Abstract
Background
Primary Open Angle Glaucoma is a multi-factorial disease with a devastating impact on the quality of life of the patient in the moderate and severe stages of the disease. Identifying risk factors for the development of moderate to severe visual field loss may decrease the proportion of patients that experience the severe forms of this disease.
Purpose
To evaluate whether the central corneal thickness correlates inversely with the severity of visual field loss in Primary Open Angle Glaucoma.
Methods
Retrospective review of 308 charts of patients seen during a six-week period by a glaucoma specialist in his community practice in a large Hispanic area. Patients were classified as normal, ocular hypertensive, and those with Primary Open Angle Glaucoma. Odds ratios and 95% confidence interval were calculated to evaluate risk factors associated to ocular hypertension and Primary Open Angle Glaucoma. Finally, a multivariate polytomous regression model was used to evaluate central corneal thickness as an independent predictor of outcome after adjustment for age and hypertension. Statistical significance was set at p<0.05.
Results
Patients with Primary Open Angle Glaucoma show a statistically significant inverse correlation between central corneal thickness and the severity of the visual field damage.
Conclusion
Thinner corneas could be considered a risk factor for the severity of visual field loss in Primary Open Angle Glaucoma.
Keywords: thin corneas, potential risk factors, severity of glaucoma
INTRODUCTION
Glaucoma has a potential devastating effect on the quality of life of those affected. Approximately 66.8 million suffered this chronic disease in the year 2000, ten percent of whom were blind.1,2 It is one of the major causes of blindness in the US, and the first cause among blacks.3 Primary Open Angle Glaucoma (POAG) is the more frequent form of the disease in the Western world; of the major risk factors associated with the development of the disease, the Intraocular Pressure (IOP) remains the most important and the only one that is treatable.
From one quarter to a third of the patients treated keep losing visual field while maintaining pressures below 21 mm Hg.4,5 Twenty-seven percent of the patients with glaucoma may go blind in one eye after 20 years of treatment. These figures demand an early identification of those patients at high risk of progression to advanced stages in order to establish a more aggressive control of the IOP, and achieve a better preservation of the visual field and the quality of life of this population.
A thin central cornea is a well-known risk factor for the development of POAG6,7 and probably represents a predictor of greater severity of visual field loss in established patients.8,9,10,11,12 The purpose of this study is to determine the relationship between central corneal thickness (CCT) and the severity of POAG in Hispanics as determined by the severity of the visual field loss.
MATERIALS AND METHODS
We reviewed all clinical charts of patients evaluated consecutively during a six-week period by a glaucoma specialist at his community practice located in San Juan, the major city of the island of Puerto Rico.
Inclusion criteria (all needed):
Minimum of three visits.
An average CCT of the right eye recorded from multiple measurements on the same day with an Accutome Pachymeter V (Accutome, Malvern, Pennsylvania, USA). In the case of one-eyed patients, we use for the analysis the remaining eye.
At least one reliable Visual Field less than 18 months old for patients with Ocular Hypertension and POAG. We selected the latest study. All tests were 30-2, done with a Humphrey Field Analyzer (Carl Zeiss, Dublin, California, USA).
Exclusion criteria:
Secondary Glaucoma.
Angle closure Glaucoma.
Co-existence of retina or neurologic disease that affect the visual field.
Corneal disease that prevent an accurate measurement of CCT and the performance of a reliable Visual Field.
The selected patients were divided into three groups:
Normal: these patients had IOPs below 21 mm Hg (Goldmann Applanation Tonometry: GAT) in all visits. In addition, they had healthy looking optic nerves and a normal visual field and/or optic nerve imaging study if present, plus a recorded CCT.
Ocular Hypertension: this group of patients had at least one documented visit with an IOP greater or equal to 21 mm Hg (GAT). They also had healthy looking optic nerves accompanied by a visual field graded as stage 1 or less and a normal RNFL as tested with the Time Domain OCT (Stratus; Carl Zeiss, Dublin, California, USA).
POAG: this group of individuals had at least one visit with an IOP greater or equal to 21 mm Hg (GAT) with evidence of RNFL loss as tested with the Time Domain OCT, and/or visual field loss.
The glaucoma specialist classified the extent of visual field loss using the Glaucoma Visual Field Staging System described by Miles et al.13
The database construction and the statistical analysis were done using the statistical software STATA Version 11 (STATA Corp, College Station, TX, USA) and Graph Pad Prism version 5.1. Descriptive statistics were used to describe the study population by group (normal, OHT, and POAG). Continuous variables were described as mean, standard deviation, median, and range. Categorical variables were described using frequencies and proportions.
Correlation analyses of visual field (VF) stage and the study parameters CCT and age were done using the Spearman’s correlation coefficient.
