Abstract
Combined with medium-pressure liquid chromatography (MPLC) and preparative high-pressure liquid chromatography (Prep-HPLC), high-speed countercurrent chromatography (HSCCC) was successfully applied for separation and purification of isoflavonoids from the extract of belamcanda. HSCCC separation was performed on a two-phase solvent system composed of methyl tert-butyl ether -ethyl acetate - n-butyl alcohol – acetonitrile −0.1% aqueous trifluoroacetic acid at a volume radio of 1:2:1:1:5. Semi-purified peak fractions from HSCCC separation were further purified by Prep-HPLC. Nine well-separated fractions were analyzed by HPLC-UV absorption spectrometry to determine their purities and characterized with ESI-MSn. Except for peaksland VII (unknown) seven compounds were identified as apocynin (peak II), mangiferin (peak III), 7-O-methylmangiferin (peak IV), hispidulin (peak V), 3′-hydroxyltectoridin (peak VI), iristectorin B (peak VII), isoiridin (peak IX).
Keywords: high-speed countercurrent chromatography, preparative high-pressure liquid chromatography, medium-pressure liquid chromatography, Belamcanda, isoflavonoids
INTRODUCTION
Belamcanda is a perennial shrub growing on the hill sides in the East Asia including the Korean peninsula and has been used as Chinese traditional medicine for treating throat ailment such as asthma and tonsillitis. From this plant a number of flavonoids have already been isolated [1], and the flavonoids isolated from Belamcanda chinensis showed strong anti-inflammatory effect.[2].
High-speed countercurrent chromatography (HSCCC) is a form of liquid–liquid partition chromatography without solid support[3], and the separation is based on partitioning solutes between the two immiscible liquid phases: the mobile phase and the support-free liquid stationary phase. Without solid matrix, the stationary phase is retained in the column with the aid of a centrifugal force, so that the method eliminates irreversible adsorption of samples onto the solid support. Therefore, HSCCC is very suitable for separation of active components from traditional Chinese herbs and other natural products[4–6].
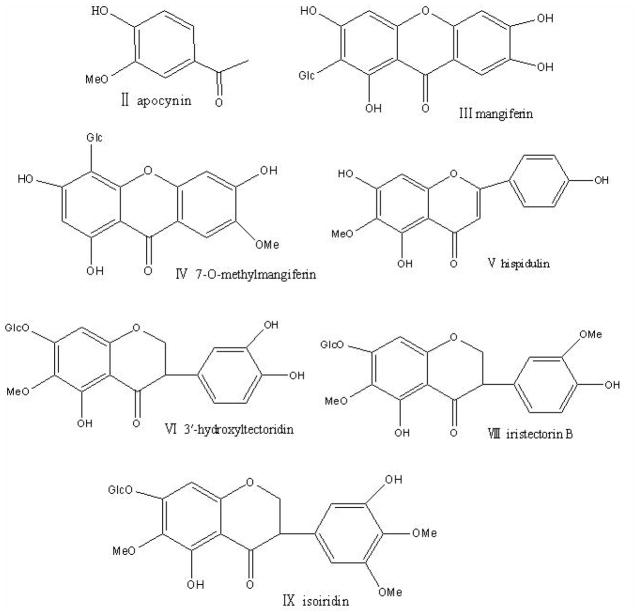
Preparative high-pressure liquid chromatography (Prep-HPLC) is a powerful tool with its high partition efficiency and good sample recovery. In this paper, combined with MPLC and Perp-HPLC, HSCCC was employed for isolation and purification of seven components including apocynin, mangiferin, 7-O-methylmangiferin, hispidulin, 3′-hydroxyltectoridin, iristectorin B, and isoiridin from belamcanda, and their structures (Fig. 1) were identified by ESI-MSn.
Figure 1.
Chemical structures of target compounds from Belamcanda
EXPERIMENTAL
Apparatus
The HSCCC instrument employed in present study was GS10A high-speed countercurrent chromatograph (Beijing Institute of Technology Application, Beijing, China) with multilayer coil separation columns connected in series (I.D.. of the tubing = 1.6 mm, total volume = 230 mL) and a 20 mL sample loop. The β value of the preparative column varied from 0.5 at the internal terminal to 0.7 at the external terminal (β = r/R, where r is the rotation radius or the distance from the coil to the holder shaft, and R is the revolution radius or the distances between the holder axis and central axis of the centrifuge). The revolution speed of the apparatus can be regulated with a speed controller in the range between 0 and 1000rpm. The system was also equipped with one S-1007 constant flow pump, a Model 8823A-UV monitor operating at 254 nm, a Yakogawa 3057 recorder.
