Abstract
Background:
To compare the efficacy and safety of intrathecal sufentanil or fentanyl as adjuvants to hyperbaric bupivacaine in patients undergoing major orthopaedic lower limb surgeries in terms of onset and duration of sensory block, motor block and post-operative pain relief.
Patients & Methods:
Ninety patients were recruited in this Prospective, randomized double blind study to receive either intrathecal sufentanil 5 μg (Group S), fentanyl 25 μg (Group F) or normal saline 0.5 ml (Group C) as adjuvants to 15 mg of 0.5% hyperbaric bupivacaine. The onset and duration of sensory and motor block were assessed intraoperatively. The pain scores were assessed postoperatively. Duration of complete and effective analgesia was recorded. The incidence of side effects such as nausea, vomiting, pruritus, shivering and PDPH was recorded.
Results:
The Demographic data, hemodynamic and respiratory parameters were comparable in the three groups. There was a significantly earlier onset and prolonged duration of sensory block in the sufentanil and fentanyl groups. The duration of complete and effective analgesia were also significantly prolonged in the fentanyl and sufentanil groups. Pruritus was noticed in the study groups (Groups S&F).
Conclusions:
Intrathecal sufentanil (5 μg) and fentanyl (25 μg), as adjuvants lead to an earlier onset and prolonged duration of sensory block. The duration of effective analgesia with intrathecal sufentanil and fentanyl as adjuvants to hyperbaric bupivacaine is longer than that of bupivacaine alone.
Keywords: Intrathecal adjuvant, Fentanyl; Sufentanil, lower limb surgeries
Acute postoperative pain is a complex physiological reaction to tissue injury, visceral distention or disease, which may result in unpleasant, unwanted sensory and emotional experiences. Various pharmacological & non-pharmacological methods have been used for providing pain-relief.
Spinal anaesthesia is a widely used regional technique for lower limb surgeries because of its ease and safety. Hyperbaric bupivacaine has been a popular choice for spinal anaesthesia. The limitations of its use include relatively short duration of the pain relief provided, necessitating early analgesic requirement in the postoperative period; and motor blockade, which may lead to urinary retention causing discomfort and delayed discharge of the patient. Intrathecally opioids act synergistically with local anaesthetics.1 They improve the quality of intraoperative anaesthesia, permit lower doses of local anesthetics, provide faster onset of surgical block and prolong the duration of postoperative analgesia.2
In the past, intrathecal morphine had been used for postoperative analgesia as it provides excellent and long lasting effect after surgical procedures. But its use is associated with unwanted effects such as nausea, vomiting, itching, urinary retention and respiratory depression.3,4
Nowadays, newer phenylpiperidine compounds like fentanyl and sufentanil are being increasingly used to provide segmental analgesia. Being highly lipid soluble and having higher affinity for opioid receptors, these drugs provide quicker onset of the block, improve the quality of intraoperative anaesthesia and prolong postoperative analgesia with fewer side effects.
Sufentanil, a mu (µ) receptor compound, provides intense and almost instantaneous analgesia lasting for 5-7 hours, thus sufficiently covering both intraoperative and early postoperative periods.5,6 We hypothesized that intrathecal fentanyl and sufentanil lead to an earlier onset and prolong the postoperative analgesia with fewer side effects. Thus, the present study was undertaken to compare the role of intrathecal sufentanil versus fentanyl as adjuvants to hyperbaric bupivacaine for orthopaedic lower limb surgeries.
PATIENTS & METHODS
This prospective double blind, randomized, controlled study was undertaken, after the approval by ethics committee and written informed consent from each patient. Ninety patients of either sex between 18 to 65 years of age belonging to ASA I and II, undergoing elective, major orthopaedic lower limb surgery were included. Patients with contraindications to subarachanoid block, chronic opioid users, severe chronic obstructive pulmonary disease {Forced expiratory volume in 1 second (FEV1) less than 600 ml}, height <150 cms or >180 cms were excluded. A routine pre-anaesthetic assessment was done and the height (cm) and weight (kg) were recorded. During pre-anaesthetic checkup, patients were familiarized with the concept of Visual Analogue Scale (VAS) for pain assessment. Pain was scored at regular intervals postoperatively using the VAS scale with VAS - 0 as no pain and 10 as worst imaginable pain. All patients were kept fasting from 10.00 pm on night before surgery and were premedicated with tablet diazepam 0.2 mg kg-1 in the night and 0.1 mg kg-1 on the morning of surgery.
