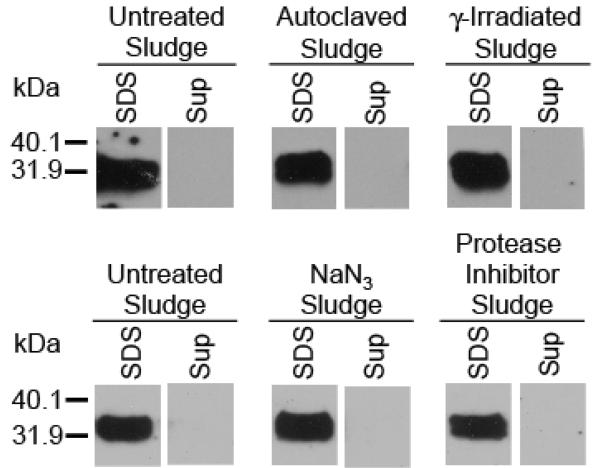
Partitioning experiments conducted with the anaerobic digestor sludge gave similar results as the aerobic sludge, with all detectable PrPTSE partitioning to the solid portion (Figure 3). None of the physical and chemical treatments employed to manipulate the sludge had any detectable effect on protein partitioning.
FIGURE 3.
Extractability and partitioning of PrPTSE in un-manipulated and manipulated anaerobic digester sludges. Abbreviations: SDS, extraction from settled anaerobic digester solids with 1% SDS; Sup, supernatant fraction from settled anaerobic digester solids.
Survival of PrPTSE during Simulated Mesophilic Anaerobic Digestion
We examined the survival of PrPTSE through a WWT facility using a laboratory model designed to simulate the secondary treatment and sludge solids handling processes used at the Nine Springs WWT facility (Figure 4A).
FIGURE 4.
(A) Flow chart of simulated mesophilic anaerobic sludge experiments. (B) PrPTSE recovery after 0-20 day incubations with un-manipulated and manipulated anaerobic digester sludge. Day indicates the time infected brain homogenate was exposed to anaerobic sludge under mesophilic conditions (i.e., 37°C).
BRIEF: The disease-associated form of the prion protein partitions to activated sludge solids and survives simulated mesophilic anaerobic sludge digestion.
A significant fraction of PrPTSE survived both 10-d and 20-d anaerobic sludge digestion (Figure 4B). No loss of PrPTSE was noted during the 7-h simulated activated sludge incubation (Figure 2A) and, as expected, PrPTSE partitioned primarily to the solids. Recovery of PrPTSE from anaerobic digester sludge was not complete at t = 0 (Figure 4B), suggesting that some PrPTSE was associated with the solids in a non-extractable state. Although we observed a substantial decline in detectable PrPTSE over the entire 20-d simulated anaerobic sludge digestion (Figure 4B), significant levels of PrPTSE were still present. Two processes could have contributed to the decline in detectable PrPTSE during the duration of the anaerobic digestion: (1) changes in the interaction between PrPTSE and the sludge solids and (2) microbial degradation. Declines in extractability could be due to the strengthening of interactions between PrPTSE molecules and anaerobic digester sludge solids during the 20 d. If the decline was due to degradation, our results suggest that although anaerobic digester sludge flora can degrade PrPTSE,, microbial activity was insufficient to completely degrade PrPTSE within 20 d. This may reflect degradation of more labile PrPTSE and the persistence of more recalcitrant PrPTSE (29). Any PrPC in the brain homogenate would be expected to have been degraded during the 7-h activated sludge digestion.
Effect of Sludge Inactivation
To obtain insight into whether the observed decrease in detectable PrP was the result of microbially mediated degradation or physico-chemical processes influencing the ability to extract and/or detect the protein, we altered microbial activity in the anaerobic digester sludge by γ-irradiation, protease inhibition, autoclaving, and poisoning with NaN3. γ-Irradiation destroys nucleic acids while leaving the cells intact and appears to be the least intrusive means to sterilize complex environmental samples (20). This method is not expected to inactivate extracellular proteases. We, therefore, added a cocktail of protease inhibitors to a second set of anaerobic digester sludge samples. In a previous study, Kirchmayer et al. (17) attempted to recover PrPTSE from anaerobic digester sludge inactivated by either steam sterilization (121°C) or poisoned with 1% NaN3. Inexplicably, PrPTSE degradation appeared to increase after these treatments (17). We, therefore, examined both autoclaved and NaN3-poisoned anaerobic digester sludge to compare our findings with those of Kirchmayer et al. (17). The inactivation methods employed did not substantially alter PrPTSE extractability by 1% SDS (Figure 3).