The MPLC equipment was an EZ purifier equipped with a dual λ UV absorbance detector (Shanghai LiSui E-Tech Co. Ltd., Shanghai). The analytical HPLC equipment was a Shimadzu LC-10ATvp consisted with a Shimadzu SPD-M10Avp UV detector, a sample injection valve (Model 7726) with a 20 mL loop, a system controller (SCL-10AVP) and a Shimadzu LC Solution Workstation (Shimadzu, Kyoto, Japan). The column applied in this work was a Diamonsil C18 column (250 mm × 4.6 mm, i.d., 5 μm, Dikma Technologies China). The preparative HPLC equipment was waters 600 equipped with a waters 2487 dual λ absorbance detector (waters, USA). The column applied in this work was an YMC-Pack ODS-A column (250 mm × 10 mm, i.d., 5 μm, YMC Co. Ltd., Japan). ESI-MSnwas carried out with an LCQ Advantage (Thermo Finnigan, USA) and the NMR equipment was Varian NMR 400 system(Varian, USA).
Reagents
All organic solvents used for HSCCC were of analytical grade (Tianjing Damao Reagent Factory, Tianjing, China). Methanol used for HPLC analysis was of chromatographic grade (DIKMA filiale in Guangzhou, China). AB-8 macroporous resin for MPLC separation was purchased from Tianjin University.
Belamcanda was purchased from Guangzhou Traditional Chinese Medicine Company, Guangzhou, China.
Preparation of Crude Sample
Belamcandae obtained from a local drug store in Guangzhou was dried at a constant temperature of 60°C for 6 hours, then ground to powder (about 30-mesh) with a disintegrator. The powder (300 g) was extracted with 1 L of 80% ethanol at 85°C for 3 hours(four times). The filtrate was pooled and condensed by evaporating the solvent under reduced pressure. The dried extract was dissolved in water (0.5 L) and water-insoluble matter was discarded. Then it was extracted in turn by petroleum ether (boiling point: 60 °C −90 °C), ethyl acetate and 1-butanol. The 1-butanol extract was evaporated to dryness and stored in a refrigerator at 4C° for further purification by HSCCC.
MPLC Separation
AB-8 macroporous resin was soaked with 95% ethanol for 24 h and subsequently rinsed with pure water thoroughly until no spirituous odor is present followed by loading into the chromatographic column (three plastic columns of 180 mm×30 mm connected in series) prepared by wet-packed method. The total column volume(CV) was 380 mL. Then the column was washed in turn with twice column volume of 2% sodium hydroxide aqueous solution, water, twice column volume of 5% hydrochloric acid and water. Each time the column was rinsed by water until the effluent becomes neutral.
The 1-butanol extract was subjected to MPLC. First, the column was washed by water until the effluent liquid became colorless. Then, it was eluted in turn by 10%, 15% and 20% industrial alcohol aqueous solution, and the effluent liquid was analyzed by HPLC. The analyses were performed with a Diamonsil C18 column where methanol-water was used as the mobile phase at a flow rate of 1.0 mL·min−1 in a gradient mode (methanol-water, 5%–100%, 60min). Each fraction of the industrial alcohol aqueous solution elute was evaporated to dryness, and stored in a refrigerator at 4°C for further purification.
HSCCC Separation
In this work, the 10%, 15% and 20% industrial alcohol aqueous solution eluates were separated by HSCCC with the same two-phase solvent system composed of methyl tert-butyl ether - ethyl acetate - n-butyl alcohol – acetonitrile −0.1% aqueous trifluoroacetic acid at a volume radio of 1:2:1:1:5. The solvent system was prepared by adding the solvent mixture to a separatory funnel according to their volume ratios and thoroughly equilibrated by repetitive vigorous shaking, and the two phases were separated shortly before use. The sample solution was prepared by dissolving the crude extract in a mixture of each phase (1:1, v/v) of the solvent system used for HSCCC separation.
In each separation the multilayer coiled column was first entirely filled with the upper phase as a stationary phase. Then the apparatus was rotated at 800 rpm. The lower phase was then pumped into the head end of the column inlet at a flow-rate of 1.8 mL·min−1. After the hydrodynamic equilibrium was reached throughout the column, the sample solution (crude extract dissolved in 2 mL of a 1:1 mixture of each phase of the solvent system was injected through the sample port. The effluent from the tail end of the column was continuously monitored with a UV detector at the wavelength of 254 nm. Each peak fraction was manually collected according to the elution profile and analyzed by HPLC.