In the operation theatre, monitoring for heart rate (HR), noninvasive blood pressure (NIBP), ECG, & oxygen saturation (SpO2) were initiated. Following i/v cannulation, preloading with 12 ml kg-1 of intravenous Lactated Ringer solution was done over a period of 10-15 minutes. Using a sealed envelope technique, patients were randomly allocated to one of the three groups (S,F,C) each comprising of 30 patients. Under all aseptic precautions, subarachanoid block was administered with 23G Quincke needle via midline approach in sitting position after local infiltration and intrathecal drug was injected over 10-15 seconds. Patients were placed in supine position after completion of the block. Patients received the drug intrathecally according to the group allocated:
Group S: Sufentanil 5μg (0.5ml) ± 15mg of 0.5% hyperbaric bupivacaine (3.0 ml).
Group F: Fentanyl 25μg (0.5ml) ± 15 mg of 0.5% hyperbaric bupivacaine (3.0 ml).
Group C: Normal saline (0.5 ml) ± 15 mg of 0.5% hyperbaric bupivacaine (3.0 ml).
A total volume of 3.5ml was injected intrathecally in all patients, irrespective of their height (which ranged from 150-180cms). Normal saline was used to dilute the study drug. Another anesthesiologist involved in the study prepared the test solution.
All patients received balanced salt solution at the rate of 2 ml kg-1 hr-1 as the maintenance fluid. Oxygen, at the rate of 5 l min-1 was administered by facemask. Blood loss till maximum permissible limit was replaced using crystalloids in the ratio 3:1.
The blinded observer made all the observations in the intraoperative and postoperative period.
I. Haemodynamic parameters
HR and NIBP were recorded initially, then every 2.5 minutes for 15 minutes after SAB, then at 15 minutes interval for 1 hour and then hourly for next 6 hours. Hypotension was defined as decrease in systolic blood pressure below 90 mmHg or a fall in blood pressure by more than 20% of the baseline value. It was treated with additional boluses of intravenous fluids. Intravenous increments of 3mg Mephent-ermine were administered if hypotension persisted. Brady-cardia was defined as a heart rate less than 60 beats per minute and was treated with 0.6mg of intravenous atropine.
II. Respiratory parameters
RR and SPO2 were recorded initially, then every 5 minutes for 15 minutes following SAB and then every 15 minutes for next 2 hours. Decrease in respiratory rate (RR) to less than 9 per minute and/or SpO2 less than 90% were considered as respiratory depression.
III. Sensory Block parameters
The onset and duration of sensory block, was assessed by loss of pinprick sensation to 23G hypodermic needle. Dermatomal level was tested every 2 minutes after SAB until level was stabilized for 4 consecutive readings. The time from intrathecal injection to the highest sensory level (Maximal Block height) was noted. Also level was tested every 15 minutes till regression by two segments from the highest level (Two segment regression) was noted.
IV. Motor block parameters
The onset and duration of motor block, was assessed initially, then every 5 minutes for 20 minutes following SAB and then every 30 minutes till full recovery using modified Bromage criteria.7
v. Sedation Score
Sedation was scored using 4-point rating score every 5 minutes after SAB for 2 hours.8
vi. Pain Score
Postoperatively, pain scores were recorded using VAS, after shifting the patient to PACU-initially every 30 minutes for 2 hours, then every 2 hours for next 8 hours and then after every 4 hours till 24 hours.9
Duration of complete analgesia, was defined as time from intrathecal injection to VAS greater than 0. Duration of effective analgesia was defined as time from intrathecal injection to a VAS greater than or equal to 3, at which a patient received 75mg of i/m diclofenac sodium as a rescue analgesic.
vii. Side Effects:
Episodes of nausea, vomiting, pruritus or shivering during postoperative period (within 24 hours) were recorded. Injection Ondansetron 0.1mg kg-1 intravenously was used to treat vomiting. Injection pheniramine maleate 25mg intravenously was used to treat itching. The presence of post dural puncture headache (PDPH), urinary retention and backache were also recorded till 24 hours.
Continuous data was analyzed by using one-way analysis of variance (ANOVA) Mann-Whitney U Test was used for comparison between two groups. Pearson Chi-Square and Fisher's Exact Test were used to evaluate the significant difference of categorical variables. P value of less than 0.05 was taken as level of significance. Data was analyzed by using SPSS version 12.0.
