INTRODUCTION
Clonidine is an imidazoline compound with the molecular formula C9H9Cl2N3. The structural formula1 is as shown in figure 1. It is the prototype of alpha-2 adrenoceptor agonists that has been extensively studied with an alpha-2: alpha-1 ratio of 200:11. The drug is licensed for the treatment of hypertension, migraine and menopausal flushing.2 It is also an analgesic, sedative and anxiolytic.1,3 These properties along with its ability to maintain peri-operative haemodynamic stability make clonidine a useful agent in anaesthesia and intensive care.1
Figure 1.
Clonidine
We set out to perform a systematic review on the efficacy of clonidine as a sedative agent in adult patients on the intensive care unit. A comprehensive literature search was performed. Medline, Embase, AMED, British nursing index, CINAHL, PsychINFO, Bandolier, Cochrane Library, HTA database and the DARE was searched for the following terms “Clonidine”, “Intensive care”, “Critical care”, “Sedation” from the period 1951 to January 2009. The references of the retrieved reviews and original articles were also manually searched. Only studies in English for adult patients were included in our review.
This literature search revealed 2 randomized controlled trials (1 unpublished), 1 single centre prospective interventional study, 1 observational study, 1 dose finding study and 4 case reports [Table 1Fig. 2]. Since the paucity of studies precluded a systematic review, this is therefore a narrative review of the use of clonidine focussing on its role as a sedative agent on the intensive care unit.
Table 1.
Characteristics of included studies in the Review
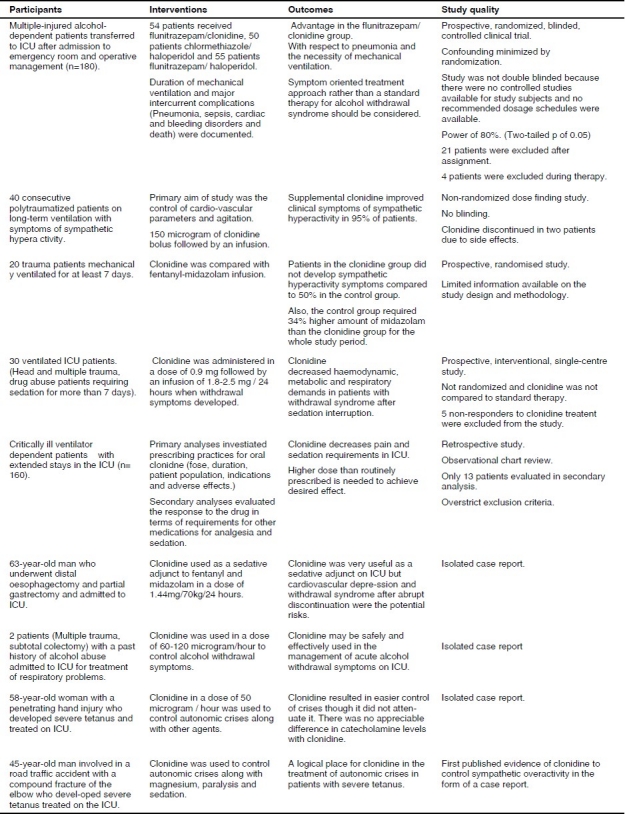
Figure 2.
