Abstract
Average blood loss after total knee arthroplasty (TKA) usually ranges from 1500 to 1900 cc, including both the postoperative drain and hidden blood loss. This represents about 46% of TKA patients requiring postoperative blood transfusion. Not only the risks of disease transmission but also those of ABO incompatibility, infection due to immunosupression, increased procedure costs, and increased length of hospital stay, are potential problems that foster blood saving strategies. In this study, 71 unilateral TKAs using a multimodal protocol to decrease blood loss were compared to 61 historical cases. Patients in both groups underwent cemented TKA with the same system, surgical technique, and multimodal protocol (MIS approach, plug in the femoral canal, tourniquet removal after wound closure and compressive bandage, analgesic periarticular infiltration with vasoconstrictor, postoperative drain at atmospheric pressure, opened 2 hours after the end of the surgical procedure and removed after 24 hours). The study series incorporated intravenous tranexamic acid (TXA) infusion in 2 doses of 10-15 mg/kg, 15 minutes before tourniquet release and 3 hours later. Results showed no transfusion requirements in the TXA series (0%), with 23/61 (37.7%) transfusions in the control, with an average cost decrease of 240 euros per patient. Visible bleeding in 24h significantly decreased from 553.36 cc (range 50-1500) to 169.72 cc (range 10-480) in the TXA series. As a conclusion, implementing a TXA-based multimodal protocol produced significant decrease in the transfusion rate, visible blood loss, and cost per patient, thus proving effectiveness and efficiency in the surgical management of TKA.
Keywords: Blood saving surgery, transfusion rate, TKA, tranexamic acid, effectiveness.
INTRODUCTION
Current surgical technique in total knee arthroplasty (TKA) usually includes the use of tourniquet, resulting in unapparent intraoperative bleeding but substantial postoperative blood loss. Postoperative drainage ranges 500 to 1000 cc, with additional hidden blood loss up to 700 cc [1]. Therefore, total blood loss is calculated, based on pre and postoperative hematocrit values, within a range of 1500 to 1900 cc [2]. Blood loss in TKA patients is multifactorial. Ischemia increases fibrinolysis, related to the proteolytic action of plasmin, with subsequent fibrinogen scission which limits postoperative coagulation and favors bleeding [3-5]. Factors that may influence bleeding after TKA include patient co-morbidities (cardiovascular, respiratory diseases, hepatic and coagulation disorders), medication (NSAIDs, salicilates, LMWH, antiagregants), anesthesia (spinal versus general), postoperative control of blood pressure (systolic under 150 mmHg), surgical technique (use of cement, incision, tourniquet time, haemostasia, tissue damage).
Allogenic blood transfusion associates the currently decreased risk of infectious disease transmission. Some of these cannot be detected by the laboratory (Chagas disease, Lyme disease, malaria, prion-mediated diseases), while others support a quantified risk, ranging from VHC (0.8/million), HIV (0.14/million), VHB (1.66/million), to bacterial contamination (2/million) [2, 6, 7]. Besides, iatrogenic severe error due to ABO discrepancy is also a potential risk, ranging from 1/400 units in Belgium or 1/3400 in France, to 1/36000 units in Germany [8]. Allogenic blood transfusion in Europe was evaluated in a multicentric study after hip and knee unilateral replacement surgery on 3996 patients, and 46% transfusions were required, in correlation with low preoperative hemoglobin. Infection rate in those patients raised to 4%, compared with 1% in case of auto-logous transfusion [9], possibly related to immunodepresion associated with allogenic transfusion [9, 10].
The mentioned risks, including increased morbidity and longer Hospital stays, but also refusals due to religious beliefs (as in the case of Jehovah’s witnesses [11]) justify clinical strategies to minimize exposure to allogenic blood transfusion. As hyperfibrinolysis is considered the major cause of postoperative bleeding after TKA surgery [3, 4, 12-14], antifibronolytic drugs have been proposed, including aprotin, aminocaproic acid, and tranexamic acid. Aprotin, derived from bovine lung, inhibits the serinprotease at the last stage of fibrinolysis, but allergies, thrombosis, nephrotoxicity, and spongiform encephalopathy [6] lead this drug out of the Spanish market. Aminocaproic acid is less effective, more expensive and less supported by the literature than tranexamic acid [3, 15]. Tranexamic acid (TXA) is a synthetic analog of serin than reversibly inhibits fibrinolysis by blocking lysine union sites in the plasmin and plasminogen activator molecules. It has been used during more than 20 years in cardiac surgery, urology, ginecology, liver transplants, etc.