RESULTS
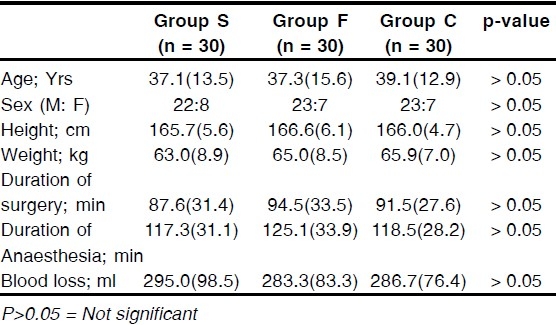
The demographic profile such as mean age, sex ratio, height and weight among the three groups were comparable. The mean duration of surgery, mean duration of anaesthesia and the mean total blood loss during surgery were also comparable between the groups (Table 1)
Table 1.
Characteristics of patients receiving either Sufentanil, Fentanyl or NS (Group S,F or C respectively). Values are mean (SD)
Hemodynamic parameters:
None of the patients developed significant changes in heart rate or blood pressure during the intraoperative period.
Respiratory parameters:
The respiratory parameters (RR and SpO2) were comparable at varying time intervals in the three groups. Baring 2 patients in Group S who had RR < 9/minute 30 mins after intrathecal injection; there was no incidence of respiratory depression in other groups.
Sensory block
The median maximal block height (T-6 dermatomal level) achieved was comparable in all the three groups The time to reach T-10 dermatomal level and maximal block height was significantly less in Group S (4.0 ± 1.5 min) and Group F (4.73 ± 1.77 min) as compared to Group C (7.26 ± 2.10 min) (p=0.000) {Fig. 1}.
Figure 1.
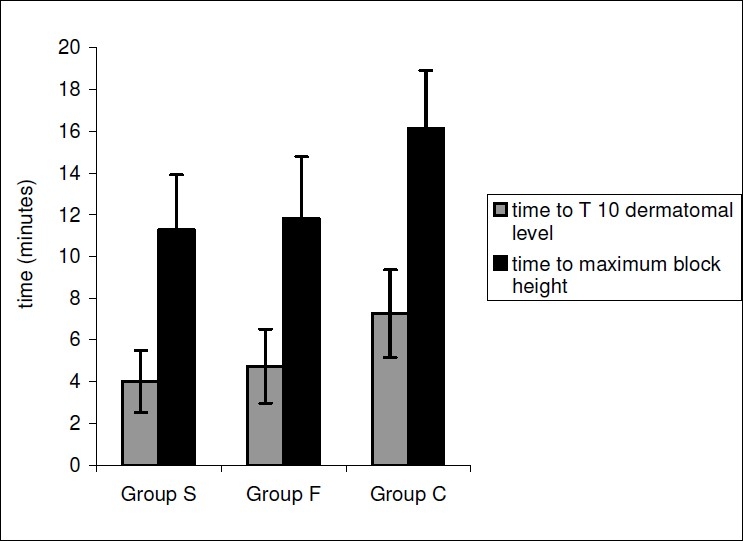
Time to reach T-10 dermatomal level and maximum block height in the Groups S,F or C.
The time to two-segment regression was significantly prolonged in Group S(150.2 ± 21.8 min) and Group F (143.2 ±. 17.3 min)as compared to Group C (116.6 ± 13.7 min)(p=0.000) {Fig. 2}.
Figure 2.
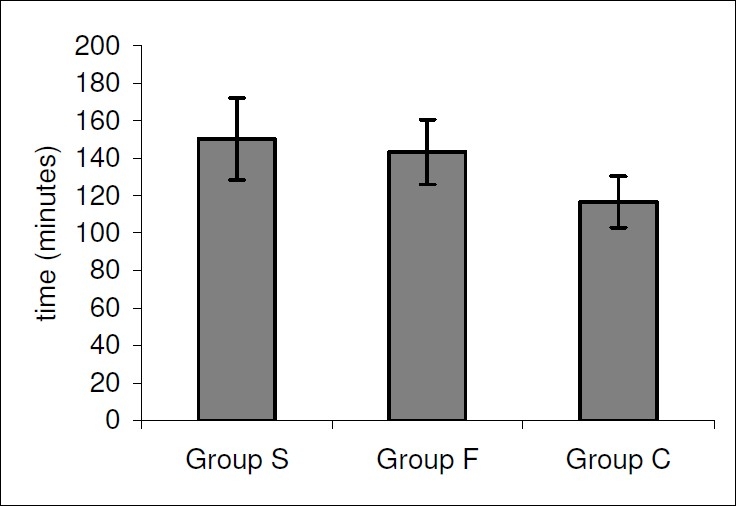
Time to two segment regression in the Groups S,F or C.