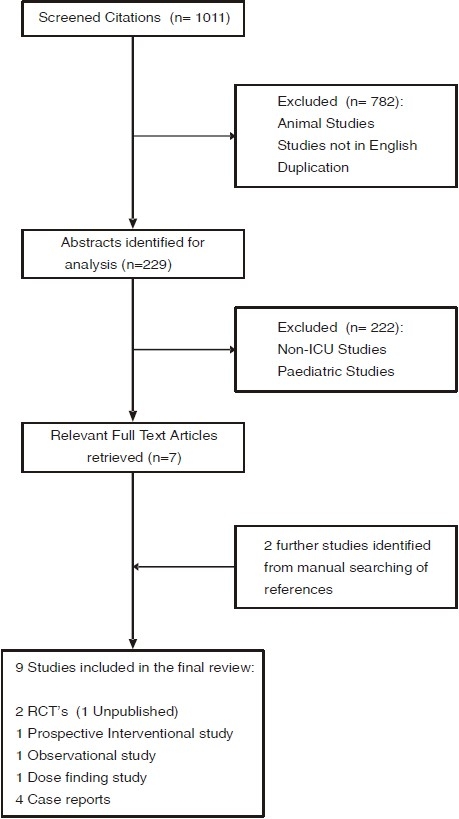
The Flowchart of studies in the Review
PHARMACOLOGY
Mechanism of action
Clonidine acts by stimulating the pre-synaptic alpha 2 adrenoceptors, thereby decreasing noradrenaline release from both central and peripheral sympathetic nerve terminals.3 The effects of clonidine occur due to its action both at spinal and supraspinal sites, including depression of thalamic transmission of impulses to the cerebral cortex as well as enhancement of descending inhibitory pathways to the dorsal horn.3,4 Supraspinally, the locus coeruleus in the floor of the fourth ventricle is the major site for the sedative and analgesic actions as revealed by radioligand studies.5 Clonidine markedly decreases noradrenaline concentrations in the locus coeruleus. The efferents from the locus coeruleus act on the descending fibres of the reticulospinal tracts that inhibit pain transmission at the spinal level1. The dorsal nucleus of vagus has high densities of alpha 2 receptors responsible for the bradycardic and hypotensive effects.6 Alpha 2 adrenoceptors are found postjunctionally on the dorsal horn neurons of the spinal cord and acts by inhibiting the release of substance P.7 Clonidine also acts on the cholinergic, purinergic and serotonergic pain systems causing analgesia.8 Clinical and experimental studies have shown that alpha 2 agonists given together with opioids into the spinal cord act synergistically to reduce pain.8–11
Pharmacokinetic actions
Clonidine is rapidly and well absorbed orally with a bioavailability of 100%. It is very lipid soluble and penetrates the central nervous system. It is 20% protein bound in the plasma with a volume of distribution of 1.7 -2.5 l kg-1. It is metabolised in the liver to inactive metabolites and 65% of the dose is excreted unchanged in the urine, 20% is excreted in the faeces. The clearance is 1.9-4.3ml kg-1 min-1 and the elimination half-life is 6-23 hours (average 7.7 hours).12 The half-life is markedly increased in renal impairment requiring a dose reduction.4,13 The peak action occurs in 10 minutes and lasts for 3-7 hours after single intravenous dose. On oral administration, it reaches a peak plasma level within 60-90 minutes.8
Pharmcodynamic actions
Central nervous system: Clonidine produces dose-related sedation, analgesia, anxiolysis and a reduction in the requirements of other anaesthetic agents and opioids.8–11 It also reduces cerebral blood flow, cerebral metabolic rate of oxygen consumption and intraocular pressure.1 It also exerts a depressant effect on both spontaneous sympathetic outflow and afferent A delta and C fibre mediated somatosympathetic reflexes.4
Cardio respiratory systems: Clonidine causes an initial shor t lived increase in blood pressure and systemic vascular resistance and a decrease in cardiac output due to activation of post junctional alpha 2 receptors on the peripheral vasculature. This is accompanied by a longer lasting decrease in heart rate and blood pressure resulting from a centrally mediated decrease in sympathetic tone and an increase in vagal activity.1 It has no effect on cardiac contractility and cardiac output is well maintained. There is a decrease in coronary and systemic vascular resistance.4,14 Clonidine causes very minimal respiratory depression along with good sedation. There is no change in respiratory rate, PaCO2, SpO2 on administration.13–16
Renal, Metabolic and Endocrine: Clonidine decreases renovascular resistance and produces diuresis. It causes a decrease in plasma catecholamine concentrations and plasma renin activity. Blood sugar concentrations may increase secondary to alpha-adrenergic stimulation.4
Dosage and administration
It is presented as a clear colourless solution for injection containing 150 micrograms in 1 ml ampoule. Clonidine is administered intravenously in boluses (50-150 microgram 8-hourly) or by continuous infusion. The dose may be extremely variable when given by infusion. However, the usual dose is in the order of 100-microgram hour -1. The oral dose is 50-600 microgram 8-hourly. The onset of action after an intravenous dose is within 10 minutes and lasts for 3-7 hours.4 It is also administered by extradural, intrathecal, intramuscular, transdermal and by nebulised routes.1
Side effects
Dryness of mouth (50% of patients), dose related sedation, depression, fluid retention and constipation has been reported.3,8 Rapid withdrawal of the drug leads to life-threatening rebound hypertension and tachycardia.3,4 Colonic Pseudoobstruction (Ogilive's syndrome), an unusual complication of high dose clonidine infusion for the treatment of delirium tremens has been reported.17
Clinical applications
Clonidine was introduced into clinical practice in the 196618 as a centrally acting anti hypertensive agent. It was first used for neurolptanalgesia in 1978.19 It is used for the treatment of migraine and menopausal flushing and also as an anaesthetic adjuvant (sedation, analgesia, anxiolysis, premedication).3
Peri-operatively it obtunds hypertensive reflexes during endotracheal intubation and results in the decreased incidence of myocardial ischaemia.20 Along with local anaesthetic agents, it is an adjunct in regional anaesthesia and in the treatment of chronic pain.21
The efficacy of clonidine as a sedative agent on the intensive care unit.
