Summary
Glucose infusion into rats causes skeletal muscle insulin resistance that initially occurs without changes in insulin signaling. The aim of the current study was to prolong glucose infusion and evaluate other events associated with the transition to muscle insulin resistance. Hyperglycemia was produced in rats by glucose infusion for 3, 5 and 8 h. The rate of infusion required to maintain hyperglycemia was reduced at 5 and 8 h. Glucose uptake into red quadriceps (RQ) and its incorporation into glycogen decreased between 3 and 5 h, further decreasing at 8 h. The earliest observed change in RQ was decreased AMPKα2 activity associated with large increases in muscle glycogen content at 3 h. Activation of the mTOR pathway occurred at 5 h. Akt phosphorylation (Ser473) was decreased at 8 h compared to 3 and 5, although no decrease in phosphorylation of downstream GSK-3 (Ser9) and AS160 (Thr642) was observed. White quadriceps showed a similar but delayed pattern, with insulin resistance developing by 8 h and decreased AMPKα2 activity at 5 h. These results indicate that, in the presence of a nutrient overload, alterations in muscle insulin signaling occur, but after insulin resistance develops and appropriate changes in energy/nutrient sensing pathways occur.
Keywords: Glucose infusion, Insulin signaling, Glucose uptake, Akt, AMPK, Glycogen
Introduction
Hyperglycemia and insulin resistance are hallmarks of type 2 diabetes. It is also clear that hyperglycemia by itself can cause, and/or exacerbate, insulin resistance in multiple tissues [1]. Skeletal muscle is quantitatively the most important tissue target of glucose postprandially and accounts for 70–80 % of insulin stimulated glucose uptake [2].
Early studies revealed that insulin resistance develops in skeletal muscle of rats infused with glucose at a high, but constant rate for 1–7 days [3–5] and that it occurs as early as day 1 of a glucose infusion [4]. Laybutt et al [5] reported that skeletal muscle of these animals had an increased long chain acyl-CoA (LCACoA) content, as well as activation of PKC, potentially implicating elevated diacylglycerol (DAG) levels as a pathogenic factor. They proposed that increased intramyocellular fatty acid metabolites may feed back onto the insulin signaling pathway to inhibit glucose uptake [5].
More recent studies in which variable rates of infusion were used to clamp blood glucose levels at ~11 mM revealed that insulin resistance develops at 5 h [6, 7]. They also revealed that at this time point it was accompanied by increases in the concentrations of malonyl-CoA and DAG and decreased AMPK activity, providing further evidence of a link between fatty acid metabolites and the hyperglycemia-induced insulin resistant state [7]. However, a subsequent study revealed that the insulin resistance generated by an acute glucose infusion at 5 h is not associated with decreased phosphorylation of proteins in the insulin signaling pathway (insulin receptor, Akt and AS160). Furthermore, it occurred without changes in maximal insulin-stimulated glucose transport capacity [6]. These findings suggested that the insulin resistance caused by a hyperglycemic insult initially may be due to excess glycogen storage and metabolic feedback, rather than a defect in insulin signaling [6].
A lack of an insulin signaling defect in the setting of insulin resistance is not in keeping with current thinking, which is based on the concurrent presence of abnormalities in insulin signaling and insulin resistance in a variety of circumstances [8]. The present study has two aims. The first is to determine if insulin signaling defects occur during a more prolonged exposure to hyperglycemia and if so whether they are associated with a further exacerbation of insulin resistance in skeletal muscle. The second is to identify other factors modified during a glucose infusion that could account for the decrease in muscle glucose uptake prior to changes in insulin signaling.
Materials and Methods
Animals
All surgical and experimental procedures performed were approved by the Garvan Institute/St Vincent’s Hospital Animal Ethics Committee and were in accordance with the National Health and Medical Research Council of Australia’s guidelines on animal experimentation.
Adult male Wistar rats (Animal Resources Centre, Perth, Australia) were communally housed in temperature controlled (22 ± 0.5 C) 12 h light-dark cycle rooms. Rats were fed ad libitum a standard chow diet (Rat Maintenance Diet; Gordon Specialty Feeds, Sydney, Australia). After a 1 week acclimatization period, cannulae were inserted into both jugular veins [6]. Average body weight on the day of experiment was 319 ± 8 g.
Glucose Infusion
Seven days after surgery, rats were randomly divided into treatment groups. After a basal blood sample was taken, a 50 % (w/v) glucose infusion commenced. Rats were infused for either 0, 3, 5 or 8 h using a peristaltic roller pump (101U/R; Watson-Marlow, Falmouth, UK). Blood samples were taken every 30 min and the glucose infusion rate was altered to maintain blood glucose concentration at ~11 mM. Red blood cells from each sample were resuspended in heparinised saline and returned to the animal. In one cohort, 2-deoxy-d-[2,6-3H]glucose and [U-14C]glucose (Amersham Biosciences, Buckinghamshire, UK) were administered as an intravenous bolus in the last 30 min of the glucose infusion. Blood samples were taken 2, 5, 10, 15, 20 and 30 min after administration of the tracer bolus for estimation of tracer clearance and blood glucose. Animals were then euthanized with an overdose of pentobarbital sodium (Nembutal; Abbott Laboratories, Sydney, Australia) and tissues were rapidly removed, freeze-clamped, and stored at −80 C for later analysis.
In the second cohort, rats were euthanized after the glucose infusion period ended (no tracer period). The soleus and EDL muscle from each leg was carefully dissected into 3 longitudinal strips from tendon to tendon with the use of a 25G needle and two strips of a similar weight from each leg were used. The third strip was immediately freeze-clamped (without further incubation) and stored at −80 C for later immunoblotting analysis. Glucose transport was assayed in isolated muscles from one leg in sealed vials containing pregassed (95% O2-5% CO2) Krebs-Henseleit bicarbonate buffer supplemented with 4 mM sodium pyruvate, 8 mM mannitol, and 0.1 % wt/vol BSA at 30°C. Muscles were preincubated for 30 min and were then incubated with or without insulin for 30 min. Insulin-stimulated glucose transport capacity was then assayed for 15 min using [3H]2DG (1 mM, 0.128 Ci/ml) in the presence or absence of 300 U/ml insulin, a dose similar to that reached in vivo (Table 1) [6].
Table 1.