Motor block
The Time to reach Bromage 1 was found to be comparable among the groups (9.6 ± 3.4, 9.3 ± 2.8 and 9.5 ± 4.2 min in Groups S, F and C respectively) (p>0.05). The Time to resolution to Bromage 6 was significantly prolonged in Group S (224.3 ± 24.3 min ) as compared to Group C (207.1 ± 22.2 min) (p=0.016 between Groups S and C). It was prolonged though not significantly in Group F (211.5 ± 23.7 min) (p >0.05 between Groups F and C).
Sedation scores
After the intrathecal injection, sedation scores assumed an initial increasing trend in all the three groups but never exceeded 2 in any patient. Minimum sedation score (0±0) was achieved at 120 minutes in Group S, at 105 minutes in Group F and at 75 minutes in Group C implying prolonged sedation in Group S.(Fig 3)
Figure 3.
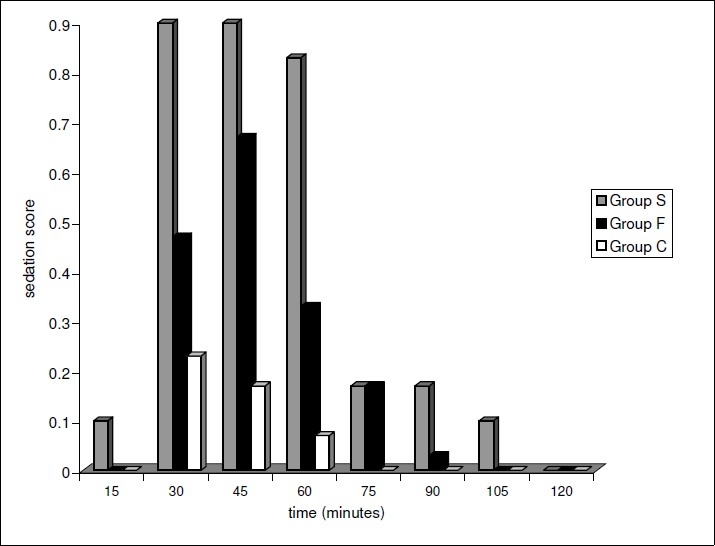
Comparison of the Sedation Scores in the Groups S,F and C.
Pain scores (VAS)
Fig. 4 shows the VAS score at varying time interval in three groups in the postoperative period. The baseline VAS scores at 0 hours (i.e. time of shifting the patient to postoperative room) were similar in Group S, Group F and Group C (0±0, 0±0 and 0.03 ± 0.1 respectively). Maximum VAS scores were reached within 2 hours in Group C (2.9 ± 2.1), as compared to 6 hours in Group F (3.2 ± 1.7) and 8 hours in Group S (2.6 ± 1.6). Group C had significantly higher VAS scores in the early (2 hours) postoperative period as compared to Group S and Group F (p ranging from 0.003 to 0.000 at different time intervals). Also the VAS scores in Group S were lower than Group F throughout the 24 hours postoperative period, though statistically insignificant (p>0.05).
Figure 4.
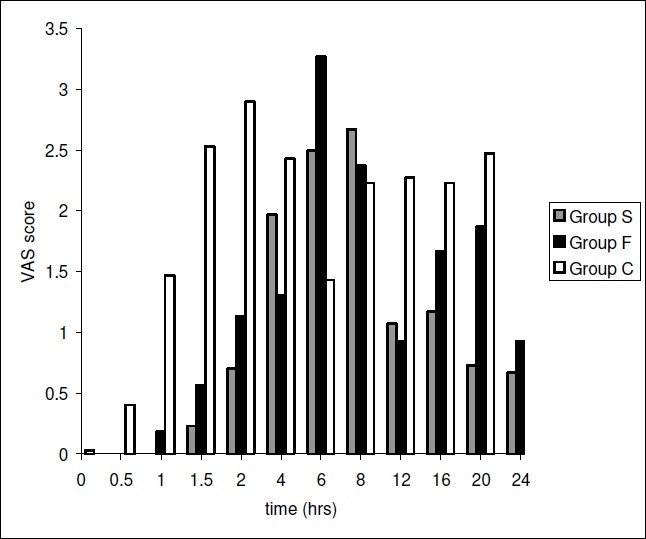
Comparison of the VAS score in the Groups S, F or C.