In recent years, clonidine, the prototype of alpha 2 agonists has been used as an analgosedative in intensive care both in ventilated and spontaneously breathing patients.12,14 The first reported use of clonidine for sympathetic overactivity on the ICU was for the treatment of autonomic dysfunction in tetanus in 198922 [Table 1]. It plays a significant role in the treatment of a variety of conditions such as hypertension, delirious syndromes and withdrawal syndromes (opioid, alcohol and nicotine addiction).23–32 It is also used as an agent to facilitate weaning from long-term mechanical ventilation and in the prevention of resistance to opioids and benzodiazepines.33–36
Mechanically ventilated patients on the ICU experience stress, agitation, anxiety and pain. The goals of ICU sedation are to provide analgesia and anxiolysis. It should also prevent cardiorespiratory and metabolic stress produced by catecholamines and achieve a state where a patient cooperates with interventions and health care providers.37 Propofol, opioids, benzodiazepines and neurolept agents are used to achieve sedation but are associated with significant side effects and cause global unconsciousness. A survey of sedation practices on ICU in Britain suggested that most units are driven towards achieving a lightly sedated and cooperative patient and at the same time fulfilling sedation goals. This national postal survey revealed that alfentanil, midazolam, morphine and propofol were the most commonly used sedative agents. Also, the sedation regimes changed depending on the length of stay on the ICU.38 Alpha-2 adrenoceptor agonists may achieve this state without significant side effects.37
A prospective, randomized, blinded, controlled clinical trial by Spies CD et al in a university hospital ICU assessed three different therapeutic regimens for the treatment of withdrawal syndromes in traumatized chronic alcoholic patients39 [Table 1]. The authors studied the treatment with regards to duration of mechanical ventilation, frequency of pneumonia and cardiac disorders during their ICU stay. They randomised patients who developed actual alcohol withdrawal syndrome to one of the following treatment regimens: flunitrazepam-clonidine (n=54); chlormethiazole-haloperidol (n=50) or flunitrazepam-haloperidol (n=55). A validated measure of the severity of alcohol withdrawal (Revised Clinical Institute Withdrawal Assessment for Alcohol Scale) was used to determine the need for administration of medication. Four patients in the Flunitrazepam-clonidine group were excluded from the study as they continued to hallucinate. Though there was no difference in the length of ICU stay between the three groups, the authors found that mechanical ventilation was significantly prolonged in the chlormethiazole/haloperidol group due to an increased frequency of pneumonia and cardiac complications were significantly increased in the flunitrazepam-clonidine group. The authors concluded that a symptom oriented treatment approach rather than a standard therapy should be considered. However, 21 patients were excluded from the study after assignment and a further 4 patients during therapy. The results are therefore subject to exclusion bias. Furthmore the study was not double-blinded and therefore is prone to observer bias. The study is also limited in that no predetermined dosage schedules were available.
The only other randomised prospective study involving the use of clonidine in ICU is unpublished35 [Table 1]. This study is described in an article on the use of clonidine for the prevention and treatment of withdrawal. We could not find any formal publication of this study even in abstract form. The efficacy of clonidine was assessed in reducing withdrawal symptoms in patients who were mechanically ventilated for more than 7 days. Clonidine was compared to Fentanyl-midazolam infusion. Patients in the treatment group received clonidine in addition to a fentanyl-midazolam infusion as compared to a fentanyl -midazolam infusion only in the control group. 20 patients were included in the study. The total amount of midazolam administered during the whole study period was 34% higher in the control group than in the clonidine group. No sympathetic hyperactivity symptoms were seen in the clonidine group compared to 60% in the control group. A systematic appraisal of this study could not be undertaken because of the limited information available on study design and methodology.