In this context, the aim of our study was to compare transfusion rate, postoperative bleeding, and additional costs, in a consecutive cohort of patients under a multimodal blood saving protocol using tranexamic acid, with a historical consecutive cohort in the same settings.
PATIENTS AND METHOD
A cohort of 71 consecutive patients (study group) with unilateral TKA was prospectively studied. These patients received 2 doses of tranexamic acid (Amchafibrin, Rottapharm, S.A. Barcelona, Spain). A first dose of 15mgr/kg weight in 100cc saline was slowly infused in 15-20 minutes, before tourniquet release. A second identical dose was administered after 3 hours. In a case-control study, this group was compared with a consecutive series of 61 patients that underwent TKA before the clinical protocol was introduced. Surgical technique, pre- and postoperative management, and transfusion criteria were the same between both series. Both series included patients in the same geographic area (North Madrid, Spain), and underwent surgery by the same group of experienced surgeons and anesthesiologists, with special dedication to this surgery. Variables under comparison included hematocrit and hemoglobin determinations, visible blood loss, transfusion requirements, and additional costs generated by transfusion. This observational study was approved by the local ethical committee and all the patients signed the informed consent.
Surgeries in the studied series with tranexamic acid were performed between October 2008 and May 2009, and the control series was included in the prior 6 months. Exclusion criteria were previous thromboembolic disease, previous myocardium infarct, hematologic disease, and retinopathy. Four patients were excluded in the study period. Anesthesia in all cases was hyperbaric intradural with 0.5% bupivacaine, fentanyl, and continuous IV infusion of midazolam and/or propofol. Ischemia was performed with pneumatic tourniquet at 100mmHg above systolic arterial pressure. Limited anterior incision followed by parapatelar medial approach without patellar eversion and minimally invasive surgical instrumentation were used in all cases. The technique included cemented posterostabilized NexGen® (Zimmer, Warsaw, Indiana, USA) prosthesis with systematic patellar replacement, except in 3 cases of the TXA group that received a non cemented NexGen® prosthesis with tantalium in the tibial and titanium mesh in the femoral component. Cement in use was Palacos R+G (Heraeus Medical, Hanau, Germany), and autologous bone was used to fill the drill of the intramedullary guide into the femoral canal before cementing. All cases underwent a multimodal analgesia protocol, as well as intra- and periarticular injection of 80cc of saline with adrenalin 300µgr, morphic chloride 10mgr, tobramicin 100mgr, betametasone sodic phosphate 6mgr, betametasone acetate 6mgr, ropivacaine 200mgr (30cc in the posterior capsule, collateral ligaments, and medial wall before cementing, 50cc in the arthrotomy after closure and intraarticularly). One single intraarticular drain (Drenofast CH-12/4,0 min Iberhospitex, S.A. Barcelona, Spain) was used per surgery, at atmospheric pressure without vacuum, opened one hour after closure and systematically retrieved after 24 hours. Tourniquet release occurred after wound closure and knee bandage in all cases. Antithromboembolic prophylaxis in both groups was performed with enoxaparin (Clexane 40mg, laboratorio Sanofi-Aventis, S.A., Barcelona, Spain), 40 mgr via subcutaneous injection during 3 weeks. Antibiotic prophylaxis included cephalosporin 2gr 1 hour before surgery and 1gr/8h during 48 hours. In allergic patients, vancomicin 1gr pre and 1gr/12h postop were used.