The Duration of complete analgesia was significantly longer in Group S (312.3±86.6 min) and Group F (282.1± 59.7 min)as compared to Group C (189.3 ± 29.9 min)(p=0.000). The Duration of effective analgesia was significantly prolonged in Group S (529.3 ± 96.6 min) and Group F(485.1 ± 82.7 min) as compared to Group C (256.3 ± 60.2 min)(p=0.000) {Fig. 5} Though, the durations of complete and effective analgesia were prolonged in Group S as compared to Group F, these were statistically insignificant (p>0.05). {Fig. 5}
Figure 5.
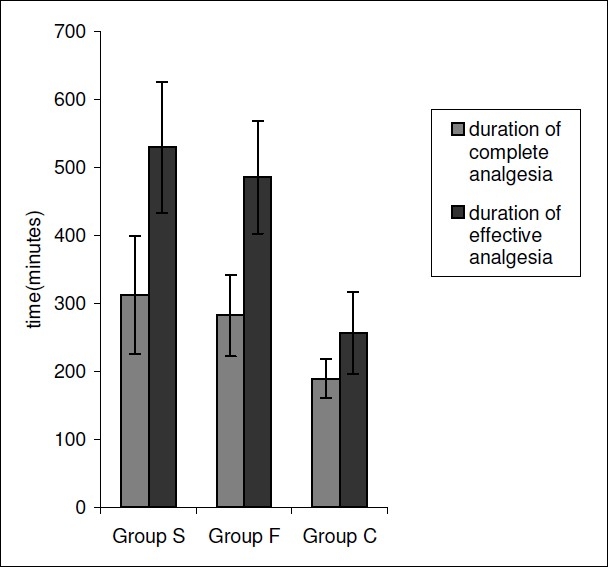
Duration of analgesia in the Groups S,F or C.
The requirement of rescue analgesic was significantly higher in Group C (mean dose=2.67) as compared to Group S (mean dose=1.27) or Group F (mean dose=1.60) during the postoperative period. (p=0.00)
Side Effects:
Figure 6 shows the comparison of side effects in the three groups. The groups were comparable in terms of side effects such as nausea and vomiting, shivering, urinary retention and PDPH. The incidence of pruritis was significantly higher in Group S as compared to Group C.
Figure 6.
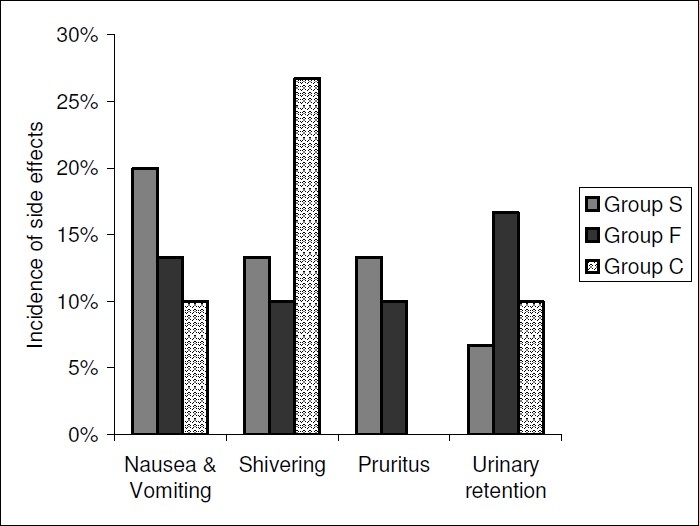
Incidence of Side Effects in the Groups S,F or C.
DISCUSSION
Subarachanoid block is a frequently used, simple and effective method of producing anaesthesia and early postoperative analgesia in patients undergoing lower limb orthopaedic surgeries. Various adjuvants have been used along with bupivacaine for prolonging the duration and improving the quality of analgesia. Intrathecal morphine provides pain relief upto 24 hr but its use has been associated with delayed respiratory depression.4,10 Newer lipophilic opioids like fentanyl and sufentanil are gaining popularity and have been used intrathecally by various workers.