Gillison M and colleagues performed a retrospective study where clonidine-prescribing practices (dose, duration, patient population, indications and adverse effects) were analysed40 [Table 1]. The charts of 160 critically ill ventilator dependent patients with extended stays on the medical-surgical ICU who received clonidine were reviewed. Primary analyses of this review investigated prescribing practices for oral clonidine and the secondary analyses evaluated the response to the drug in terms of requirements of other medications for analgesia and sedation. The mean dose was 0.26mg and opioid and benzodiazepine requirements were decreased. The authors concluded that clonidine decreases pain and sedation requirements and also suggested that a higher dose than routinely prescribed is required to achieve the desired effect. Other case reports have also suggested clonidine to be a useful analgo-sedative that potentiates the effects of fentanyl and midazolam. They also conclude that clonidine is safe and effective in the treatment of established delirium tremens and that it facilitates weaning from mechanical ventilation41–42 [Table 1].
The dose of clonidine is very variable when administered as an intravenous infusion. Tryba and colleagues conducted a dose-finding study to investigate the feasibility and dosage requirement of clonidine for the treatment of sympathetic symptoms in ventilated polytraumatized patients (18-70 years) with chronic alcohol intake35. Forty consecutive polytraumatized patients on long-term mechanical ventilation with symptoms of sympathetic hyperactivity receiving sedation with fentanyl and midazolam were included in the study. A bolus of clonidine followed by infusion was administered to control the cardiovascular parameters (HR< 120/min and systolic BP<130 mmHg). Haloperidol was supplemented up to a maximum dose of 100 mg per day if agitation was not controlled with clonidine. The dose administered to control cardiovascular parameters varied extremely with the lowest effective dosage of 0.45 mg and the highest dosage of 15.45 mg. Sweating improved in most patients within 2-3 hours and disappeared within a maximum of 13 hours after start of clonidine. A reduction in HR to less than 120/min occurred in 90% of patients within 24 hours. After initiation of clonidine treatment, sedation became adequate in 24 patients and fentanyl-midazolam requirement could be reduced by 38 (+/- 19) % within 24 hours. The authors concluded that supplemental clonidine improved clinical symptoms of sympathetic hyperactivity in 95% of patients.
Abrupt sedation interruption on ICU can result in the development of withdrawal symptoms (agitation, sweating, hyperventilation, hypertension and tachycardia) with a surge of catecholamines resulting in haemodynamic instability and increased metabolic demand. Domniki Liatsi and colleagues conducted a prospective, interventional study on 30 ventilated ICU patients with withdrawal symptoms treated with clonidine following sedation interruption. The authors state that clonidine significantly decreased haemodynamic, metabolic and respiratory demands and facilitated patient coordination with the ventilator and early weaning along with inducing mild sedation in patients with withdrawal syndrome. Clonidine also significantly lowered Ramsay sedation scores and patients were extubated 24-48 hours after starting treatment. However this was an interventional cohort study. There was no randomisation; no blinding and the 5 non-responders to clonidine treatment were excluded from the analyses36 [Table 1].
The treatment of autonomic dysfunction in tetanus consists of heavy sedation, paralysis, adrenergic blockade and magnesium sulphate43,44 [Table 1]. Clonidine has been successfully used to treat severe autonomic dysfunction in a 45-year-old patient with severe tetanus who was refractory to magnesium sulphate.22 Turner and colleagues also describe that the use of clonidine resulted in easier control of autonomic crises in a patient with severe tetanus when used along with other agents.43
Critically ill patients with alcohol and opiate withdrawal symptoms present significant treatment difficulties especially following sedation interruption. Several studies23–32 performed outside of the ICU setting indicate that oral clonidine may be effective in controlling autonomic and psychologic manifestations of alcohol and opiate withdrawal symptoms. In a double blind, placebo-controlled, crossover trial, clonidine eliminated signs and symptoms of opiate withdrawal for 240-360 minutes in eleven addicts in a hospital setting.25 In another prospective, randomised, double blind study involving 50 patients over a 4-day study period, the efficacy of transdermal clonidine was compared with that of chlordiazepoxide in the treatment of severe acute alcohol withdrawal syndrome.31 The group receiving transdermal clonidine had a more significant response globally for the signs and symptoms of alcohol withdrawal, as measured by the Alcohol Withdrawal Assessment Scale. The authors concluded that transdermal clonidine is effective treatment for the treatment of acute alcohol withdrawal syndrome. Although the above studies have been conducted in the hospital setting rather than in an ICU setting, they give an indication that clonidine may be safe and efficacious for the treatment of withdrawal syndromes.