Bivariate analyses were performed both ways, as continuous and categorical variables. For data evaluated in a continuous manner, one-way analysis of variance (ANOVA) or the Kruskal-Wallis test when applicable (for data with no Gaussian distribution), were employed to evaluate mean (or median) differences of study parameters by glaucoma group. Post-hoc analyses (by Bonferroni’s method for ANOVA or the Dunn’s test for Kruskal-Wallis) were subsequently performed to establish all possible pair wise comparisons. For data evaluated in a categorical manner, Pearson’s chi-square test or Fisher’s exact test (when applicable) were used. In addition, contingency tables and odds ratios (OR) with their 95% confidence intervals (CI) were also calculated to evaluate the association of study parameters with disease.
Finally, a multivariate polytomous logistic regression model was performed to evaluate CCT as a predictor of disease (OHT or POAG). All variables statistically (p<0.05) associated with disease (OHT or POAG) were included in the final model for adjustment purposes.
Statistical significance of all analysis performed was set at p<0.05.
RESULTS
Based on the selection criteria mentioned above, we included 308 patients. Table 1 shows the general characteristics of the subjects studied. POAG patients were significantly older and had thinner corneas as compared to both normal subjects and OHT patients. In addition, 32% of the POAG patients had a visual field stage equal or greater than three.
TABLE 1.
Characteristics of the study group.*
| Variable | Normal subjects (n = 53) |
OHT (n = 69) |
POAG subjects (n = 186) |
P-value |
|---|---|---|---|---|
| Age, years | ||||
| < 65 | 17 (32.1) | 22 (32.9) | 29 (15.6) | <0.0001† |
| 65–74 | 17 (32.1) | 33 (47.8) | 68 (36.6) | |
| 75+ | 19 (35.9) | 14 (20.3) | 89 (47.9) | |
| Sex | ||||
| Female | 38 (71.7) | 44 (63.8) | 107 (57.5) | 0.156 |
| Male | 15 (28.3) | 36 (36.2) | 79 (42.5) | |
| CCT, microns | ||||
| > 515 | 45 (84.9) | 58 (84.1) | 126 (67.7) | <0.0001† |
| ≤ 515 | 8 (15.1) | 11 (15.9) | 60 (32.3) | |
| HBP (by History) | ||||
| No | 39 (73.6) | 29 (42.0) | 92 (49.5) | 0.001† |
| Yes | 14 (26.4) | 40 (58.0) | 94 (50.5) | |
| DM (by History) | ||||
| No | 39 (73.6) | 54 (78.3) | 140 (75.3) | 0.822 |
| Yes | 14 (26.4) | 15 (21.7) | 46 (24.7) | |
| VF stage | ||||
| 0–2 | 53 (100.0) | 69 (100.0) | 127 (68.3) | <0.0001§ |
| 3–5 | 0 | 0 | 59 (31.7) |
Values are number of subjects (percentages) unless otherwise indicated.
OHT: Ocular Hypertension; POAG: Primary Open Angle Glaucoma; HBP: Hypertension; DM: Diabetes Mellitus; VF stage: Visual Field stage.
p<0.05-Pearson’s chi square;
p<0.05-Fisher’s exact test.
Patients with OHT had higher prevalence of Systemic Hypertension (HBP) when compared to both POAG patients and normal subjects (p<0.05). No statistical differences were observed by sex and Diabetes Mellitus (DM) status between OHT and POAG patients when compared to normal subjects (p>0.05).
Table 2 shows the odds ratio and 95% CI of selected risk factors. Older age was associated with a significant increased risk (p<0.05) of POAG; patients in the age groups of 65–74 years and 75 years of age and older had 2.3 times and 2.7 times the risk of a diagnosis of POAG, respectively, as compared to normal subjects. Conversely, age was not statistically associated with OHT patients.
TABLE 2.
Odd ratio (OR) and 95% confidence interval (95% CI) for the association of risk factors for glaucoma.
| Variables | OHT OR (95% CI) | POAG OR (95% CI) |
|---|---|---|
| Age, years | ||
| < 65 | 1.0 | 1.0 |
| 65–74 | 1.50 (0.63–3.55) | 2.34 (1.05–5.22) |
| 75 + | 0.57 (0.22–1.45) | 2.74 (1.26–5.97) |
| Sex | ||
| Female | 1.0 | 1.0 |
| Male | 1.44 (0.66–3.12) | 1.87 (0.96–3.64) |
| CCT, microns | ||
| > 515 | 1.0 | 1.0 |
| ≤ 515 | 1.07 (0.40–2.87) | 2.68 (1.19–6.04) |
| HBP | ||
| No | 1.0 | 1.0 |
| Yes | 3.84 (1.77–8.34) | 2.85 (1.45–5.59) |
| DM | ||
| No | 1.0 | 1.0 |
| Yes | 0.77 (0.34–1.79) | 0.92 (0.46–1.84) |
Reference category of multinomial logistic regression: normal subjects
In addition, a lower CCT was statistically associated with POAG. Patients with POAG had 2.7-increased risk of having a CCT below 515 microns as compared to normal subjects (p<0.05). However, this association was not found in OHT patients when compared to normal subjects. HBP was associated with both diagnosis, POAG and OHT. Patients with HBP had 2.9 the risk and 3.8 times the risk of having POAG and OHT, respectively, when compared to normal subjects (p<0.05).