In the present study 90 patients received either 5μg of sufentanil, 25μg fentanyl or 0.5 ml normal saline with 3 ml of 0.5% hyperbaric bupivacaine intrathecally. A total volume of 3.5ml was injected, irrespective of height (which ranged from 150-180cms).
Patients in the three study groups were comparable with respect to demographic profile. The average duration of surgery, anaesthesia and the mean blood loss were also comparable in the three study groups.
Various dose-response studies on laboring patients have documented the dose of intrathecal fentanyl to be 5 times more than intrathecal sufentanil.11,12 Taking into account these considerations; we administered 25μg fentanyl or 5μg of sufentanil as adjuvants to bupivacaine intrathecally in patients undergoing lower limb surgeries.
A comparable decrease in the SBP after SAB was observed in all the groups. Decrease in BP is a known occurrence after intrathecal bupivacaine due to a decrease in sympathetic afferent activity and is dose related. No episode of hypotension was observed in any patient despite the maximum block height (by pin prick method) of T4 in 25 patients. Reuben et al and Chen Wang et al also documented stable hemodynamic parameters following intrathecal fentanyl.1,13
A major concern with the intrathecal use of lipophilic opioids (fentanyl/sufentanil) is respiratory depression. Hansdottir et al observed that, intrathecal sufentanil is rapidly cleared from cerebrospinal fluid within an hour.14 Therefore, we monitored respiratory rate and oxygen saturation for 2 hours after the intrathecal injection of the drug .In our study, two patients (6.6%) in the sufentanil group had respiratory depression (respiratory rate <9 /minutes) 30 minutes after intrathecal injection. No incidence of respiratory depression was seen in other groups. No episode of oxygen desaturation was observed in any patient. This could have been attributed to our routine practice of administering supplemental oxygen with facemask both in the intraoperative and early postoperative period. Hay et al also reported respiratory depression following 15 μg of intrathecal sufentanil.15 whereas Lu et al reported two cases of respiratory arrest occurring after administering 10μg of intrathecal sufentanil.16
We found a significantly quicker onset of sensory block in the patients receiving either sufentanil (4.0 ± 1.5 min) or fentanyl (4.7 ± 1.7 min) as compared to control group (7.2 ± 2.1 min)(p=0.00). Similar results have been reported by previous authors.17,18,19 The maximal block height achieved was similar (T6) in the sufentanil, fentanyl and control groups. Dahlgren et al have also reported similar maximal block height in patients receiving sufentanil, fentanyl or placebo as adjuvants for cesarean section.20
In our study, time to two-segment regression was significantly prolonged in sufentanil (150.2 ± 21.8 minutes) and fentanyl group (143.2 ± 17.3 minutes) as compared to the control group {p=0.00 }. Singh et al,21 Goel et al,22 Ben David et al23 have also reported a similar prolongation of sensory block.
The time to reach Bromage 1 motor block (unable to move feet or knees) was comparable in all the groups in our study (p>0.05). However, time to resolution to Bromage 6 motor block (able to perform partial knee bend) was prolonged in the sufentanil and fentanyl groups as compared to the control group. This was in contrast to other authors who have reported similar onset time and time to resolution of motor block following intrathecal opioids.20,21,23 Our findings may have been related to a higher dose of bupivacaine used in our study.
In our study higher sedation scores were observed in patients receiving intrathecal opioids as compared to those in control group. There was an earlier onset of sedation in the sufentanil group (within 15 minutes), with significant degree of sedation occurring at 30-60 min. Sedation score never exceeded 2 in fentanyl group and sufentanil group.(Fig. 6) In a study by Fournier et al, the sedation score never exceeded 2 (somnolent, responds to tactile stimuli) in patients receiving 7.5μg of intrathecal sufentanil or 40μg of intrathecal fentanyl.24 Cowan et al however, observed a significant degree of early(within 1 hr) sedation following intrathecal fentanyl.25
In our study the VAS scores and 24 hr requirement of the rescue analgesic were significantly higher in control group as compared to sufentanil or fentanyl group over 24 hours postoperative period. The duration of complete analgesia and effective analgesia were significantly prolonged in patients of fentanyl or sufentanil group as compared to control group. Dahlgren et al also found the duration of complete analgesia to be significantly prolonged in patients receiving intrathecal 10μg fentanyl, 2.5μg or 5μg sufentanil compared to control group[20].