Dexmeditomidine is a newer Alpha-2 adrenoceptor agonist.45–49 The drug shares similar pharmacodymanic actions to that of its predecessor clonidine. Two recent multicentre randomised controlled clinical trials have assessed the efficacy of dexmeditomidine as a sedative agent in adult ICU patients.48,49 These studies are of a robust methodological design; adequately powered, randomised, and blinded thereby reducing the potential for bias. Dexmedetomidine compared favourably to the control drugs (lorazepam and midazolam) with a lower incidence of delirium or coma and a reduced duration of mechanical ventilation.
CONCLUSION
The efficacy of clonidine as a sedative agent for adult patients in the ICU is yet to be established. The paucity of studies evaluating the efficacy of clonidine as a sedative agent on the intensive care unit precluded a systematic review. Whilst clonidine is used as a sedative agent on the ICU, the evidence underpinning this practice is of a relatively poor quality. In this era of clinical governance and evidence based medicine the continued use of clonidine as a sedative agent in adult patients on the ICU cannot be justified. If the drug is to establish its place as a safe and effective sedative agent in adult ICU patients then it should be subjected to the rigors of well designed randomised controlled clinical trials first. However it is much more likely that the newer alpha-2 adrenoceptor agonist dexmeditomidine will replace clonidine as a sedative agent having already shown promise in two recent multicentre randomised controlled clinical trials.48–49
REFERENCES
- 1.Peden CJ, Prys-Roberts C. International Practice of Anaesthesia. 6th. Oxford (UK): PracticeofAnaesthesia; 1996. The alpha-2 adrenoceptors and anaesthesia. Cedric Prys-Roberts and Burnell R. Brown. [Google Scholar]
- 2.Joint Formulary Committee. London: British Medical Association and Royal Pharmaceutical Society of Great Britain. (57th ed) 2008 Sep; British National Formulary, [Google Scholar]
- 3.Calvey T.N, Williams N.E. 4th ed. Oxford (UK): Blackwell Science Ltd; 2003. Principles and Practice of Pharmacology for Anaesthetists. [Google Scholar]
- 4.Martin Sasada, Susan Smith. 3rd. Oxford (UK): Oxford University Press; 2003. Drugs in Anaesthesia and Intensive care; pp. 76–77. [Google Scholar]
- 5.Scheinin M, Schwinn D. The Locus Coeruleus: Site of hypnotic actions of alpha 2 adrenoceptor agonists? Anesthesiology. 1992;76:873–875. doi: 10.1097/00000542-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Quintin L, Gonon F, Buda M, Ghignone M, Hilaire G, Pujog J F. Clonidine modulates metabolic hyperactivity induced by stress in behaving rats. Brain Res. 362:366–369. doi: 10.1016/0006-8993(86)90465-8. [DOI] [PubMed] [Google Scholar]
- 7.Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- 8.Maze M, Tranquilli W. Alpha 2 adrenoceptor agonists: defining the role in clinical anaesthesia. Anesthesiology. 1991;74:581–605. [PubMed] [Google Scholar]
- 9.Aantaa R, Scheinin M. Alpha 2 adrenergic agents in Anaesthesia. Acta Anaesthesiol Scand. 1993;37:433–438. doi: 10.1111/j.1399-6576.1993.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 10.Flacke JW, Flacke WE. The use of alpha 2 adrenergic agonists during general anaesthesia. Anaesth Pharmacol Rev. 1993;1:268–283. [Google Scholar]
- 11.Hayashi Y, Maze M. Alpha 2 adreneceptor agonists and Anaesthesia. Br J Anaesth. 1993;71:108–118. doi: 10.1093/bja/71.1.108. [DOI] [PubMed] [Google Scholar]
- 12.Keranen A, Nykanen S, Taskinen J. Pharmacokinetics and side effects of clonidine. Eur J Clin Pharmacol. 1978;13:97–101. doi: 10.1007/BF00609752. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Tetsuya Ise, Mikiko Yamashiro, Hideki Furuya. Clonidine as a drug for intravenous conscious sedation. Odontology. 2002;90:57–63. doi: 10.1007/s102660200009. [DOI] [PubMed] [Google Scholar]