Preparative HPLC Separation
The results of the HSCCC separation were unsatisfactory and most of the peak fractions contained a large amount of impurities, such as peak fractions 2 and 4 from HSCCC separation of the 15% industrial alcohol aqueous solution eluate. These peak fractions were further purified by preparative HPLC. The purification was performed with an YMC-Pack ODS-A column as follows: methanol −0.1 % acetic acid water was used as the mobile phase in an isocratic mode at a flow rate of 5.0 mL·min−1.
HPLC, ESI-MSn Analysis and Identification of Target Compounds
Each target compound was analyzed by HPLC. The analyses were performed with a Diamonsil C18 column as follows: methanol −0.1 % acetic acid in water was used as the mobile phase in gradient mode. The flow-rate of the mobile phase was 1.0 mL·min−1. And the effluent was monitored by a dual-wavelength absorbance detector.
Identification of target compounds was carried out by ESI-MSn. ESI-MSn was carried out using a Finingan LCQ Deca ion trap mass XP MAX spectrometer equipped with an electrospray ionization source (Thermo Finnigan, San Jose, CA, USA).
RESULTS AND DISCUSSION
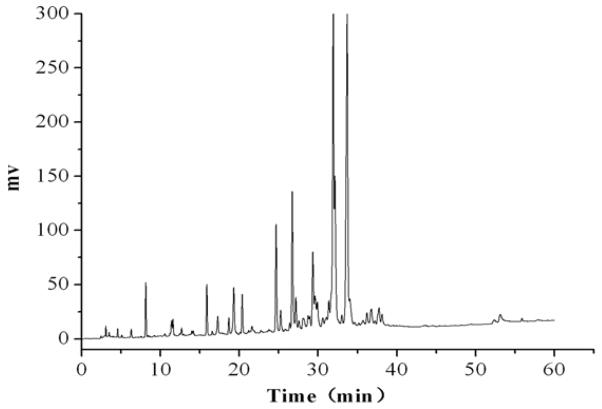
HPLC Chromatogram of 1-Butanol Extract
The crude 1-butanol extract was analyzed by HPLC with a mobile phase composed of methanol-0.1% acetic acid water (gradient elution, methanol, 5%–100%, 60 min). The chromatograms were shown in Fig. 2. From 8 to 40 min of the retention time many peaks were eluted. This indicates that the 1-butanol extract is a highly complex system, and a good result can not be expected by HSCCC separation unless it is prepurified by MPLC before subjected to HSCCC separation.
Figure 2.
HPLC chromatogram of the crude extract of Belamcanda by 1-butanol. Mobile phase: methanol-0.1% acetic acid water, gradient elution, (methanol, 5%–100%, 60 min), flow rate: 1.0 mL·min 1; detection wavelength: 254 nm.
Optimization of MPLC Conditions
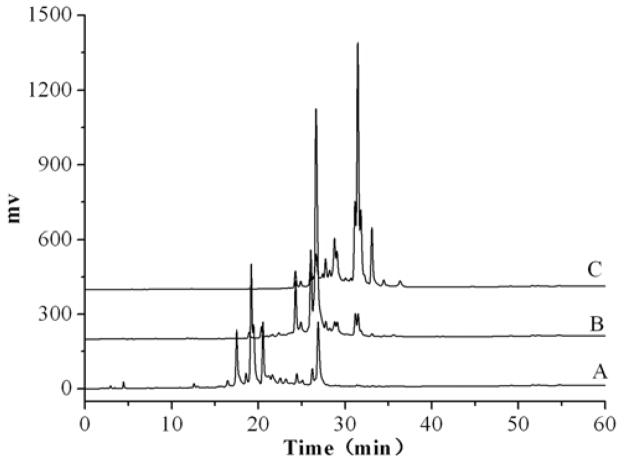
Ethanol-water solution was used as an eluent for AB-8 macroporous resin. Initially, the condition of MPLC was set as industrial alcohol-0.1% acetic acid water (industrial alcohol, 10%, 30%, 50%, 70%, 90%, 35min respectively). The result of HPLC analysis showed that the substances were mostly eluted before the elution with 30% industrial alcohol. Then, a good result was obtained in the condition of industrial alcohol-0.1% acetic acid water (industrial alcohol, 10%, 15%, 20%, 35min, respectively). The HPLC analysis result was shown in Fig. 3.
Figure 3.
HPLC chromatograms of the eluate by industrial alcohol aqueous solution. A, B and C were the 10%, 15%, 20% industrial alcohol aqueous solution eluate, respectively. Mobile phase: methanol-water (methanol, 5%–100%, 60 min), flow rate: 1.0 mL·min 1; detection wavelength: 254 nm.
From the Fig. 3, we can find that the retention time of the different concentrated industrial alcohol eluate was concentrated in different time period, i.e., 17 min to 23 min (the 10% industrial alcohol elute), 25 min to 30 min (the 15% industrial alcohol elute), 25 min to 30 min (the 20% industrial alcohol elute). The results of the HPLC analysis indicated that the pre-fractionation of the crude extract with MPLC was successful.