Transfusion was decided in both groups by the Orthopaedic surgeon and/or Anesthesiologist on call. As a general rule, hemoglobin laboratory determination under 8.0 gr/dL and/or related symptoms of acute anemia required transfusion, whereas in patients with cardiovascular or pulmonary comorbidities, the threshold was set at 9.0 gr/dL. Repeated laboratory tests including both hematocrit and hemoglobin determination were performed preoperatively and postoperatively in the recovery unit, and days 1 and 2). The direct cost of a single unit of red cell concentrate in our Hospital was 300 euros. The direct cost of the administered TXA was 3.05 euros.
In the statistical analysis, quantitative variables are expressed as mean and standard deviation, and qualitative variables by absolute and relative frequencies. The quantitative variables were compared with Student’s t-test, when Kolmogorov-Smirnov test confirmed the normal distribution. The comparison of qualitative variables was performed by Chi-square and Fisher’s exact test. The sequential evolution of hematocrit and hemoglobin determinations was analyzed by two-factor ANOVA for repeated measurements. Differences between groups in evolution profiles were also evaluated. Bonferroni post-hoc test was used when required. SPSS 15.0 was used as statistical software (SPSS Inc., Chicago IL).
RESULTS
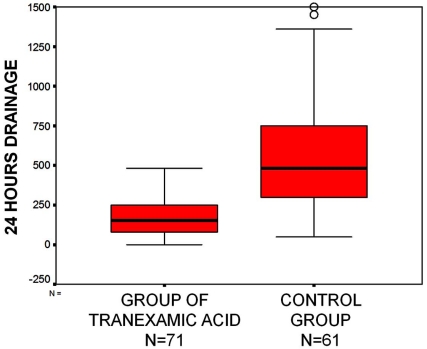
Both study groups were similar, with no statistically significant differences in demographics, surgical variables, and preoperative laboratory parameters, as seen in Table 1. Statistically significant differences were found in the amount of visible bleeding at the drain in 24h, and in the percentage of transfused patients (Table 2), with significantly less bleeding and less transfused patients in the TXA group (Fig. 1). While no patients received transfusion in the group treated with tranexamic acid, 23 out of 61 patients required transfusion in the control group (37.7%), 2 of which received pre-donated autologous blood. These 23 patients consumed in total 49 units of concentrated red cells, 4 of them requiring 1 unit, 14 required 2 units, 4 required 3 units and one patient required 5 units.
Table 1.
Demographics and Surgical Variables in Both Studied Groups of Patients (Mean (Range); p Values After Statistical Comparison, Student t-Test or Fischer’s Exact Test)
| Control Group (N = 61) | Tranexamic Acid Treatment Group (N = 71) | p Value | |
|---|---|---|---|
| Age (years) | 69 (52-82) | 71 (53-85) | p=0.144 |
| Sex ratio: women/men | 43/18 | 55/16 | p=0.426 |
| Weight (kg) | 78.07 (53-118) | 76.69 (53-108) | p=0.486 |
| ASA I ASA II ASA III |
2 37 22 |
1 47 23 |
p=0.639 |
| Tourniquet time | 86.2 minutes (55-120) | 92.2 minutes (65-120) | p=0.350 |
| Cemented implants | 61 cemented | 68 cemented/3 non-cemented | |
| Preoperative hematocrit (%) | 42.3 (34.1 -51.9) | 43.0 (31.9 - 50.5) | p=0.294 |
| Preoperative hemoglobin (g/dL) | 14.4 (11.9 - 17.9) | 14.3 (10.6 -17.2) | p=0.642 |
ASA = American Society of Anaesthesiology Status.
Table 2.
Outcome Variables in Both Studied Groups of Patients (Mean (Range); p Values After Statistical Comparison, Student t-Test or Fischer’s Exact Test)
| Control Group (N=61) | Tranexamic Acid Treatment Group (N=71) | p Value | |
|---|---|---|---|
| Bleeding in 24h drain (cc) | 553.36 (50-1500) | 169.72 (10-480) | p=0.001 |
| Transfusion (% patients) | 23 patients (37.7%) | 0 patients (0%) | p=0.001 |
Fig. (1).
Box plot (mean and error bars of standard deviation) of 24-hour drainage for both groups in the study: the group with tranexamic acid (n=71 patients) and the control group (n=61 patients).