Blood, whole body and muscle parameters of basal and glucose infused animals.
| Time of Glucose Infusion |
||||
|---|---|---|---|---|
| Basal | 3 h | 5 h | 8 h | |
| Whole Body | ||||
| Glucose (mM) | 4.4 0.2 | 11.5 0.5 a | 11.5 ± 0.2 a | 11.8 0.3 a |
| Insulin (mU/L) | 36 8 | 261 44 a | 335 24 a | 345 39 a |
| GIR (mg/min/kg) | N/A | 63 3 | 53 2b | 48 1b |
| Rd (mg/min/kg) | 12 1 | 58 3a,c,d | 52 1a,b | 48 1 a,b |
| Red Quadriceps | ||||
| Rg′ (mol/min/100g) | 8 3 | 40 2 a,c,d | 30 2 a,b,d | 23 1 a,b,c |
| Glycogen Content (mol/g) | 36 3 | 111 8 a,d | 122 6 a,d | 154 5 a,b,c |
| Glycogen Synthesis rate (mol/100g/min) | 2 1 | 24 3 a,c,d | 16 2 a,b,d | 8 1 b,c |
| White Quadriceps | ||||
| Rg′ (mol/min/100g) | 5 1 | 39 4 a,d | 35 2 a,d | 26 2 a,b,c |
| Glycogen Content (mol/g) | 38 3 | 70 5 a,d | 71 3 a,d | 94 5 a,b,c |
| Glycogen Synthesis rate (mol/100g/min) | 0.3 0.1 | 4.6 0.4 a | 4.2 0.5 a | 3.1 0.3 a |
Data are mean s.e.m.
P<0.05 vs basal,
P<0.05 vs 3 h,
P<0.05 vs 5 h,
P<0.05 vs 8 h glucose infusion. n=4–13.
GIR, glucose infusion rate; Rd, whole body glucose disposal; Rg′, glucose uptake into red or white quadriceps.
After the preincubation phase, the remaining muscle strips were transferred to buffer containing 5 mM glucose and 2 Ci of [U-14C]glucose to monitor glucose incorporation into glycogen. The resulting glycogen concentration and [14C]glucose incorporation into glycogen were measured as described previously [6, 9].
Analytic Methods
Blood and plasma glucose levels (YSI2300; Yellow Springs Instruments, Yellow Springs, OH), and plasma insulin (Linco, St Louis, MO) were measured.
Plasma and tissue levels of 3H- and 14C-labelled tracers were measured to calculate whole body glucose disposal rate (Rd) and to estimate red (RQ) and white (WQ) quadriceps glucose uptake (Rg′), [14C]glucose incorporation into glycogen and glycogen concentration. Assays and calculations are as previously described [9].
Long chain acyl-CoA (LCACoA) levels were measured as described by Antinozzi et al [10]. Malonyl-CoA was assayed radioenzymatically by a modification of the method of McGarry et al [11]. The measurement of DAG and ceramide content was determined as previously described [12]. Glucose metabolites were measured spectrophotometrically by standard enzymatic assays [13].
Western blot analysis
Protein extraction and immunoblots from RQ and WQ muscle homogenates were performed as previously described [6]. Antibodies raised against insulin receptor β were obtained from BD Biosciences (San Jose, CA), phospho-Tyr1162/63 insulin receptor from BioSource International (Camarillo, CA), phospho-Tyr612 IRS-1 from Sigma-Aldrich (St Louis, MO), Akt, phospho-Ser473 Akt, GSK-3β, phospho-Ser9 GSK-3β, IRS-1, phospho-IRS-1307 IRS-1, anti phospho-Ser2448 mTOR, anti phosphor-Thr389 p70S6K, total mTOR, p70S6K, phospho-Ser79 ACC and total ACC from Cell Signaling Technology (Danvers, MA), AS160 from Upstate Biotechnology (Lake Placid, NY), and phospho-Thr642 AS160, antibody was a gift from Symansis (Auckland, New Zealand).
AMPK activity assay
RQ and WQ muscle were homogenized in lysis buffer (50 mM Hepes (pH 7.4) 50 mM NaF, 5 mM NaPP, 10 % Glycerol, 1 mM EDTA (pH 8.0)) and incubated for 2 h at 4 C. Samples were then centrifuged at 13000 rpm for 10 min at 4 C. Protein concentrations were determined by a Bradford assay (Bio-Rad Regents Park, NSW, Australia). 100 g of the cleared lysate was incubated at 4 C with gentle mixing with an antibody to either the 1- or 2-subunit of AMPK (supplied by D. Carling, MRC, London) and with Protein G Sepharose beads (GE Healthcare BioSciences, Uppsala, Sweden). After 2 h, samples was spun at 13000 rpm, the supernatant was removed and washed in lysis buffer and then in HGE buffer (50 mM HEPES pH 7.4, 10 % glycerol, 0.1 mM EDTA). Beads were then incubated at 30 C with constant mixing in reaction buffer containing 40 mM HEPES pH 7.4, 80 mM NaCl, 5 mM MgCl2, 8 % glycerol, 800 M EDTA, 200 M AMP, 200 M AMARA peptide, 800 M dithiothreitol, 200 M ATP (0.148 MBq [-32P]ATP). After 20 min, the beads were pelleted and duplicate aliquots were spotted onto P81 Whatman photo-cellulose paper (Whatman Bioscience, Clifton, NJ), and washed 3 times in 1 % phosphoric acid before scintillation counting.
Statistical Analysis
Data are expressed as mean S.E.M. Differences between groups were determined via ANOVA and regression analysis using GraphPad Prism (Version 5.00 for Windows, GraphPad Software, San Diego, California, USA). When the ANOVA reached significance, a Newman-Keuls multiple comparison post hoc test was conducted. Significance was set at P≤0.05.
Results
Blood and plasma parameters
Blood glucose and plasma insulin levels were similar in all 4 groups in the basal state. Blood glucose was maintained at ~11 mM with a variable glucose infusion which also caused a 7–9 fold increase in plasma insulin levels (Table 1).
Whole body
The glucose infusion rate (GIR) required to maintain hyperglycemia was decreased significantly at 5 h (P<0.05; Table 1) and tended to decrease further by 8 h. Whole body glucose disposal (Rd) showed a similar pattern of change (P<0.05; Table 1).