Optimization of HPLC Conditions
Several elution systems were tested in HPLC separation of the 10%, 15% and 20% ethanol-water eluates such as methanol–water, methanol–0.05% aqueous phosphoric acid, isocratic elution and gradient elution of methanol-0.1% aqueous acetic acid. When methanol-0.1% aqueous acetic acid was used as the mobile phase, a good result was obtained. The optimized HPLC mobile phase conditions of the 10%, 15% and 20% industrial alcohol elute were respectively as follows:
10%: methanol-0.1% acetic acid water (isocratic elution, methanol, 13%);
15%: methanol-0.1% acetic acid water (gradient elution, methanol, 30%–37%, 30min);
20%: methanol-0.1% acetic acid water (gradient elution, methanol, 30%–40%, 45 min).
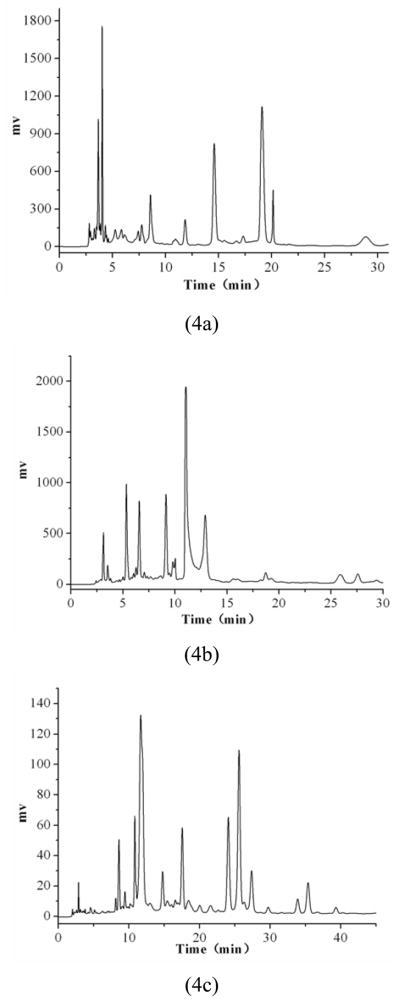
The chromatograms obtained using the above mobile phases are shown in Fig. 4..
Figure 4.
HPLC chromatograms (a): the 10% industrial alcohol aqueous solution eluate. Mobile phase: methanol-0.1% acetic acid water, isocratic elution, (methanol, 13%, 30 min): (b) the 15% industrial alcohol aqueous solution elute. Mobile phase: methanol-0.1% acetic acid water, gradient elution, (methanol, 30%–37%, 30 min); (c): the 20% industrial alcohol aqueous solution eluate. Mobile phase: methanol-0.1% acetic acid water, gradient elution, (methanol, 30%–40%, 45 min); flow rate: 1.0 mL·min 1; detection wavelength: 254 nm.
Selection of Two-Phase Solvent System of HSCCC
In an HSCCC experiment, selection of the two-phase solvent system is the first and most critical step. A solvent system that can provide an ideal partition coefficient (K) for the target compounds should be selected. For the colored sample, the two-phase solvent system which provide a suitable K values can be easily selected by comparing the color density between the upper and lower phases. In this work, many kinds of two-phase solvent system were examined, but the color of upper phase was always darker than that of the lower phase indicating that the polarity of solvent system was not high enough. Then we have tested a more polar two-phase solvent system composed of methyl tert-butyl ether - 1-butanol – acetonitrile – water (2:2:1:5)[7,8], which has been successfully used for separation of anthocyanins. Finally we have found that when the composition of the above solvent system is modified to methyl tert-butyl ether - ethyl acetate butanol- – acetonitrile −0.1% trifluoroacetic acid aqueous solution at a volume radio of 1:2:1:1:5, suitable K values for the target compounds were obtained.