Hemoglobin and hematocrit evolution in the 48h of observation was significantly different between the two groups (p=0.001). The studied values decreased more in the control group (Table 3) than in the TXA group (Table 4), even if the control group received an average of 0.8 units of concentrated red cells.
Table 3.
Blood Loss Evolution (48 h) in the Control Group (without Tranexamic Acid), n=61 Patients
| Preoperative | 6 h | 24 h | 48 h | |
|---|---|---|---|---|
| Hemoglobin g/dL | 14.4(11.9 - 17.9) | 12.2 (9.0 - 14.1) | 10.9 (7.5 - 13.3) | 10.3 (7.4 - 12.8) |
| Hematrocrit % | 42.3 (34.1 -51.9) | 36.3 (27.3 -41.8) | 32.0 (24 - 42.6) | 30.7 (25.3 -36.1) |
Table 4.
Blood Loss Evolution (48 h) in the Tranexamic Acid Group, n=71 Patients
| Preoperative | 6 h | 24 h | 48 h | |
|---|---|---|---|---|
| Hemoglobin g/dL | 14.3 (10.6 -17.2) | 12.7 (9.7 - 15.1) | 12.1 (8.9 - 12.8) | 11.3 (8.7 - 12.0) |
| Hematrocrit % | 43.0 (31.9 - 50.5) | 38.0 (29.0 -42.3) | 36.3 (25.9 -37.8) | 33.7 (27.0 -35.6) |
The cost of one single unit of red cells in our Hospital being 300 euros, the total cost of red cell units for the control series was 14.700 euros. Because an average of 0.8 units was transfused per patient in the control, the average direct cost per patient was 240 euros.
Special care was taken in the diagnosis of deep venous thrombosis (DVT). Patients with clinical signs of calf circumference increase underwent venous eco-doppler performed by an experienced vascular radiologist. This was required in 7 patients of the control group and 3 in the TXA group. In all of them, the deep venous system was permeable. Nevertheless, these patients received one month treatment of low-molecular weight heparin at therapeutic dosage. No patient presented with pulmonary embolism. One patient in the control group suffered from deep periprosthetic infection by S. aureus 4 months after surgery, and required two-stage revision surgery. Two patients in the study group underwent intense nausea related to tranexamic acid infusion in less than 15 minutes. Slow infusion (more than 15 minutes) of tranexamic acid is required to avoid this complication.
DISCUSSION
Different strategies have been established to decrease the risk of allogenic blood transfusion in the postoperative patient. In our study, we significantly decreased the risk of allogenic blood transfusion through a protocol that included two doses of tranexamic acid. Other techniques include predonation, blood recovery, preoperative treatments, or hemodilution.
Preoperative autologous blood donation followed by autotransfusion is an expensive procedure with logistic problems in many Hospitals. Furthermore, about 45% predonated units may be discarded due to different reasons [10]. Some authors recommend at least 50% transfusion to support a predonation program [16]. Postoperative blood recovery and reinfusion is an equivalent of autologous donation [2, 17]. As the risk of transfusion depends on the preoperative hemoglobin values, preoperative treatments such as eritropoietin and intravenous iron have been proposed to avoid postoperative transfusion [15, 16]. Patients with anemia (preoperative hemoglobin under 12 g/dL) could benefit, but with a raise in the procedure cost (in our setting, 3000 euros if 3 doses are prescribed per patient). Normovolemic hemodilution has been also proposed [18], consisting on the collection of 1-2 blood units during surgery and reinfusion when hematocrit decreases under 28%. Criticisms include those of reinfusion from blood cell recovery programs, variable patient tolerance, and unnecessary blood collection in those patients not requiring postoperative transfusion, with subsequent discard of these units.