Skeletal muscle
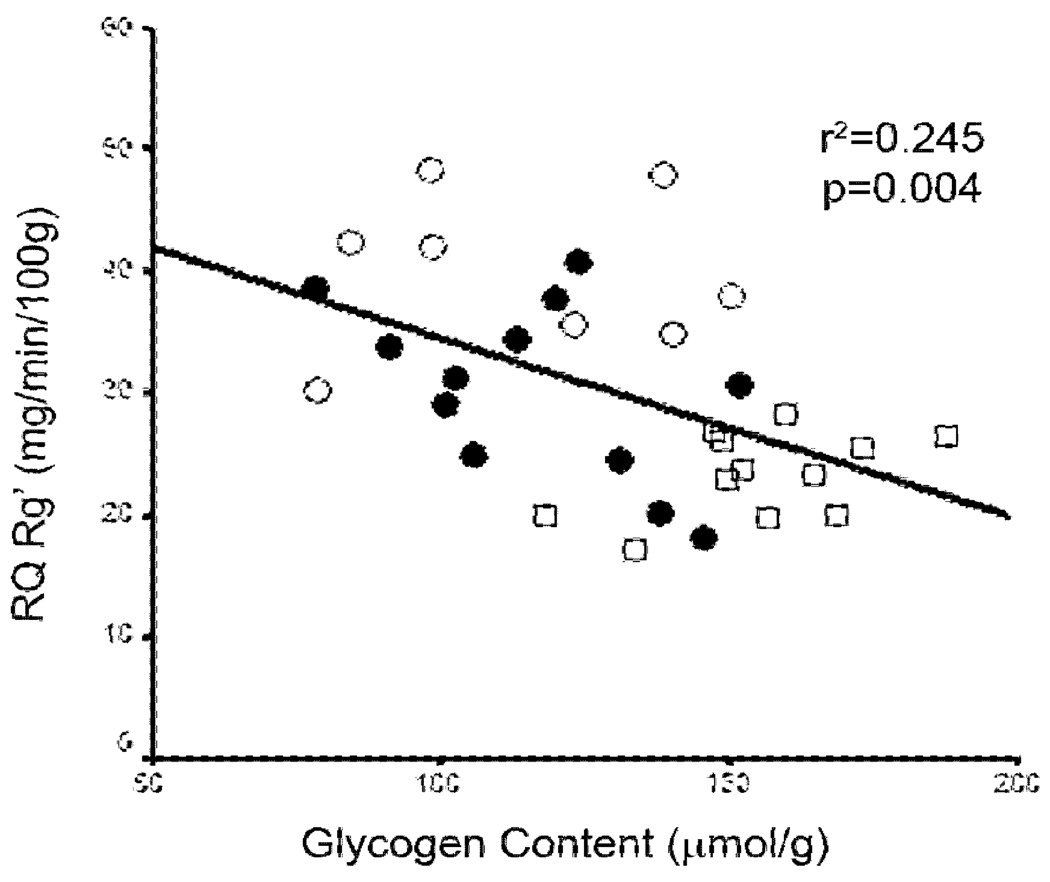
Glucose uptake (Rg′) into red quadriceps (RQ) muscle was significantly decreased after 5 h of glucose infusion compared to 3 h and was decreased further at 8 h (−22 % and −40 % vs 3 h respectively, P<0.05; Table 1). RQ glycogen content was increased ~3 fold after 3 h and 5 h of hyperglycemia with a small further increase at 8 h (Table 1). Accordingly, the rate of glycogen synthesis was significantly decreased at 5 h and was further diminished at 8 h (−32 % and −67 % vs 3 h respectively, P<0.05). When the data from the insulin stimulated groups were pooled, a negative relationship between glycogen concentration and glucose uptake in the RQ was evident (r2=0.245, p=0.004; Figure 1).
Figure 1.
Negative correlation between glucose uptake (RQ Rg`) and glycogen content in the red quadriceps muscle from insulin stimulated animals. Data expressed as individual points. Open circles 3 h, closed circles 5 h, open square 8 h.
Glucose uptake (Rg’) into white quadriceps (WQ) muscle was decreased at 8 h compared to 3 and 5 h glucose infusion (−33 % and −27 % respectively, P 0.05, Table 1) suggesting a delay in the development of insulin resistance in WQ compared to RQ. Glycogen content was increased at 3 and 5 h compared to basal with a further increase at 8 h, while the glycogen synthesis rate showed only a small, non-significant decrease at 8 h (Table 1). As in the RQ, when the data from the insulin stimulated groups were pooled, there was a negative relationship between glycogen content and glucose uptake, albeit weaker (r2=0.121, P=0.05).
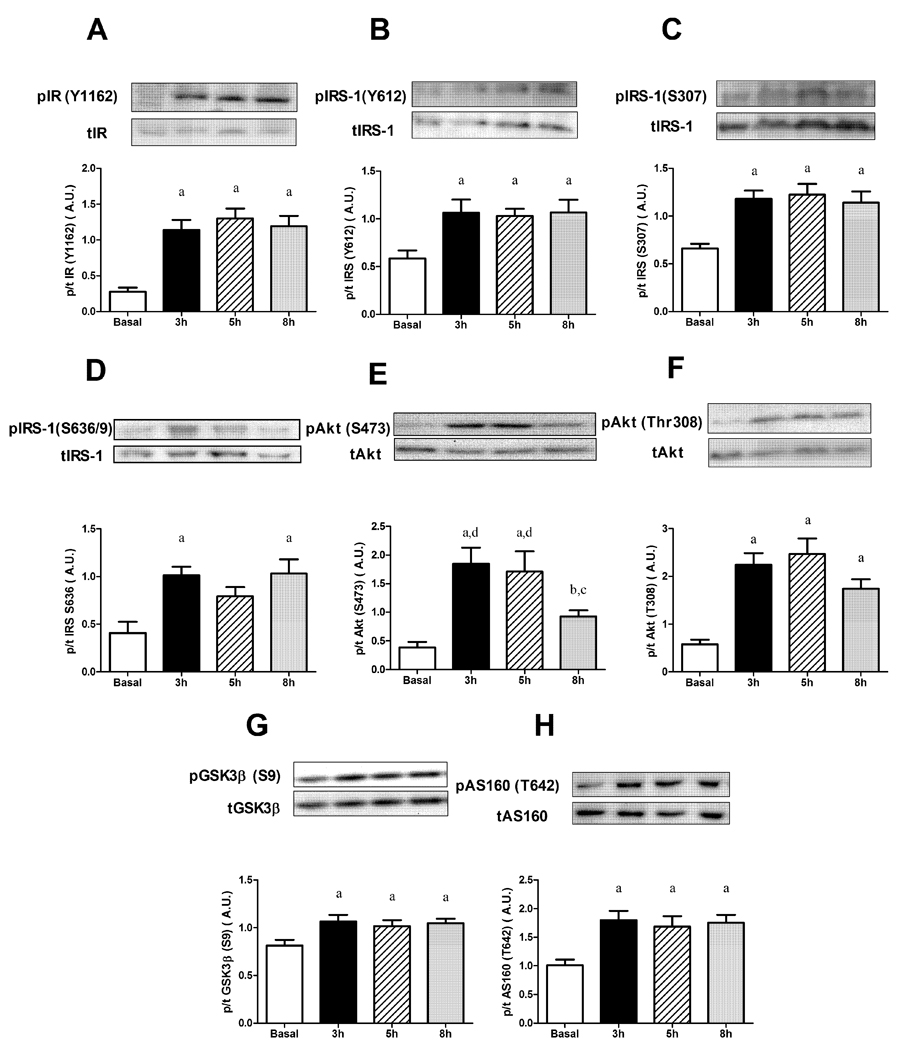
During the entire 8 h glucose infusion, in the RQ, no defects in either the tyrosine phosphorylation of the insulin receptor (Figure 2A), nor insulin receptor substrate-1 (IRS-1; Figure 2B) were observed, although serine phosphorylation of IRS-1 at Ser307 was increased (Figure 2C), compared to basal at 3, 5, and 8 h. Phosphorylation of IRS-1 at Ser636/639 showed a similar pattern (Figure 2D). An alteration in the insulin signaling cascade did occur at the level of Akt, with its phosphorylation at Ser473 decreased at 8 h of glucose infusion compared to 3 and 5 h (−50 %, P<0.05; Figure 2E). Phosphorylation of Akt at Thr308 showed a similar trend to be decreased at 8 h; however, it did not reach significance (Figure 2F). Two well described targets of Akt are GSK-3 and AS160. As shown in Figures 2G and H, the phosphorylation state of these proteins was increased compared to basal values at 3, 5 and 8 h of glucose infusion (P<0.05).