Incubation of PrPTSE in anaerobic sludge inactivated by techniques designed to reduce the count of living cells (viz. γ-irradiation and autoclaving) appears to increase the amount of PrPTSE recovered after 10 and 20 d compared to untreated sludge (Figure 4B). This increase in signal suggests that the γ-irradiation and autoclaving either inactivated bacteria responsible for degradation of PrPTSE or inhibited processes resulting in the uptake of protein, thus increasing extractability. Azide and protease inhibitor treatment of the sludge had little effect on the amount of PrPTSE recovered compared to untreated sludge. We note that most anaerobic digester sludge microorganisms are obligate anaerobes (30). Sodium azide is not effective in inhibiting the respiration of strict anaerobes, and may actually improve their growth conditions by eliminating competition from any aerobes present; NaN3 has been used as a selective agent for anaerobes (31). This may explain the increased loss of PrPTSE in NaN3-treated anaerobic sludge previously reported (17).
Implications for Management of Prion-contaminated Influent
Our results indicate that most PrPTSE would pass through WWT facilities employing activated sludge treatment followed by mesophilic anaerobic sludge digestion. Prions would most likely be confined to biosolids and not discharged into local bodies of water, provided that the secondary clarifier effectively removes suspended solids. Contamination of treated biosolids represents the primary risk associated with entry of prions into municipal WWT facilities. While PrPTSE degradation was observed in our simulated anaerobic sludge digestion, a significant amount of detectable PrPTSE survived this treatment. Our conclusions are based on immunochemical measurements of PrPTSE and PrPTSE-to-sludge ratios far exceeding those expected to occur in a WWT facility. The time-dependent decline in PrPTSE extractability from anaerobic digester sludge solids may reduce the oral transmissibility of biosolids-associated prions. Further studies using additional animal bioassays are warranted to assess retention of infectivity of biosolids-associated prions.
While this study was motivated by the need to understand the fate of CWD and BSE agents in WWT systems, reports of urinary prion excretion may raise concern about the introduction of human prions into WWT facilities. We note that some reports of urinary excretion of PrPTSE by CJD patients and TSE-infected animals (32) have not been substantiated by others (33), and may have been artifactual (34). However, urinary prion excretion at levels typically ≤ 0.5 intracerebral infectious units (IU50)·mLurine−1 has been demonstrated in scrapie-infected mice that have concurrent kidney infection (35). To put the potential risk of urinary shedding of human prions by CJD patients into context, consideration of the amount of prions potentially entering WWT facilities and ultimately land applied in biosolids is instructive. The incidence of sporadic CJD is 0.5 to 1.0 case in a million (36). For this analysis, we assume that one CJD patient lives in a population served by a 150,000 m3·d−1 WWT plant (the size of the MMSD plant serving a population of ∼300,000) and excretes 2 L of urine per day containing the same prion concentration as reported for scrapie-infected rodents with concurrent nephritis (35). Because oral exposure rather than intracerebral inoculation is relevant for our analysis, we express prion infectivity in terms of oral IU50. Based on data from rodent models, oral transmission of prion disease is less efficient than intracerebral inoculation by a factor of ∼105 (37). Under this scenario, acquisition of a sufficient dose of prions to cause disease (i.e., 1 IU50) would require consumption of > 1010 Lwastewater. To calculate the amount of human prions in biosolids-amended agricultural soils, we assume that all prions entering the plant partition to activated sludge solids and survive biosolids treatment, the plant produces ∼21,000 kgbiosolids·d−1, and an application rate to agricultural fields of ∼4,000 kgbiosolids·ha−1. These latter two values are based on the performance of the Nine Springs treatment plant and the practice of MMSD. Assuming an ingestion rate of 5 gsoil·d−1 (considered a reasonable upper bound for a child with habitual pica; ref. 38) and that all soil ingested originated from a biosolids-amended field, intake over a 70-y period would not exceed 1 oral IU50, even accounting for possible enhanced transmission of soil particle-bound prions (39). These analyses lead us to conclude that any risk associated with the entry of human prions into WWT facilities is exceedingly small.
We further emphasize that, to date, prions have not been reported in wastewater influent, effluent or biosolids. Immunochemical methods (e.g., immunoblotting, enzyme-linked immunosorbent assays) lack the sensitivity needed to detect prion protein in wastewater, biosolids and other environmental media. Recent advances in prion detection (e.g., refs 40-42) may lead to methods that are sufficiently sensitive to measure prions in environmental matrices.