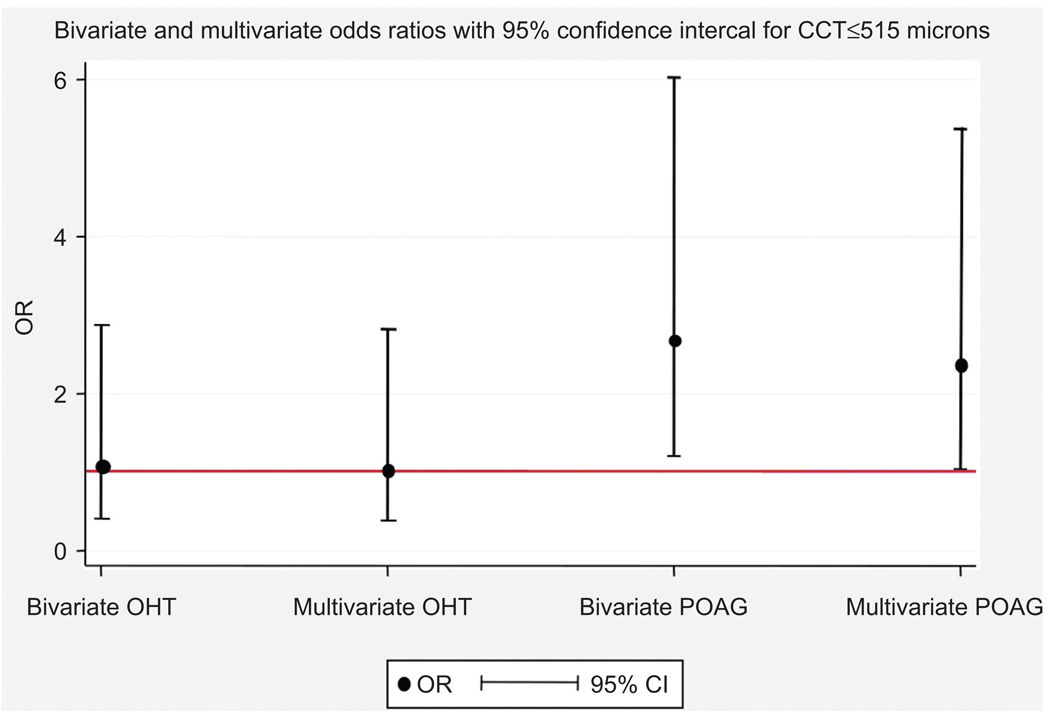
In multivariate polytomous regression analysis (Figure 1), CCT remains as an important independent predictor of POAG (p<0.05) after adjustment for age and HBP; patients with CCT ≤ 515 microns had 2.4 times the risk of POAG as compared to normal subjects. This association was not observed in patients with OHT when compared to normal subjects (p>0.05).
FIGURE 1.
Increased risk of POAG in patients with CCT ≤ 515 microns after adjustment for age and hypertension. Numbers in graph represents odds ratios and 95% confidence intervals: OR (95% CI) OHT: Ocular Hypertension; POAG: Primary Open Angle Glaucoma † Multivariate model is adjusted for age and hypertension.
Finally, Table 3 shows the statistically significant inverse correlation between CCT and visual field loss in POAG.
TABLE 3.
Correlations between CCT and VF stage in OHT and POAG.
| Group | N | Spearman’s ρ | p-value |
|---|---|---|---|
| OHT | 69 | −0.19 | 0.111 |
| POAG | 186 | −0.317* | <0.001 |
| Total | 308 | −0.372* | <0.001 |
DISCUSSION
The rate of progression in POAG although strongly related to the IOP is also influenced by other factors. Those patients with severe visual field loss need a more aggressive IOP control to avoid further loss of function.14 Early identification of patients at risk could modify the course of the disease, and in turn decrease its future morbidity.
In line with the current literature, we found that POAG patients had thinner corneas than OHT and normal subjects. However, in this study we did not find a statistically significance difference between the CCT of normal and OHT patients. The limited representation of normal subjects in our study may explain this result. The difference in CCT between POAG patients and OHT is in agreement to the studies published. 6,15 Systemic Hypertension seems to be a risk factor for the presence of OHT and POAG. Diabetes Mellitus does not present a similar risk. However, the self-reported nature of both diagnoses limits the value of these observations.