Figure 2.
Effects of glucose infusion on phosphorylation of insulin signaling intermediates in red quadriceps muscle. (A) Insulin receptor (IR Tyr1162); (B) Insulin receptor substrate-1 (IRS-1 Tyr612); (C) IRS-1 (Ser307); (D) IRS-1 (Ser636/9); (E) Akt (Ser473); (F) Akt (Thr308); (G) Glycogen synthase kinase-3 (GSK-3 ; Ser9); (H) Akt substrate of 160kDa (AS160; Ser642). Data expressed as mean s.e.m. n=6–15 rats per group aP<0.05 vs basal, bP<0.05 vs 3 h, cP<0.05 vs 5 h, dP<0.05 vs 8 h.
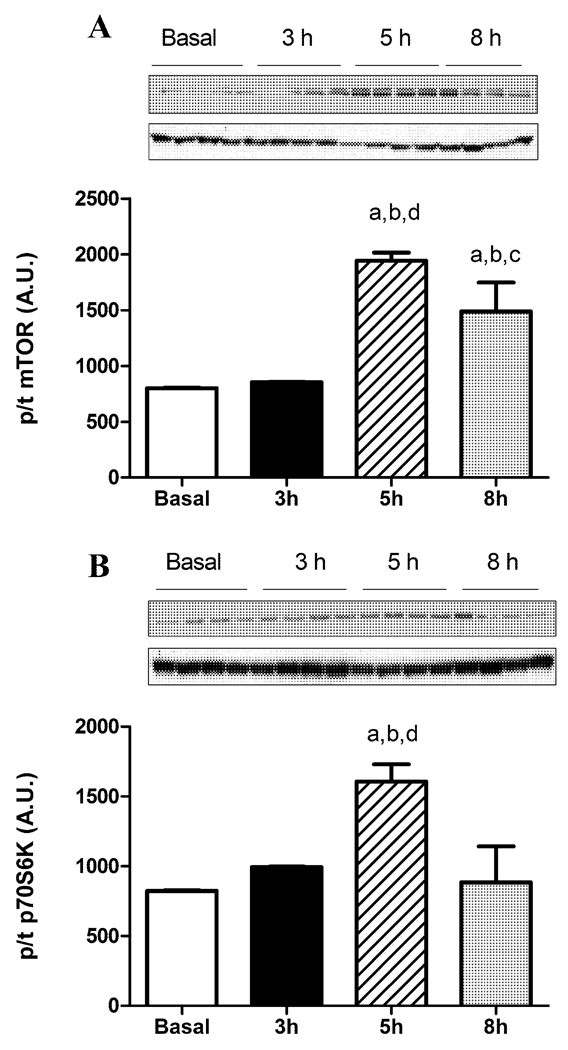
mTOR, a nutrient sensor and target of Akt involved in protein synthesis and many other events, showed an increased phosphorylation at 5 h glucose infusion when compared to basal and 3 h but was decreased slightly from 5 h levels at 8 h (Figure 3A). p70S6K, a target of mTOR, showed similar changes to that of mTOR (Figure 3B).
Figure 3.
Phosphorylation of (A) mTOR and (B) p70S6K after glucose infusion. Data expressed as mean s.e.m. n=4 rats per group aP<0.05 vs basal, bP<0.05 vs 3 h, cP<0.05 vs 5 h, dP<0.05 vs 8 h.
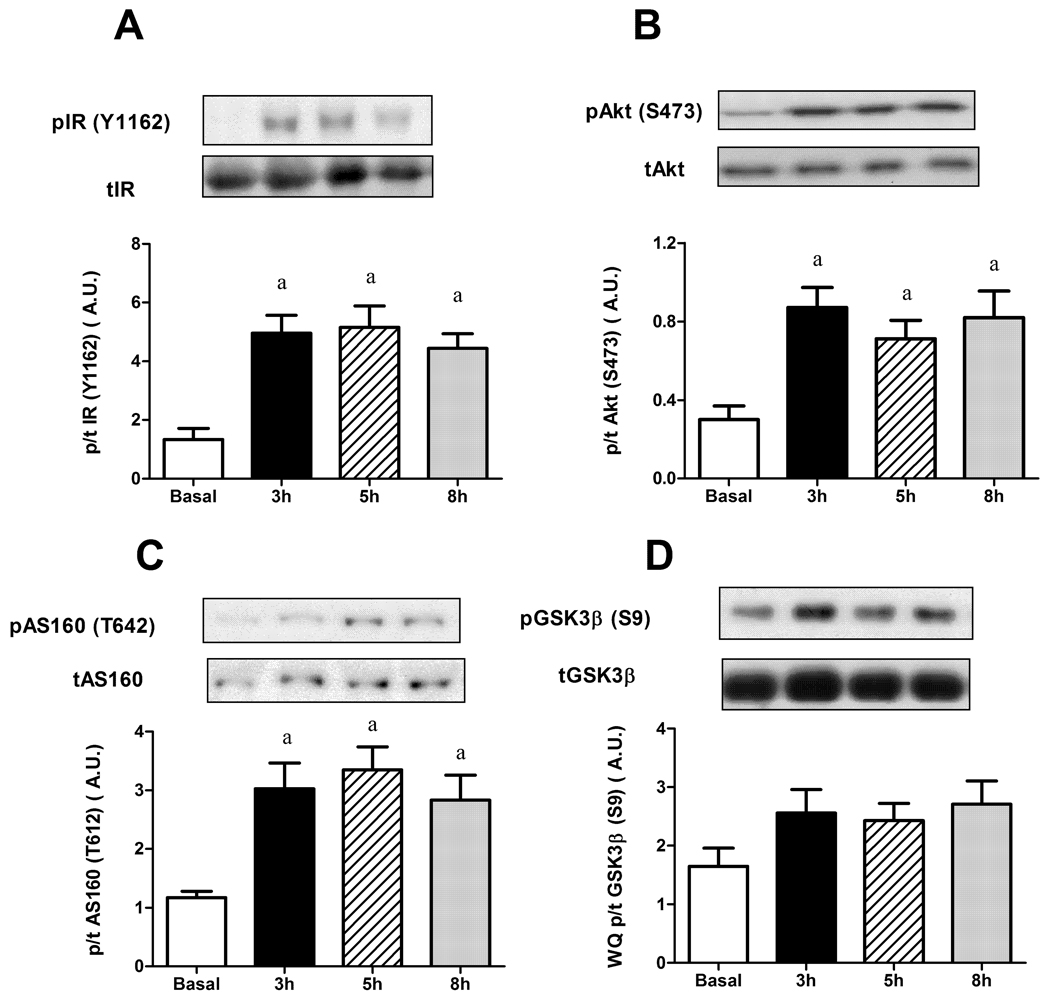
In the WQ, consistent with the delay in the development of insulin resistance, there was no decrease in the phosphorylation status of IR (Figure 4A), AS160 (Figure 4C) and more importantly, Akt (Figure 4B). While GSK3 looked as if it increased at all time points over basal, this did not reach statistical significance (Figure 4D).