Side Effects:
The incidence of Pruritus, a documented side effect with intrathecal opioids varies widely from 0 to 100% and is dose related.26 In our study, there was a higher incidence of mild itching in the sufentanil group (p<0.05).Fournier et al also reported a higher incidence of itching in patients receiving 7.5μg of intrathecal sufentanil as compared to those receiving 40μg of intrathecal fentanyl.24
The incidence of nausea and vomiting following intrathecal opioids is approximately 30% and may be dose related.10,27 In our study, a comparable percentage of patients in the 3 groups complained of mild nausea not requiring treatment(p>0.05). Fournier et al reported a comparable incidence of nausea and vomiting following intrathecal sufentanil and fentanyl.24
In our study there was a comparable low incidence of shivering in the study groups (sufentanil and fentanyl groups) (p>0.05). Other authors have also reported a lower incidence of shivering following intrathecal opioids when compared with bupivacaine alone.8,21 Incidence of shivering may also be attributed to factors such as difference in surrounding temperature, intravenous fluids, etc.
A comparable incidence of urinary retention was seen in the three groups in our study (p.0.05). Other authors have reported a higher incidence of urinary retention in patients receiving intrathecal opioids as adjuvants.23,28
We conclude that intrathecal sufentanil (5μg) and fentanyl (25μg) as adjuvants to bupivacaine lead to an earlier onset and prolong the duration of sensory block and effective analgesia in patients undergoing lower limb orthopaedic surgeries.
The duration of motor block is prolonged with the use of intrathecal sufentanil (5μg) as compared to bupivacaine alone. Duration of motor block with intrathecal fentanyl (25μg) is comparable to use of bupivacaine alone.
Higher incidence of pruritus (13.3%) and respiratory depression (6.7%) was observed in patients receiving sufentanil as adjuvant to hyperbaric bupivacaine as compared to fentanyl-bupivacaine and bupivacaine alone.
Authors disclosure: Authors have no conflict of interest & financial consideration.
Acknowledgments
We wish to acknowledge the constant guidance and support of Dr Sharmila Ahuja, an eminent professor in the Department of Anaesthesia and Critical care in the execution of this study.
REFERENCES
- 1.Wang C, Chakrabarti MK, Whitwam JG. Specific enhancement by fentanyl of the effects of intrathecal bupivacaine on nociceptive afferent but not on sympathetic efferent pathways in dogs. Anesthesiology. 1993;79:766–773. doi: 10.1097/00000542-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Hunt CO, Naulty JS, Barder AM. Preoperative analgesia with subarachanoid fentanyl: bupivacaine for cesarean delivery. Anesthesiology. 1989;71:335–540. doi: 10.1097/00000542-198910000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Gustavsson L, Wiesenfeld-Hallin Z. Spinal opioid analgesia a critical update. Drugs. 1988;35:597–603. doi: 10.2165/00003495-198835060-00001. [DOI] [PubMed] [Google Scholar]
- 4.Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–151. doi: 10.1097/00000542-197902000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Leyson JE, Gommeren W, Niemegeers CJE. H-Sufentanil, a superior ligand for mu opioid receptors: binding properties and redistribution in rat brain and spinal cord. Eur J Pharmacol. 1983;87:209–225. doi: 10.1016/0014-2999(83)90331-x. [DOI] [PubMed] [Google Scholar]
- 6.Ionescus TI, Taverne RHT, Houweling FL, et al. Pharmacokinetic study of extradural and intrathecal sufentanil anaesthesia for major surgery. Br. J Anaesth. 1991;66:458–464. doi: 10.1093/bja/66.4.458. [DOI] [PubMed] [Google Scholar]
- 7.Bromage PR, Burfoot MF, Ceowell DE. Quality of epidural block. Br J Anaesth. 1964;36:342–352. doi: 10.1093/bja/36.6.342. I Influence of physical factors. [DOI] [PubMed] [Google Scholar]
- 8.Techanivate A, Urosopone P, Kiatgungwanglia P, et al. Intrathecal fentanyl in spinal anesthesia for appendectomy. J Med Assoc Thai. 2004;87(5):525–530. [PubMed] [Google Scholar]
- 9.Huskisson EC. Visual Analogue Scale. In: Melzack R, editor. Pain Measurement and Assessment. New York: Raven Press; 1983. pp. 33–37. [Google Scholar]