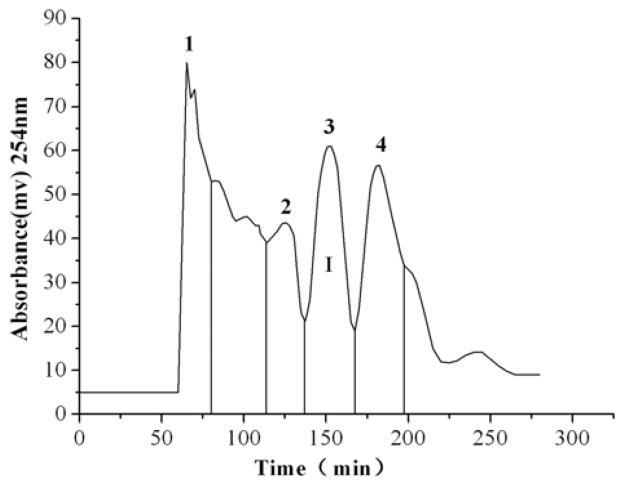
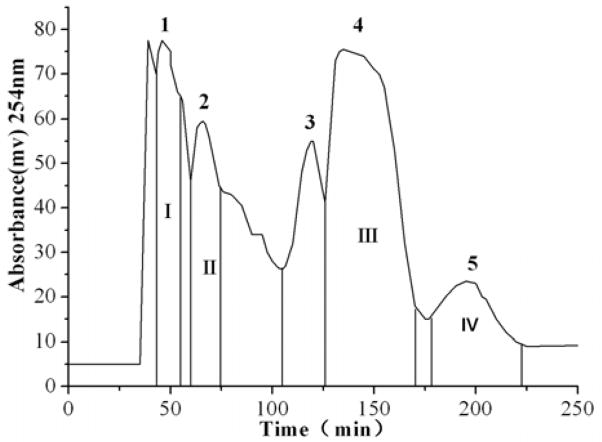
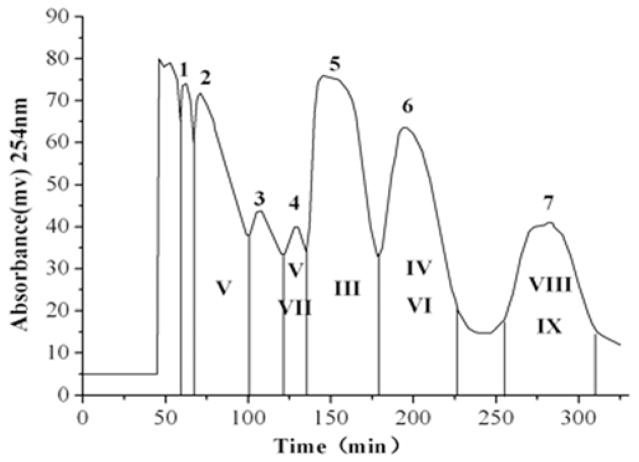
The separation of the crude extract dissolved in 2 mL of an isometric two-phase mixture was performed by eluting the mobile phase of the above two-phase solvent system at a flow rate of 1.8 mL/min under 800 rpm of revolution speed. The crude extract from Belamcanda was separated and purified under these optimum HSCCC conditions. The typical HSCCC chromatogram is shown in Fig. 5. Only peak fraction 3 from the 10% and 15% industrial alcohol aqueous solution elute HSCCC separation were pure. HSCCC peak fraction 3 from the 10% industrial alcohol aqueous solution eluate separation was an unknown compound while peak fraction 1 from the 15% industrial alcohol aqueous solution eluate was apocynin.
Figure 5.
HSCCC chromatograms (a): the 10% industrial alcohol aqueous solution eluate; (b) the 15% industrial alcohol aqueous solution eluate; (c): the 20% industrial alcohol aqueous solution eluate; Solvent system: methyl tert-butyl ether - ethyl acetate -1-butanol – acetonitrile – water (0.1% trifluoroacetic acid aqueous solution) at a volume ratio of 1:2:1:1:5. Mobile phase: the lower phase; flow rate: 1.8 mL/min; revolution speed 800 rpm; detection wavelength: 254 nm; sample size: crude sample dissolved in 2 mL of mixture solution of lower phase and upper phase (1:1, v/v) of the solvent system.
Optimization of Prep-HPLC Conditions
In order to obtain pure target compounds, the peak fractions from HSCCC separation were subjected to prep-HPLC
The prep-HPLC mobile phase conditions of the peak fractions 1, 2 and 4 from HSCCC separation of the 15% industrial alcohol aqueous solution eluate were respectivelyas follows;
methanol-0.1% acetic acid water (isocratic elution, methanol, 40%, 18min);
methanol-0.1% acetic acid water (isocratic elution, methanol, 20%, 20min);
methanol-0.1% acetic acid water (isocratic elution, methanol, 30%, 20min).
The prep-HPLC mobile phase conditions of the peak fraction 2, 4, 6 and 7 from HSCCC separation of the 20% industrial alcohol aqueous solution elute were respectivelyas follows:
methanol-0.1% acetic acid water (isocratic elution, methanol, 32%, 20min);
methanol-0.1% acetic acid water (isocratic elution, methanol, 40%, 20min);
methanol-0.1% acetic acid water (isocratic elution, methanol, 38%, 20min);
methanol-0.1% acetic acid water (isocratic elution, methanol,40%, 20min);
The chromatograms obtained from the above mobile phases are shown in Fig 6.