Figure 4.
Effects of glucose infusion on phosphorylation of insulin signaling intermediates in white quadriceps muscle. (A) Insulin receptor (IR Tyr1162); (B) Akt (Ser473); (C) Glycogen synthase kinase-3 (GSK-3 ; Ser9); (D) Akt substrate of 160kDa (AS160; Ser642). Data expressed as mean s.e.m. n=6–13 rats per group aP<0.05 vs basal.
Incubated muscles
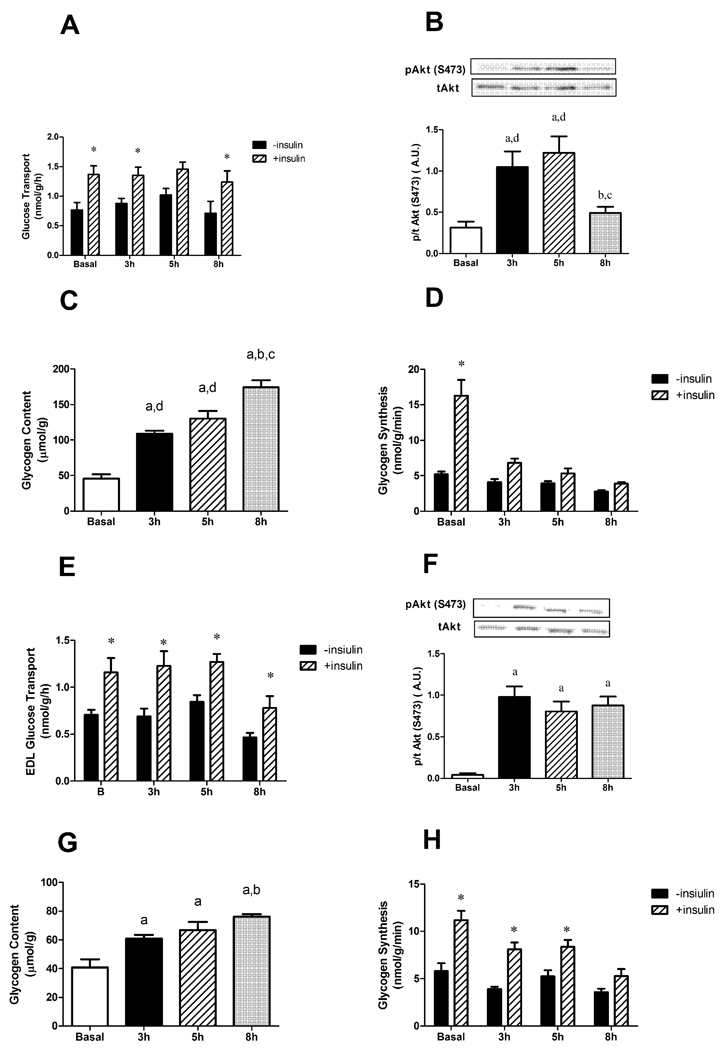
We next conducted ex vivo experiments to assess glucose transport in isolated soleus muscles, obtained from rats infused with glucose. This would allow us to determine if the first step in glucose uptake is affected by hyperglycaemia (i.e. glucose transport across the membrane). These studies revealed that insulin stimulated glucose transport was similar at all time points (Figure 5A). Likewise, as was observed in the red quadriceps in vivo, the isolated soleus showed decreased phosphorylation of Akt Ser473 at 8 h, and increased glycogen content and decreased glycogen synthesis at 3, 5, 8 h (Figure 5B, C and D).
Figure 5.
Effects of glucose infusion on (A, E) glucose transport, (B, F) Akt (Ser473) phosphorylation, (C, G) glycogen content, and (D, H) glycogen synthesis rate in response to insulin in isolated soleus (A,B,C,D) or EDL (E,F,G,H) muscles. Data expressed as mean s.e.m. n=4–6 rats per group aP<0.05 vs basal, bP<0.05 vs 3 h, cP<0.05 vs 5 h, *P<0.05 vs –Insulin.
Similar to the soleus, the EDL showed no alterations in glucose transport across the membrane in any of the time points (Figure 5E). Although at 8 h there seemed to be a decrease, this was not significant and more importantly, the ability of insulin to stimulate transport across the membrane (i.e. the change from basal) was not different. As with the in vivo WQ, ex vivo insulin stimulation of EDL strips showed no decrease in Akt phosphorylation, an increase in the glycogen content and delayed decrease in glycogen synthesis (5F, G and H).