- 15.Ooi R, Pattison J, Feldman SA. The effects of Intravenous clonidine on ventilation. Anaesthesia. 1991;46:632–633. doi: 10.1111/j.1365-2044.1991.tb09709.x. [DOI] [PubMed] [Google Scholar]
- 16.Bailey PL, Sperry RJ, Johnson GK, et al. Respiratory effects of clonidine alone and combined with morphine in humans. Anesthesiology. 1991;74:43–8. doi: 10.1097/00000542-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Stieger DS, Cantieni R, Frutiger A. Acute colonic pseudoobstruction (Ogilvie's syndrome) in two patients receiving high dose clonidine for delirium tremens. Intensive Care Med. 1997;23:70–782. doi: 10.1007/s001340050409. [DOI] [PubMed] [Google Scholar]
- 18.Helmut Stahle. A historical perspective: development of clonidine. Baillieres Clin Anaesthesiol. 2000;14:237–246. [Google Scholar]
- 19.Kaukinen S, Kaukinen L, Eerola R. Preoperative and postoperative use of clonidine with neurolept anaesthesia. Acta Anaesthesiol Scand. 1978;23:113–120. doi: 10.1111/j.1399-6576.1979.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 20.Talke PO, Mangano DT. Alpha 2 adrenergic agonists and perioperative ischaemia. Anaesth Pharmacol Rev. 1993;1:310–315. [Google Scholar]
- 21.Tamsen A, Gordh T. Epidural Clonidine produces analgesia. Lancet II. 1984:231–232. doi: 10.1016/s0140-6736(84)90523-3. [DOI] [PubMed] [Google Scholar]
- 22.Sutton DN, Tremlett MR, Woodcock TE, Nielsen MS. Management of autonomic dysfunction in severe tetanus: the use of magnesium sulphate and clonidine [review] Intensive Care Med. 1990;16:75–80. doi: 10.1007/BF02575297. [DOI] [PubMed] [Google Scholar]
- 23.Gold MS, Pottash AC, Sweeney DR, Kleber HD. Opiate withdrawal using clonidine.A safe, effective, and rapid nonopiate treatment. JAMA. 1980;243:343–348. [PubMed] [Google Scholar]
- 24.Gupta AK, Jha BK. Clonidine in heroin withdrawal syndrome: a controlled study in India. Br J Addict. 1988;83:1079–1084. doi: 10.1111/j.1360-0443.1988.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 25.Gold MS, Redmond DE, Jr, Kleber HD. (1978) Clonidine blocks acute opiate withdrawal symptoms. Lancet. 1978;2:599-602–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- 26.Kahn A, Mumford JP, Rogers GA, Beckford H. Double-blind study of lofexidine and clonidine in the detoxification of opiate addicts in hospital. Drug Alcohol Depend. 1997;44:57–61. doi: 10.1016/s0376-8716(96)01316-6. [DOI] [PubMed] [Google Scholar]
- 27.Taschner KL. A controlled comparison of clonidine and doxepin in the treatment of the opiate withdrawal syndrome. Pharmacopsychiatry. 1986;19:91–5. doi: 10.1055/s-2007-1017162. [DOI] [PubMed] [Google Scholar]
- 28.Lin SK, Strang J, Su LW, Tsai CJ, Hu WH. Double-blind randomised controlled trial of lofexidine versus clonidine in the treatment of heroin withdrawal. Drug Alcohol Depend. 1997;48:127–133. doi: 10.1016/s0376-8716(97)00116-6. [DOI] [PubMed] [Google Scholar]
- 29.Manhem P, Nilsson LH, Moberg Al, Wadstein J, Hokfelt B. Alcohol withdrawal: Effects of Clonidine on sympathetic activity, the renin aldosterone system, and clinical symptoms. Alcohol Clin Exp Res. 1985;9:238–243. doi: 10.1111/j.1530-0277.1985.tb05743.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins AJ, Jenkins WJ, Steiner JA. Efficacy of clonidine in treatment of alcohol withdrawal state. Psychopharmacology. 1983;81:78–80. doi: 10.1007/BF00439278. [DOI] [PubMed] [Google Scholar]
- 31.Walinder J, Balldin J, Bokstrom K, Karlsson I, Lundstrom B, Svensson TH. Clonidine suppression of the alcohol withdrawal syndrome. Drug Alcohol Depend. 1981;8:345–348. doi: 10.1016/0376-8716(81)90043-0. [DOI] [PubMed] [Google Scholar]