Figure 6.
Preparative HPLC chromatograms. (a), (b) and (c): the peak fractions 2, 1 and 4 from the 15% industrial alcohol aqueous solution elute HSCCC separation; (d), (e), (f) and (g): the peak fractions 2, 4, 6 and 7 from the 20% industrial alcohol aqueous solution eluate HSCCC separation; Mobile phase: methanol-0.1% acetic acid water, isocratic elution, elution condition: (a): methanol, 20%, 20 min; (b): methanol, 40%, 18 min; (c): methanol, 30%, 20 min; (d): methanol, 32%, 20 min; (e): methanol, 40%, 20 min; (f): methanol, 38%, 20 min; (g): methanol, 40%, 20 min; flow rate: 5.0 mL·min 1; detection wavelength: 254 nm.
Analysis of Preparative HPLC Separations
Combined with prep-HPLC, nine compounds were obtained from the MPLC fractions by HSCCC. The yields and purities of these compounds were 1.2 mg at 98.36% purity (compound I: unknown), 0.8 mg at 94.26% purity (compound II: apocynin), 2.5 mg at 98.40% purity (compound III, mangifenin), 3.4 mg at purity 98.40% (compound IV: 7-O-methylmangiferin), 1.2 mg at 94.13% purity (compound V: hispidulin), 1.6 mg at 95.37% purity (compound VI: 3′-hydroxyltectoridin), at 88.93% purity (compound VII: unknown), 3.9 mg at 98.97% purity (compound VIII iristectorin B ), 2.8 mg at 98.58% purity (compound IX: isoiridin:) as analyzed by HPLC-UV. The HPLC chromatograms are shown in Fig. 7. The background of the target compounds was summarized in Table 1.
Figure 7.
HPLC chromatograms of target compounds. Peak I to Peak III was from peak fractions 2, 1 and 4 in HSCCC separation of the 15% industrial alcohol aqueous solution eluate. Mobile phase: methanol-0.1% acetic acid water, gradient elution, (methanol, 30%–37%, 30 min); Peak IV to Peak IX was from the peak fractions 4, 2, 6 and 7 in HSCCC separation of the 20% industrial alcohol aqueous solution eluate, Mobile phase: methanol-0.1% acetic acid water, gradient elution, (methanol, 30%–40%, 45 min); flow rate: 1.0 mL·min 1; detection wavelength: 254 nm.
Table 1.
The background of the target compounds
| serial number | background |
|---|---|
| Compound I | peak fraction 3 from A HSCCC separation and peak fraction 2 from B preparative HPLC separation |
| Compound II | Peak fraction 1 from B HSCCC separation |
| Compound III | Peak fraction 4 from B preparative HPLC separation, peak fraction 5 from C preparative HPLC separation |
| Compound IV | Peak fraction 5 from B preparative HPLC separation, peak fraction 6 from C preparative HPLC separation |
| Compound V | Peak fraction 2 and Peak fraction 4 from C preparative HPLC separation |
| Compound VI | peak fraction 6 from C preparative HPLC separation |
| Compound VII | peak fraction 4 from C preparative HPLC separation |
| Compound VIII | peak fraction 7 from C preparative HPLC separation |
| Compound IX | peak fraction 7 from C preparative HPLC separation |
A, B and C are the 10%, 15%, 20% industrial alcohol aqueous solution eluate, respectively.
Identification of Target Compounds
The chemical structure of each known compound was identified according to its EIS-MSn data and their possible fragmentation pathways are also illustrated for peak II, peak VI and peak IX and so on.
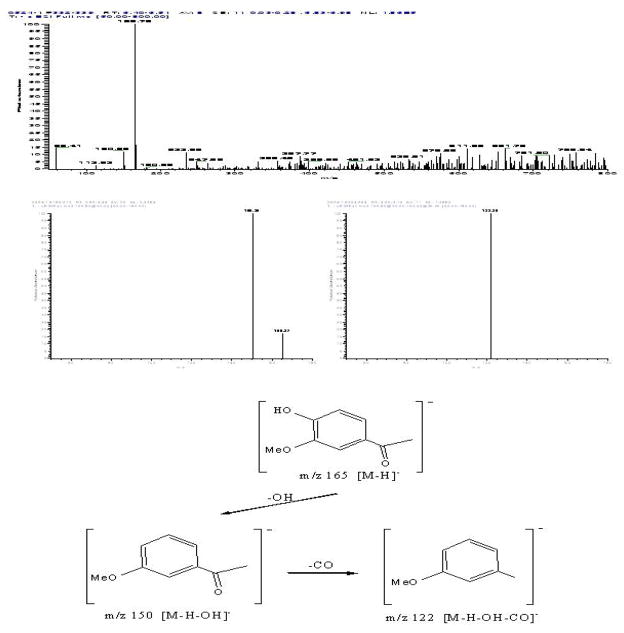
Peak II in Fig. 8: negative ESI-MS, m/z 165 [M-H]−, EIS-MS2: m/z 150, EIS-MS3: m/z 122. The mass spectrogram of Peak II showed that it was apocynin[9].