AMPK activity, lipid content and glycolytic intermediates in vivo
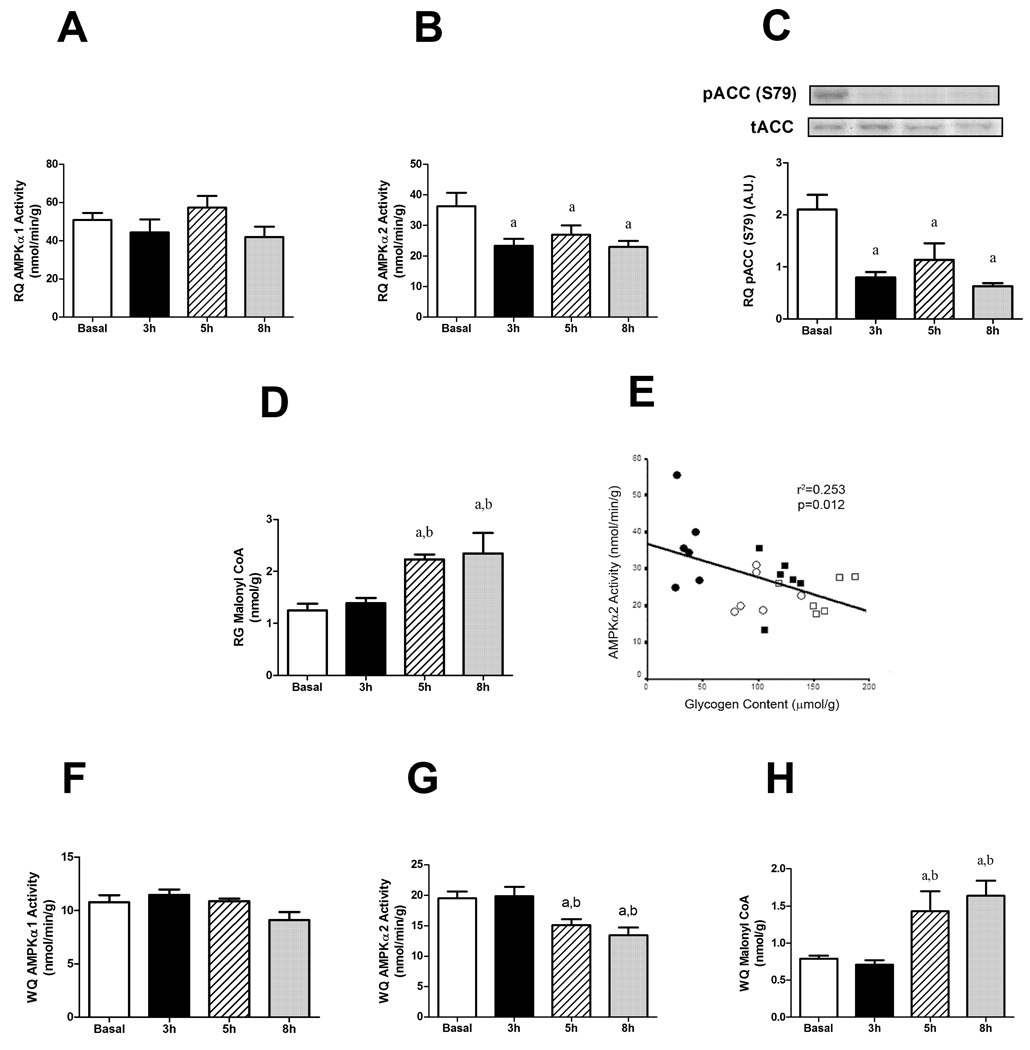
AMP-activated protein kinase (AMPK) is known to be an important nutrient sensor and has been linked to the regulation of glycogen synthesis [14]. In the RQ we found no change in AMPK 1 activity (Figure 6A); however, AMPK 2 activity was decreased at all three time points compared to baseline values in glucose infused animals (Figure 6B). In keeping with this finding, the phosphorylation of ACC was decreased at 3, 5 and 8 h (Figure 6C) and the concentration of malonyl CoA was increased at 5 and 8 h of glucose infusion (Figure 6D). When data were pooled from all groups, a negative relationship was found between AMPK 2 activity and glycogen content (r2=0.253, P=0.012; Figure 6E). As with the RQ, AMPK 1 activity did not change in the WQ (Figure 6F). Consistent with the delay in insulin resistance development in WQ, there was a decrease in AMPK 2 activity at 5 and 8 h (Figure 6G). Unlike the RQ there was no relationship between AMPK 2 activity and glycogen content (P=0.973, r2<0.001).
Figure 6.
AMPK 1 (A) or 2 (B) activities, malonyl CoA content (C) phosphorylation of ACC (D) and (E) negative correlation between AMPK 2 activity and glycogen content in red quadriceps following glucose infusion. AMPK 1 (F) or 2 (G) activities, malonyl CoA content (H) in white quadriceps muscle. Data expressed as mean s.e.m., except (D) individual points. n=4–6 rats per group aP<0.05 vs basal, bP<0.05 vs 3 h. Closed circles basal, open circles 3 h, open square 5 h, closed square 8 h.
To investigate if changes in lipid intermediates might have played a role in the decreased phosphorylation of Akt in RQ at 8 h, LCACoA, DAG and ceramide levels were measured. Table 2 shows that the hyperglycemic insult reduced LCACoA levels to a similar extent at each time point (P<0.05) and increased DAG levels at 5 and 8 h when compared to basal and 3 h (P<0.05). Interestingly, DAG-Kinase activity was decreased after 5 and 8 h of glucose infusion which could account for the increased DAG levels observed. Since malonyl CoA is an inhibitor of CPT1 and therefore of mitochondrial fatty acid oxidation, the increase in malonyl CoA at 5 and 8 h infusion (Fig 6D) could lead to an increase in the cytosolic pool of lipids and therefore contribute to the increase in DAG content seen here. Ceramide levels were increased by 50 % at 5 and 8 h; however the increases were not statistically significant (Table 2). There was no relationship between either DAG or ceramide content and Akt phosphorylation (P=0.535, r2=0.04 and P=0.457, r2=0.05 respectively). Glucose metabolites were also measured and there was a significant increase in cellular citrate levels after 8 h of glucose infusion and trends for increased G6P and decreased FDP at this time, neither of which were statistically significant (Table 2).
Table 2.
Lipid intermediates, DAG-Kinase activity and glucose metabolites in red muscle of basal and glucose infused animals.
| Time of Glucose Infusion |
||||
|---|---|---|---|---|
| Basal | 3 h | 5 h | 8 h | |
| Lipid Intermediates | ||||
| LCACoA (nmol/g) | 21 2 | 5 1a | 5 1a | 8 1a |
| Ceramide (nmol/g) | 25 7 | 27 8 | 43 8 | 40 9 |
| DAG (nmol/g) | 100 24 | 112 43 | 290 18a,b | 310 18a,b |
| DAG-Kinase (a.u.) | 2434 66 | 2240 149 | 1461 64a,b | 1407 22a,b |
| Glucose Metabolites | ||||
| Citrate (mol/g) | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.27 ± 0.02a,b,c |
| G6P (mol/g) | 1.54 ± 0.06 | 1.87 ± 0.15 | 1.53 ± 0.05 | 1.96 ± 0.30 |
| F6P (mol/g) | 0.20 ± 0.01 | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.19 ± 0.03 |
| FDP (mol/g) | 0.40 ± 0.06 | 0.29 ± 0.03 | 0.36 ± 0.05 | 0.35 ± 0.02 |
Data are mean s.e.m.
P<0.05 vs basal;
P<0.05 vs 3 h;
P<0.05 vs 5 h glucose infusion. n=4–13.
LCACoA, long chain acyl-CoA; DAG, diacylglycerol; G6P, glucose-6-phosphate; F6P, Fructose-6-phosphate; FDP, Fructose-1,6-bisphosphate. LCACoA measured in RQ, rest measured in RG.