- 10.Samiik, Chauvin M, Viars P. Postoperative spinal analgesia with morphine. Br J Anaesth. 1981;53:817–820. doi: 10.1093/bja/53.8.817. [DOI] [PubMed] [Google Scholar]
- 11.Herman NL, Calicott R, Van Decar TK, et al. Determination of dose-response relationship for intrathecal sufentanil in laboring patients. Anesth Analg. 1997;84:1256–1261. doi: 10.1097/00000539-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Palmer CM, Cork RC, Hays R, et al. The dose-response relation of intrathecal fentanyl for labor analgesia. Anesth Analg. 1994;88:355–361. doi: 10.1097/00000542-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Reuben SS, Dunn SM, Dupart KM, O'sullivan P. An intrathecal fentanyl dose-response study in lower extremity revascularization procedure. Anesthesiology. 1994;81:1371–1375. doi: 10.1097/00000542-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Hansdottir V, Hedner T, Woestenborghs R, Nordberg C. The CSF and plasma pharmokinetics of sufentanil after intrathecal administration. Anesthesiology. 1991;74(2):264–269. doi: 10.1097/00000542-199102000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Hay RL, Palmer CM. Respiratory depression after intrathecal sufentanil during labor. Anesthesiology. 1994;81:511–512. doi: 10.1097/00000542-199408000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Lu JK, Mahullang TR, Staples MH, Kern SE, Bailey PL. Maternal respiratory arrests, severe hypotension and fetal distress after administration of intrathecal sufentanil and bupivacaine after intravenous fentanyl. Anesthesiology. 1997;87:170–172. doi: 10.1097/00000542-199707000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Bano F, Sabbar S, Zafar S, Rafeeq N, Haider S, et al. Intrathecal fentanyl as adjunct to hyperbaric bupivacaine in spinal anesthesia for caesarean section. JCPSP. 2005;16(2):87–90. [PubMed] [Google Scholar]
- 18.Khan FA, Hamdani GA. Comparison of intrathecal fentanyl and buprenorphine in urological surgery. JPMA. 2006;56(6):277–281. [PubMed] [Google Scholar]
- 19.Braga Ade F, Braga FS, Poterio GM, Pereira RI, Reis E, Cremonesi E. Sufentanil added to hyperbaric bupivacaine for subarachanoid block in caesarean section. Eur J Anaesthesiol. 2003;20:631–634. doi: 10.1017/s0265021503001017. [DOI] [PubMed] [Google Scholar]
- 20.Dahlgren G, Hultstrand C, Jakobsson Jan, Norman M, et al. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997;85:1288–1293. doi: 10.1097/00000539-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Singh H, Yang J, Thornton K, Adolph H. Intrathecal fentanyl prolongs sensory bupivacaine spinal block. Can J Anaesth. 1995;42(11):987–991. doi: 10.1007/BF03011070. [DOI] [PubMed] [Google Scholar]
- 22.Goel S, Bhardwaj N, Grover VK. Intrathecal fentanyl added to intrathecal bupivacaine for day case surgery: a randomized study. Eur J Anaesth. 2003;20:294–297. doi: 10.1017/s0265021503000462. [DOI] [PubMed] [Google Scholar]
- 23.Ben-David B, Solomon E, Levin H, et al. Intrathecal fentanyl with small-dose dilute bupivacaine: Better analgesia without prolonging recovery. Anesth Analg. 1997;85:560–565. doi: 10.1097/00000539-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Fournier R, Weber A, Gessel EV, Gamulin Z. A comparison of intrathecal analgesia with fentanyl or sufentanil after total hip replacement. Anesth Analg. 2000;90:918–922. doi: 10.1097/00000539-200004000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Cowan CM, Kendall JB, Barelay PM, Wilkes RG. Comparison of intrathecal fentanyl and diamorphine in addition to bupivacaine for caesarean section under spinal anaesthesia. Br J Anaesth. 2002;89:452–458. [PubMed] [Google Scholar]
- 26.Chaney MA. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42(10):891–903. doi: 10.1007/BF03011037. [DOI] [PubMed] [Google Scholar]
- 27.Bailey PL, Rhondeau S, Schafer PG, et al. Dose-response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59. doi: 10.1097/00000542-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Chiu AA, Carpenter RL, et al. Fentanyl prolongs lidocaine spinal anesthesia without prolonging recovery. Anesth Analg. 1995;80:735–739. doi: 10.1097/00000539-199504000-00014. [DOI] [PubMed] [Google Scholar]