Figure 8.
The mass spectrogram and the structure analysis of the target compound II.
Peak III: negative ESI-MS, m/z 421 [M-H]−, EIS-MS2: m/z 331, 301, EIS-MS3: m/z 313, 301, 271, 259. The mass spectrogram of Peak III showed that it was mangiferin [10].
Peak IV: negative ESI-MS, m/z 435 [M-H]−, EIS-MS2: m/z 353, 345, 315. IS-MS3: m/z 300, 272. The mass spectrogram of Peak IV showed that it was 7-O-methylmangiferin [11].
Peak V: negative ESI-MS, m/z 299 [M-H]−, EIS-MS2: m/z 284, 217. EIS-MS3: m/z 256, 240, 227, 191. The mass spectrogram of Peak V showed that it was hispidulin [1].
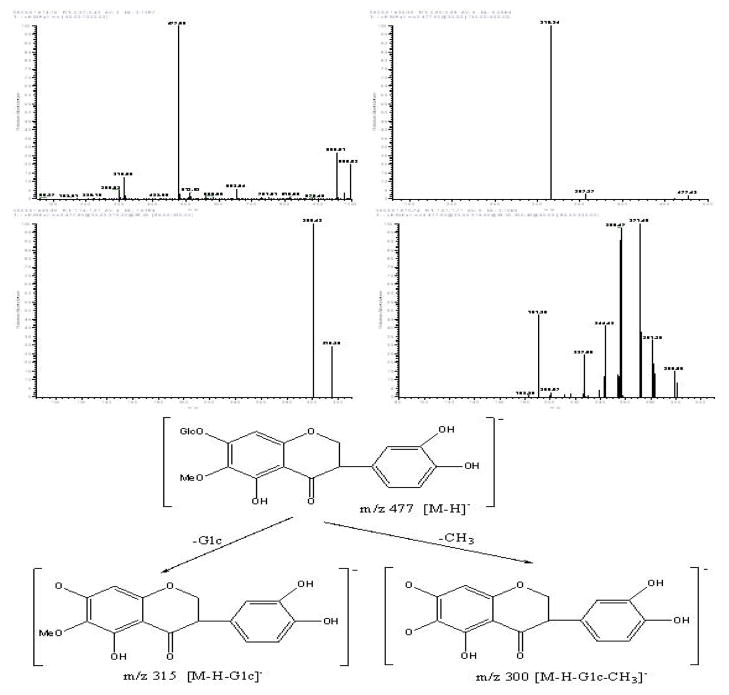
Peak VI in Fig. 9: negative ESI-MS, m/z 477 [M-H]−, EIS-MS2: m/z 315, 300. EIS-MS3: m/z 281, 271, 256, 244, 227, 191. The mass spectrogram of Peak VI showed that it was 3′-hydroxyltectoridin [12].
Figure 9.
The mass spectrograms and the structure analysis of the target compound VI.
Peak VIII: negative ESI-MS, m/z 491 [M-H]−, EIS-MS2: m/z 329, EIS-MS3: m/z 314, EIS-MS4: m/z 299, 271. The mass spectrogram of Peak VIII showed that it was iristectorin B [10].
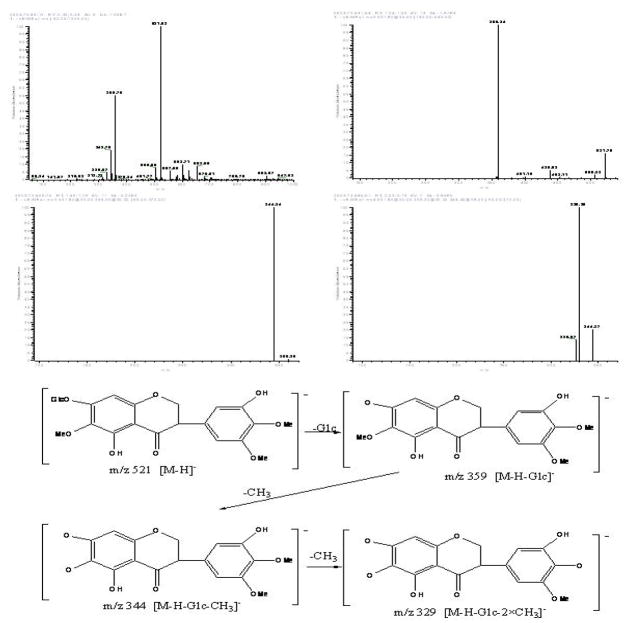
Peak IX in Fig. 10: negative ESI-MS, m/z 521 [M-H]−, EIS-MS2: m/z 359, EIS-MS3: m/z 344, EIS-MS4: m/z 329. The mass spectrogram of Peak IX showed that it was isoiridin [10].