Acknowledgements
We thank David Taylor (MMSD) for providing the sludges used in this study. We thank Richard Rubenstein (SUNY Downstate Medical Center) for the gift of mAb 3F4. We gratefully acknowledge the constructive comments of three anonymous reviewers. This research was supported, in part, by a grants from the U.S. Environmental Protection Agency (4C-R156-NAEX) and Department of Defense (DAMD17-03-1-0369). KHJ was supported by a NIH Training Grant (NIH 5 T32 GM08349). The content of this manuscript has not been subject to agency review. Endorsement by the sponsors is not implied and should not be assumed.
Literature Cited
- 1.DeArmond SJ, Bouzamondo E. Fundamentals of prion biology and diseases. Toxicol. 2002;181-182:9–16. doi: 10.1016/s0300-483x(02)00249-4. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Novel proteinaceous infectious particles causes scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 3.Silveria JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prusiner SB, Scott M, Foster D, Pan KM, Groth D, Mirenda C, Torchia M, Yang SL, Serban D, Carlson GA. Transgenic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 5.Caughey B, Raymond GL. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 1991;266:18217–18223. [PubMed] [Google Scholar]
- 6.Pan KM, Baldwin M, Nguyen J, Gasset M, Serban D, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, Prusiner SB. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prusiner SB. Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochem. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DM. Inactivation of transmissible degenerative encephalopathy agents: a review. Vet. J. 2000;159:10–17. doi: 10.1053/tvjl.1999.0406. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin MA. Mass spectrometric analysis of prion proteins. In: Caughey B, editor. Advances in Protein Chemistry, Volume 57, Prion Proteins. Academic Press; San Diego, CA: 2001. pp. 29–54. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen JA, McMahon KD, Benson CH. Prions: Novel pathogens of environmental concern? J. Environ. Eng. 2006;132:967–969. [Google Scholar]
- 12.Gale P, Stanfield G. Towards a quantitative risk assessment for BSE in sewage sludge. J. Appl. Microbiol. 2001;91:563–569. doi: 10.1046/j.1365-2672.2001.01466.x. [DOI] [PubMed] [Google Scholar]
- 13.Gale P, Young C, Stanfield G, Oakes D. Development of a risk assesment for BSE in the aquatic environment. J. App. Microbiol. 1998;84:467–477. doi: 10.1046/j.1365-2672.1998.00495.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Kobayashi S, Nishiguchi A, Nonaka T, Tsutsui T. Evaluation of bovine spongiform encephalopathy (BSE) infection risk of cattle via sewage sludge from wastewater treatment facilities in slaughterhouses in Japan. J. Vet. Med. Sci. 2006;68:137–142. doi: 10.1292/jvms.68.137. [DOI] [PubMed] [Google Scholar]
- 15.Scherebel C, Pichner R, Groschup MH, Müller-Hellwig S, Scherer S, Dietrich R, Märtlbauer E, Gareis M. Degradation of scrapie associated prion protein (PrPSc) by the gastrointestinal microbiota of cattle. Vet. Res. 2006;37:695–703. doi: 10.1051/vetres:2006024. [DOI] [PubMed] [Google Scholar]
- 16.Muller-Hellwig S, Groschup MH, Pichner R, Gareis M, Martlbauer E, Scherer S, Loessner MJ. Biochemical evidence for the proteolytic degradation of infectious prion protein PrPSc in hamster brain homogenates by foodborne bacteria. Sys. App. Microbio. 2006;29:165–171. doi: 10.1016/j.syapm.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Krichmayr R, Reichl HE, Schildorfer H, Braun R, Somerville RA. Prion protein: detection in ‘spiked’ anaerobic sludge and degradation experiments under anaerobic conditions. Wat. Sci. Tech. 2006;53:91–98. doi: 10.2166/wst.2006.239. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathogens. 2006;2:296–302. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman GJ. Sterilization and preservation by ionizing radiation. In: Block SS, editor. Disinfection Sterilization and Preservation. 4th ed. Lea and Febiger; Philadelphia, Pa.: 1991. pp. 556–579. [Google Scholar]
- 20.Wolf DC, Dao TH, Scott HD, Lavy TL. Influence of sterilization methods on selected soil microbiological, physical, and chemical properties. J. Envir. Qual. 1989;18:39–44. [Google Scholar]
- 21.Gautam S, Shah MR, Sabharwal S, Sharma A. Gamma irradiation of municipal sludge for safe disposal and agricultural use. Water Environ. Res. 2005;77:472–479. doi: 10.2175/106143005x67386. [DOI] [PubMed] [Google Scholar]