Discussion
The present work extends previous studies showing firstly that decreased glucose uptake in muscle in response to 5 h of hyperglycemia is not accompanied by defects in insulin signaling in skeletal muscle [6] and secondly that suppression in AMPK activity occurs in this model [7]. We confirm these findings but go on to make further novel findings that help to characterize the early onset of insulin resistance in skeletal muscle in response to excess glucose availability. We demonstrate that the AMPK activity suppression precedes the onset of insulin resistance and occurs in concert with activation of the mTOR pathway, also involved in nutrient signaling. By prolonging the hyperglycemia beyond 5 h, a further reduction in red quadriceps muscle glucose uptake occurs. This took place with a selective perturbation of one component of the insulin signaling pathway, namely a 50 % reduction in the phosphorylation of Akt at Ser473 (a key site for maximal kinase activity). Interestingly, this reduction is not associated with decreases in either GSK3 or AS160 phosphorylation in vivo and the stimulation of glucose transport by insulin in isolated soleus muscle from these rats was not diminished. In addition, by extending the infusion to 8 h we show that while similar metabolic changes occur in white muscle (quadriceps) to that in red muscle, these changes are delayed (significant after 8 h but not 5 h infusion). It is noteworthy that this occurs without significantly altered Akt phosphorylation that may occur later. Our studies do not establish causality among the early muscle AMPK and mTOR changes and later insulin resistance. However, based on the time course data obtained here, taken with a recent in vitro study where insulin resistance is prevented by maintenance of AMPK activity in the presence of glucose elevation [15], we hypothesize that AMPK plays an important regulatory role influencing insulin potency in response to increased nutrient availability.
Under conditions of glucose oversupply, in the presence of insulin (e.g. during a glucose infusion in vivo), one mediator of decreased muscle glucose metabolism may be the degree of accumulation of muscle glycogen. Here we confirm previous reports demonstrating that muscle glycogen levels correlate negatively with glucose uptake (Figure 1) [16, 17]. While the precise mechanism for this remains uncertain, a number of reports attribute this to either inhibition of glucose transport, hexokinase and/or glycogen synthase activity [18–20]. Hyperglycemia has been shown to lead to a shift of the rate limiting step from glucose transport to post-transport steps [21].
AMPK 2 but not AMPK 1 activity was decreased in both red and white muscle prior to any decrease in glucose uptake and any changes to the insulin signaling pathway (Figure 4). It recently has been hypothesized that when the pool of muscle glycogen is enlarged, more AMPK is bound via its glycogen binding domain to glycogen where it phosphorylates glycogen synthase on site 2, decreasing its activity [14]. Since glucose incorporation into muscle glycogen is perhaps its major fate in a rat infused with glucose, a decrease in glycogen synthase activity could have been a major contributor to the decreased glucose uptake seen here.
A test of the role of suppression of AMPK activity on subsequent insulin resistance could conceivably be made by interventional studies such as concomitant AICAR infusion. This was investigated in pilot studies (not shown) but these confirmed that due to the inhibitory effects of AMPK activation on the pancreatic -cell to release insulin [22, 23], systemic infusion of AICAR cannot easily be interpreted in the current context. However, studies in isolated muscle strips have shown co-incubation in high glucose (+/− insulin) and AICAR can reverse the decreased AMPK activity, Akt phosphorylation [15] and the decreased glucose uptake [24] associated with hyperglycaemia. These studies, combined with our own data, suggest a role for AMPK in the development of insulin resistance due to hyperglycemia.
The present study indicates that decreased Akt phosphorylation is a relatively late marker of the decrease in glucose uptake by the red quadriceps muscle since it developed between 5 and 8 h of glucose infusion. Akt needs to be phosphorylated on two sites for full activation [25]. Thus, the decrease in its phosphorylation at Ser473 should theoretically have been sufficient to decrease the kinase activity of the enzyme. However, there was no change in the phosphorylation state of two well described targets of Akt, GSK-3 or AS160 (Figure 2E and F). Furthermore, insulin stimulated glucose transport in isolated soleus muscle strips was unaltered at 8 h glucose infusion (Figure 3D), indicating that the glucose transport step is unaltered. Thus decreased Akt phosphorylation of the magnitude observed may not be responsible for the further decline in glucose uptake during the 5–8 h time period. The non-linearity of Akt phosphorylation and downstream responses has support in the literature both from cell and animal studies. Whitehead et al [26] showed that in cultured adipocytes, only 20 % of the total Akt pool needs to be phosphorylated for maximal glucose uptake (as measured by 2DG uptake) to occur. More recently, Hoehn and colleagues [27] showed that in L6 myotubes, GLUT4 translocation is maximal despite as little as 5 % of the Akt pool being phosphorylated. In rats, 3 days of high fat feeding caused adiponectin resistance in skeletal muscle and this occurred with a decrease in Akt Ser473 phosphorylation but without changes in glucose uptake [28]. An important point from the current study is that although we observed a defect in Akt phosphorylation, the level was still a 2-fold increase over basal and appeared sufficient to maintain the ability of insulin to stimulate glucose transport.
In the red quadriceps we have shown an increased Tyr612, Ser307 and Ser636/639 phosphorylation of IRS-1 from 3 h of glucose infusion (Figure 4). We also show an increase in phosphorylation of mTOR and p70S6K at 5 h glucose infusion when the insulin resistance is occurring (Figure 6). mTOR, and its downstream target p70S6K, are nutrient sensors that are known to negatively feedback on the insulin signaling cascade by phosphorylating IRS-1 on Ser307 and Ser636/639 [29–31]. This is interesting as multiple studies have shown that serine phosphorylation can interfere with subsequent tyrosine phosphorylation, disrupting signal transduction [32, 33]. However, a recent study by Paul et al [34] showed a dissociation between tyrosine and serine phosphorylation. That is, animals fed a high fat diet had restored IRS-1 total tyrosine phosphorylation after a single bout of exercise and this occurred without a reduction in the increased Ser307 phosphorylation [34]. Hence it may be that under certain circumstances, as in the current study, there isn’t a linear relationship between serine and tyrosine phosphorylation of IRS-1. Lastly, the reciprocal variation between AMPK 2 activity and elevated mTOR and p70S6K, approximately coincident in time, supports a possible inverse cyclic interaction between these two pathways, as supported by recent genetic downregulation studies of S6 kinase [35].
Due to the lack of alterations in the phosphorylation state of proximal signaling intermediates (IR, IRS-1) it was unclear what caused the decreased Akt phosphorylation observed in the RQ after 8 h of glucose infusion. The selectivity of this defect at Akt is similar to that seen in cells treated with palmitate, or ceramide [36, 37]. We observed a ~50 % increase in mean ceramide content in red gastrocnemius in the current study; however, neither the increase in ceramide nor its correlation with Akt phosphorylation was statistically significant.