Figure 10.
The mass spectrogram and the structure analysis of the target compound IX.
Fractions from peak I and peak VII are still unknown compounds.
CONCLUSIONS
In this paper, efficient purification of seven target compounds was achieved by combined use of MPLC separation, HSCCC and Prep- HPLC. First step of prefractionation was performed by MPLC; then the prepurified sample was fractionized by HSCCC. Although some pure substances were obtained in this step, most of the peak fractions were still impure and some fractions contained several target compounds. Finally, these partially purified fractions from HSCCC were purified by Prep-HPLC. This method would be suitable for the separation of highly complex system from natural products.
References
- 1.Ito H, Onoue S, Yoshida T. Isoflavonoids from Belamcanda chinensis. Chem Pharm Bull. 2001;49:1229–1231. doi: 10.1248/cpb.49.1229. [DOI] [PubMed] [Google Scholar]
- 2.Ahn KS, Noh EJ, Cha K, Kim YS, Lim SS, Shin KH, Jung SH. Inhibitory effects of Irigenin from the rhizomes of Belamcanda chinensis on nitric oxide and prostaglandin E2 production in murine macrophage RAW 264.7 cells. Life Sci. 2006;78:2336–2342. doi: 10.1016/j.lfs.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y. Efficient preparative counter-current chromatography with a coil plant centrifuge. J Chromatogr A. 1981;214:122–125. [Google Scholar]
- 4.Yao S, Li Y, Kong LY. Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum by high-speed counter-current chromatography. J Chromatogr A. 2006;1115:64–71. doi: 10.1016/j.chroma.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 5.Chu X, Sun AL, Liu RM. Preparative isolation and purification of five compounds from the Chinese medicinal herb Polygonum cuspidatum Sieb. et Zucc by high-speed counter-current chromatography. J Chromatogr A. 2005;1097:33–39. doi: 10.1016/j.chroma.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Cao XL, Wang C, Pei HR, Sun BG. Separation and identification of polyphenols in apple pomace by high-speed counter-current chromatography and high-performance liquid chromatography coupled with mass spectrometry. J Chromatogr A. 2009;1216:4268–4274. doi: 10.1016/j.chroma.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Degenhardt A, Knapp H, Winterhalter P. Separation and Purification of Anthocyanins by High-Speed Countercurrent Chromatography and Screening for Antioxidant Activity. 2000;48:338–343. doi: 10.1021/jf990876t. [DOI] [PubMed] [Google Scholar]
- 8.Oka H, Ito Y, Yamada S, Kagami T, Hayakawa J, Harada K, Atsumi E, Suzuki M, Suzuki M, Odani H, Akahori S, Maeda K, Nakazawa H, Ito Y. Separation of lac dye components by high-speed counter-current chromatography. J Chromatogr A. 1998;813:71–77. doi: 10.1016/s0021-9673(98)00311-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhou LX, Lin M, He LF. Studies on Chemical Constituents of the Roots of Blackberrylily(Belamcanda chinensis) Chin Tradit Herb Drugs. 1996;27(1):8–10. [Google Scholar]
- 10.Li J, Li WZM, Huang W, Cheung AWH, Bi CWC, Duan R, Guo AJY, Dong TTX, Tsim Karl WK. Quality evaluation of Rhizoma Belamcandae (Belamcanda chinensis (L.) DC.) by using high-performance liquid chromatography coupled with diode array detector and mass spectrometry. J Chromatogr A. 2009:2071–2078. doi: 10.1016/j.chroma.2008.05.082. [DOI] [PubMed] [Google Scholar]
- 11.Fujita M, Inoue T. Studies on the constituents of Iris florentina L.II. C-glycosides of xanthones and flavones from the leaves of Iris florentina L. Chem Pharm Bull. 1982;30:2342–2348. [Google Scholar]
- 12.Qiu YK, Xu BX, Gao YB, Liu K. An NMR Study of Isoflavones Extracted from Belamcanda chinensis. Chin J Magn Reson. 2006;23:443. [Google Scholar]