- 22.Lichstein HC. Studies of the effect of sodium azide on microbic growth and respiration: III. The effect of sodium azide on the gas metabolism of B. subtilis and P. aeruginosa and the influence of pyocyanine on the gas exchange of a pyocyanine-free strain of P. aeroginosa in the presence of sodium azide. J. Bact. 1944;47:239–251. doi: 10.1128/jb.47.3.239-251.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichstein HC, Soule MH. Studies of the effect of sodium azide on microbic growth and respiration: I. The action of sodium azide on microbic growth. J. Bact. 1944;47:221–230. doi: 10.1128/jb.47.3.221-230.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichstein HC, Soule MH. Studies of the effect of sodium azide on microbic growth and respiration: II. The action of sodium azide on bacterial catalase. J. Bact. 1944;47:231–238. doi: 10.1128/jb.47.3.231-238.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bessen RA, Marsh RF. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J. Gen. Virol. 1992;73:329–334. doi: 10.1099/0022-1317-73-2-329. [DOI] [PubMed] [Google Scholar]
- 26.Lerch RN, Barabarick KA, Azari P, Sommers LE, Westfall DG. Sewage sludge proteins: I. Extraction methodology. J. Envir. Qual. 1993;22:620–624. [Google Scholar]
- 27.Bolton DC, Bendheim PE, Marmorstein AD, Potempska A. Isolation and structural studies of the intact scrapie agent protein. Arch. Biochem. Biophys. 1987;258:579–590. doi: 10.1016/0003-9861(87)90380-8. [DOI] [PubMed] [Google Scholar]
- 28.Diringer H, Hilmert H, Simon D, Werner E, Ehlers B. Towards purification of the scrapie agent. Eur. J. Biochem. 1983;134:555–560. doi: 10.1111/j.1432-1033.1983.tb07602.x. [DOI] [PubMed] [Google Scholar]
- 29.Pastrana MA, Sajnani G, Onisko B, Castilla J, Morales R, Soto C, Requena JR. Isolation and characterization of a proteinase K-sensitive PrPSc fraction. Biochem. 2006;45:15710–15717. doi: 10.1021/bi0615442. [DOI] [PubMed] [Google Scholar]
- 30.Raskin L, Zheng D, Griffin ME, Stroot PG, Misra P. Characterization of microbial communities in anaerobic bioreactors using molecular probes. Antonie van Leeuwenhoek. 1995;68:297–308. doi: 10.1007/BF00874140. [DOI] [PubMed] [Google Scholar]
- 31.Forget A, Fredette V. Sodium azide selective medium for the primary isolation of anaerobic bacteria. J. Bact. 1962;89:1217–1223. doi: 10.1128/jb.83.6.1217-1223.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaked GM, Shaked Y, Kariv-Inbal Z, Halimi M, Avraham I, Gabizon R. A protease-resistant prion protein isoform is present in urine of animals and humans affected with prion diseases. J. Biol. Chem. 2001;276:31479–31482. doi: 10.1074/jbc.C100278200. [DOI] [PubMed] [Google Scholar]
- 33.Head MW, Kouverianou E, Taylor L, Green A, Knight R. Evaluation of urinary PrPSc as a diagnostic test for sporadic, variant, and familial CJD. Neurology. 2005;64:1794. doi: 10.1212/01.WNL.0000161842.68793.8A. [DOI] [PubMed] [Google Scholar]
- 34.Furukawa H, Doh-Ura K, Okuwaki R, Shirabe S, Yamamoto K, Udono H, Ito T, Katamine S, Niwa M. A pitfall in diagnosis of human prion diseases using detection of protease-resistant prion protein in urine - Contamination with bacterial outer membrane proteins. J. Biol. Chem. 2004;279:23661–23667. doi: 10.1074/jbc.M400187200. [DOI] [PubMed] [Google Scholar]
- 35.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Burkhardt S, Miele G, Aguzzi A. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen NS, Smith E. Prion diseases: Epidemiology in man. APMIS. 2002;110:14–22. doi: 10.1034/j.1600-0463.2002.100103.x. [DOI] [PubMed] [Google Scholar]
- 37.Kimberlin RH, Walker CA. Pathogenesis of scrapie in mice after intragastric infection. Virus. Res. 1989;12:213–220. doi: 10.1016/0168-1702(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 38.LaGoy PK. Estimated soil ingestion rates for use in risk assessment. Risk Analysis. 1987;7:355–359. doi: 10.1111/j.1539-6924.1987.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 39.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathogens. 2007;3:874–881. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onisko B, Dynin I, Requena JR, Silva CJ, Erickson M, Carter JM. Mass spectrometric detection of attomole amounts of the prion protein by nanoLC-MS-MS. J. Am. Soc. Mass Spectrom. 2007;18:1070–1079. doi: 10.1016/j.jasms.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Saá P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 42.Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, Onwubiko HA, Priola SA, Caughey B. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nature Methods. 2008;5:211–212. doi: 10.1038/nmeth0308-211. [DOI] [PubMed] [Google Scholar]