Our data indicates that white glycolytic muscle may be less susceptible to the onset of insulin resistance than red oxidative muscle. White quadriceps showed a significant delay in development of insulin resistance compared to red quadriceps (after 8 vs 5 h infusion). This is consistent with less glucose uptake into white muscle under equivalent conditions of insulin elevation [9]. Importantly and consistent with this finding was a delay in suppression of AMPK 2 activity in white versus red quadriceps muscle.
In conclusion, this study adds to the growing body of literature [6, 27, 38] that suggests that defects in the insulin signaling cascade and glucose transport are not always associated with impaired glucose uptake and metabolism in skeletal muscle. Thus other mechanisms appear to be responsible for the decrease in muscle glucose uptake and glycogen storage during the early stages of a glucose infusion (i.e. prolonged hyperglycemia and hyperinsulinemia). The results suggest that reduced AMPK activity and increased glycogen are possible contributors to these mechanisms.
Acknowledgments
The authors would like to thank Ms Emma Polkinghorne and Ms Jennifer Tid-Ang for their technical assistance.
Grants
GJC and EWK are supported by the National Health and Medical Research Council (NHMRC) of Australia’s Fellowship Scheme. AJH is supported by an NHMRC Biomedical Australian training fellowship. NT is supported by an NHMRC Career Development Award. This study was also funded by grants to NBR and EWK from the National Institutes of Health, USA (RO1 DK067509 and RO1 DK019514).
Abbreviations
- ACC
Acetyl CoA Carboxylase
- AMPK
5' adenosine monophosphate-activated protein kinase
- AS160
Akt substrate of 160KDa
- DAG
Diacylglycerol
- EDL
Extensor digitorum longus
- GSK-3β
glycogen synthase kinase 3 beta
- IR
Insulin receptor
- IRS-1
Insulin receptor substrate-1
- LCACoA
long chain acyl CoA
- mTOR
mammalian target of rapamycin
- p70S6K
p70S6 kinase
- Rd
whole body glucose disposal rate
- Rg’
glucose uptake
- RQ
Red quadriceps
- WQ
White quadriceps
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Unger RH, Grundy S. Diabetologia. 1985;28:119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 3.Hager SR, Jochen AL, Kalkhoff RK. Am. J. Physiol. 1991;260:E353–E362. doi: 10.1152/ajpendo.1991.260.3.E353. [DOI] [PubMed] [Google Scholar]
- 4.Laybutt DR, Chisholm DJ, Kraegen EW. Am. J. Physiol. 1997;273:E1–E9. doi: 10.1152/ajpendo.1997.273.1.E1. [DOI] [PubMed] [Google Scholar]
- 5.Laybutt DR, Schmitz-Peiffer C, Saha AK, Ruderman NB, Biden TJ, Kraegen EW. Am. J. Physiol. 1999;277:E1070–E1076. doi: 10.1152/ajpendo.1999.277.6.E1070. [DOI] [PubMed] [Google Scholar]
- 6.Hoy AJ, Bruce CR, Cederberg A, Turner N, James DE, Cooney GJ, Kraegen EW. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1358–E1364. doi: 10.1152/ajpendo.00133.2007. [DOI] [PubMed] [Google Scholar]
- 7.Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Am. J. Physiol. Endocrinol. Metab. 2006;290:E471–E479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- 8.Petersen KF, Shulman GI. Am. J. Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James DE, Jenkins AB, Kraegen EW. Am. J. Physiol. 1985;248:E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- 10.Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB. J. Biol. Chem. 1998;273:16146–16154. doi: 10.1074/jbc.273.26.16146. [DOI] [PubMed] [Google Scholar]
- 11.Saha AK, Kurowski TG, Ruderman NB. Am. J. Physiol. 1995;269:E283–E289. doi: 10.1152/ajpendo.1995.269.2.E283. [DOI] [PubMed] [Google Scholar]
- 12.Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM. J. Biol. Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- 13.Maizels EZ, Ruderman NB, Goodman MN, Lau D. Biochem J. 1977;162:557–568. doi: 10.1042/bj1620557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Diabetes. 2010;59:2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derave W, Hansen BF, Lund S, Kristiansen S, Richter EA. Am. J. Physiol. Endocrinol. Metab. 2000;279:E947–E955. doi: 10.1152/ajpendo.2000.279.5.E947. [DOI] [PubMed] [Google Scholar]
- 17.Jensen J, Jebens E, Brennesvik EO, Ruzzin J, Soos MA, Engebretsen EM, O'Rahilly S, Whitehead JP. Am. J. Physiol. Endocrinol. Metab. 2006;290:E154–E162. doi: 10.1152/ajpendo.00330.2005. [DOI] [PubMed] [Google Scholar]
- 18.Bogardus C, Lillioja S, Stone K, Mott D. J. Clin. Invest. 1984;73:1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. New Engl. J. Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 20.Hespel P, Richter EA. Biochem. J. 1992;284:777–780. doi: 10.1042/bj2840777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasserman DH, Ayala JE. Clin. Exp. Pharmacol. Physiol. 2005;32:319–323. doi: 10.1111/j.1440-1681.2005.04191.x. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Biochem. J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zhou L, Shao L, Qian L, Fu X, Li G, Luo T, Gu Y, Li F, Li J, Zheng S, Luo M. Life Sci. 2007;81:160–165. doi: 10.1016/j.lfs.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 24.Kawanaka K, Han DH, Gao J, Nolte LA, Holloszy JO. J. Biol. Chem. 2001;276:20101–20107. doi: 10.1074/jbc.M010599200. [DOI] [PubMed] [Google Scholar]
- 25.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. EMBO. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. J. Biol. Chem. 2001;276:27816–27824. doi: 10.1074/jbc.M011590200. [DOI] [PubMed] [Google Scholar]
- 27.Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullen KL, Pritchard J, Ritchie I, Snook LA, Chabowski A, Bonen A, Wright D, Dyck DJ. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008:R243–R251. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- 29.Um SH, D'Alessio D, Thomas G. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 31.Khamzina L, Veilleux A, Bergeron S, Marette A. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 32.Boura-Halfon S, Zick Y. Am. J. Physiol. Endocrinol. Metab. 2009;296:E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi CM, Emanuelli B, Kahn CR. Nature Reviews Molecular Cell Biology. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 34.Pauli JR, Ropelle ER, Cintra DE, Carvalho-Filho MA, Moraes JC, De Souza CT, Velloso LA, Carvalheira JB, Saad MJ. J. Physiol. 2008;586:659–671. doi: 10.1113/jphysiol.2007.142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. Cell Metab. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz-Peiffer C, Craig DL, Biden TJ. J. Biol. Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 37.Stratford S, Hoehn KL, Liu F, Summers SA. J. Biol. Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 38.Hoy AJ, Brandon AE, Turner N, Watt MJ, Bruce CR, Cooney GJ, Kraegen EW. Am. J. Physiol. Endocrinol. Metab. 2009:E67–E75. doi: 10.1152/ajpendo.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]