- 32.Baumgartner GR, Rowen RC. Transdermal clonidine versus chlordiazepoxide in alcohol withdrawal: a randomized controlled clinical trial. South Med J. 1991;84:312–321. doi: 10.1097/00007611-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Zalunardo M, Zollinger A, Heinzelmann M, et al. Restlessness during weaning from mechanical ventilation: successful use of clonidine [abstract] Eur J Anaesthesiol. 1993;10:67. [Google Scholar]
- 34.Kress J.P, Pohlman A.S, O'Connor M.F, Hall J.B. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 35.Michael Tryba. Alpha 2-adrenoceptor agonists in intensive care medicine: prevention and treatment of withdrawal. Baillieres Clin Anaesthesiol. 2000;14(2):459–470. [Google Scholar]
- 36.Domniki Liatsi, Basilis Tsapas, Smaro Pampori, Matthew Tsagourias, Ioannis Pneumatikos, Dimitrios Matamis. Respiratory, metabolic and hemodynamic effects of clonidine in ventilated patients presenting with withdrawal syndrome. Intensive Care Med. 2009;35:275–281. doi: 10.1007/s00134-008-1251-0. [DOI] [PubMed] [Google Scholar]
- 37.Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001;17:881–897. doi: 10.1016/s0749-0704(05)70185-8. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch S, Cohen A. Intensive care sedation: A review of current British practice. Intensive Care Med. 2000;26:922–928. doi: 10.1007/s001340051282. [DOI] [PubMed] [Google Scholar]
- 39.Spies CD, Dubisz N, Neumann T, et al. Therapy of alcohol withdrawal syndrome in intensive care unit patients following trauma. Crit Care Med. 1996;24:414–422. doi: 10.1097/00003246-199603000-00009. Results of a prospective randomised trial. [DOI] [PubMed] [Google Scholar]
- 40.Gillison M, Fairbairn J, McDonald K, Zvonar R, Cardinal P. Clonidine use in the intensive care unit of a tertiary care hospital: Retrospective analysis. Can J Hosp Pharm. 2004;57:83–89. [Google Scholar]
- 41.Bohrer H, Bach A, Layer M, Werning P. Clonidine as a sedative adjunct in intensive care. Intensive Care Med. 1990;16(4):265–6. doi: 10.1007/BF01705163. [DOI] [PubMed] [Google Scholar]
- 42.IP Yam PC, Forbes A, Kox WJ. Clonidine in the treatment of alcohol withdrawal in the intensive care unit. Br J Anaesth. 1992;68(1):106–8. doi: 10.1093/bja/68.1.106. [DOI] [PubMed] [Google Scholar]
- 43.Freshwater-Turner D, Udy A, Lipman J, et al. Autonomic dysfunction in tetanus - what lessons can be learnt with specific reference to alpha-2 agonists? Anaesthesia. 2007;62:1066–70. doi: 10.1111/j.1365-2044.2007.05217.x. [DOI] [PubMed] [Google Scholar]
- 44.Esslinger P, Kistler W, Berger T. M.Severe autonomic dysfunction in an 11-year-old girl with generalised tetanus. Eur J Pediatr Surg. 2003;13:209–212. doi: 10.1055/s-2003-41260. [DOI] [PubMed] [Google Scholar]
- 45.Bachand R, Scholz J, Pinaud M, et al. The effects of dexmedetomidine on patients in intensive care settings [abstract 622] Intensive Care Med. 1999;25 (suppl I):S160. [Google Scholar]
- 46.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 47.Dexmedetomidine: A novel agent for patients in intensive care settings [abstract 683] Intensive Care Med. 1999;25 (suppl I):623. [Google Scholar]
- 48.Riker Richard R, Shehabi Yahya, Bokesch Paula M, et al. Dexmedetomidine vs Midazolam for the sedation of Critically ill patients: A Randomized Trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 49.Pandharipande Pratik P, Pun Brenda T, Herr Daniel L, et al. Effect of sedation with Dexmedetomidine vs Lorazepam on Acute brain dysfunction in mechanically ventilated patients: The MENDS randomized Trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]