POAG patients with advanced disease have significantly thinner corneas. This finding has been recorded in other studies.8,9–12,16,17 The inverse correlation between CCT and VF stage underlines the importance of taking into consideration the corneal thickness in the long-term strategy of treatment of POAG. Underestimation of the IOP during long-term therapy is one of the possible explanations for this correlation. Future studies should address this possibility by including an average of the IOP during a long period of time with a correction for the IOP, in order to evaluate a pressure-independent risk of severe disease in POAG patients with a thin cornea as has been suggested by others.
ACKNOWLEDGMENT
This publication was possible by Grant number P20 RR11126 from the National Center for Research Resources (NCRR), a component of the National Institute of Health (NIH). We are grateful for the help and advice of Ms. Mariely Nieves-Plaza, MS, Ms. Karen J. Ortiz-Ortiz, MA, MPH, and biostatisticians from the Clinical Research Center of Medical Sciences Campus, University of Puerto Rico.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Coleman AL. Glaucoma. Lancet. 1999;354(9192):1803. doi: 10.1016/S0140-6736(99)04240-3. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’aale D, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization. November. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Ryskulova A, Turczyn K, Makuc D, Cotch M, Klein R, Janiszewski R. Self-reported age-related eye diseases and visual impairment in the United States: Results of the 2002 national health interview survey. American Journal of Public Health March. 2008;98(3):454–461. doi: 10.2105/AJPH.2006.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijl A, Bengtsson B, Hyman L, Leske MC. Early Manifest Glaucoma Trial Group. Natural history of open-angle glaucoma. Ophthalmology. 2009 Dec.116(12):2271–2276. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien, et al. IOP and the rate of of visual field loss. Am J of Ophthalmol. 1991;111:491–500. doi: 10.1016/s0002-9394(14)72386-4. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002 Jun;120(6):714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 7.Dadaci Z. Relationship between short wavelength perimetry and central corneal thickness values in ocular hypertensive subjects. Eur J Ophthalmol Sept. 2006;16(5):667–673. doi: 10.1177/112067210601600502. [DOI] [PubMed] [Google Scholar]
- 8.Meirelles SH. Relationship between corneal thickness and severity of visual field loss in primary open-angle glaucoma. Arq Bras Oftalmol. 2006 May;69(3):313–317. doi: 10.1590/s0004-27492006000300006. [DOI] [PubMed] [Google Scholar]
- 9.Rogers DL, Cantor RN, Catoira Y, Cantor LB, Dunn DW. Central corneal thickness and visual field loss in fellow eyes of patients with open-angle glaucoma. Am J Ophthalmol. 2007;143:159–161. doi: 10.1016/j.ajo.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan-Mee M. Relationship between asymmetric central corneal thickness and glaucomatous visual field loss within the same patient. Optom Vis Sci. 2006 Jul;83(7):516–519. doi: 10.1097/01.opx.0000218433.49803.e7. [DOI] [PubMed] [Google Scholar]
- 11.Patwardhan AA. The importance of central corneal thickness measurements and decision making in general ophthalmology clinics: a masked observational study. BMC Ophthalmol. 2008 Jan;8:1. doi: 10.1186/1471-2415-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimenez-Rodriguez E, Lopez-De-Cobos M, Luque-Aranda R, Lopez-Egea-Bueno MA, Vazquez-Salvi AI, Garcia-Campos JM. Relationship between central corneal thickness, intraocular pressure and severity of glaucomatous visual field loss. Arch Soc Esp Oftalmol. 2009;84:139–144. doi: 10.4321/s0365-66912009000300006. [DOI] [PubMed] [Google Scholar]
- 13.Mills RP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J of Ophthalmol. 2006;141(1):24–30. doi: 10.1016/j.ajo.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 14.Advanced Glaucoma Intervention Study. AGIS (7) Am J Ophthalmol. 2000 Oct;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 15.Singh RP, Goldberg I, Graham SL, Sharma A, Mohsin M. Central corneal thickness, tonometry, and ocular dimensions in glaucoma and ocular hypertension. J Glaucoma. 2001;10:206–210. doi: 10.1097/00061198-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Medeiros FA, Sample PA, Zangwill LM, Bowd C, Aihara M, Weinreb RN. Corneal thickness as a risk factor for visual field loss in patients with pre-perimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 17.Kniestedt C, Lin S, Choe J, Nee M, Bostrom A, Sturmer J, et al. Correlation between intraocular pressure, central corneal thickness, stage of glaucoma, and demographic patient data: Prospective analysis of biophysical parameters in tertiary glaucoma. J Glaucoma. 2006;15:91–97. doi: 10.1097/00061198-200604000-00003. [DOI] [PubMed] [Google Scholar]