Multiple surgical variables may affect postoperative blood loss, and although isolated surgical gestures may represent limited savings, the simultaneous use of these techniques may produce a substantial blood loss reduction. Tourniquet release after wound closure and bandage proved blood loss decrease in many studies [19-21]. A meta-analysis including 11 prospective randomized trials concluded that tourniquet release to perform surgical hemostasis before closure was related to an increased bleeding of 229cc in average [20]. Limited incision with minimally invasive surgery technique (MIS) was also associated to a decreased blood loss and hospital length of stay [22, 23]. Plugging the intramedullary canal of the femur after introduction of the intramedullary instrumentation guide significantly reduced blood loss in TKR up to 17% [24]. Intra- and peri-articular local infiltration to postoperatively control pain, associating vasoconstrictor medication, also reduced blood loss [25, 26]. Blood loss decreased in all-component cemented TKR due to the hemostatic effect of cement polymerization on the exposed bone [27]. The use of drainage raised the transfusion rate in a meta-analysis of 18 prospective randomized controlled trials [28, 29]. Not using drains did not raise the infection rate or local wound complications [28, 29]. In our study, we just used one single atmospheric pressure drainage system during 24h, and the drainage opening was delayed one hour after surgery. Also, our transfusion rate in unilateral, cemented primary TKR was 48%, and decreased to 37.7% after using the limited approach with MIS technique, and the vasoconstrictor in the multimodal postoperative analgesia protocol.
Although tourniquet decreases TKR intraoperative bleeding, postoperative blood loss occurs due to an increased fibrinolysis in response to exanguination [5, 13, 30, 31]. Tranexamic acid inhibits fibrinolysis through a reversible molecular block of lysine union sites in the plasmin, plasminogen, and tissue activator of plasminogen. This antifibrinolytic molecule is 10 times more effective than aminocaproic acid. Effective plasmatic levels are reached 10-15 minutes after infusion, and therapeutic level must be maintained at tourniquet release, being the initial dose administration crucial to efficacy [3-5, 13, 14, 30]. Safety was also a matter of concern regarding the possible thrombogenic effect with the use of tranexamic acid, but this was not found in different studies [3-5, 30, 31]. A meta-analysis of 8 randomized studies comparing with placebo concluded that TXA use is effective in decreasing allogenic blood transfusion requirements without increasing the thrombosis rate in TKR [3]. Effectiveness is also a matter of controversy, as more recent randomized clinical trials proved a significant early postoperative decrease in blood loss, but one single TXA dose failed to confirm a significant association with total blood loss or transfusion requirements [32]. Meanwhile, other authors also used one single intraoperative dose of TXA and reduced transfusion rates [33]. In a recent systematic review of randomized trials [34], the use of antifibrynolytic agents to reduce bleeding and transfusion risk to a 50%, without increasing thromboembolic risk, is well proven. More debate persists on the dosage and timing of administration, and heterogeneity limits the strength of conclusions on these aspects in the mentioned systematic review [34], this debate justifying further studies to maximize effectiveness. In our experience, a multimodal protocol with tranexamic acid administration in two doses of 15mg/kg, the first dose infused 15-20 minutes before tourniquet release, and repeated after 3 hours, virtually revoked the need of transfusion in our series, from a rate of 37.7% in the control group to 0% in the treatment group, while the visible bleeding in the 24h drainage also decreases from 553.3 cc in the control group to 169.7cc in the treatment group. Our conjoined strategy shows notable results in restricting transfusion requirements, and its use is simple and safe, not increasing thromboembolic complications in our hands. Saving an average of 0.8 concentrated blood cell units per patient represented direct cost savings of 240 euros per procedure in our settings, not considering the indirect savings related to allogenic transfusion risks and derived expenditure.
The results of this multimodal protocol allowed us to incorporate primary, unilateral TKR procedures without blood transfusion into our offer of services as a provider, giving an increased demand by Jehova’s witnesses and other groups of patients. Based on this study, we can conclude that two-dose IV tranexamic acid, in a multimodal protocol, can be used in TKR procedures with proven effectiveness and efficiency to decrease postoperative blood loss in our patients undergoing TKR.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Rosario Madero, statistician, Hospital La Paz, for her assistance with the statistical analysis of this study.
REFERENCES
- 1.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4 ):561–5. [PubMed] [Google Scholar]
- 2.Woolson ST, Wall WW. Autologous blood transfusion after total knee arthroplasty: a randomized, prospective study comparing predonated and postoperative salvage blood. J Arthroplasty. 2003;18(3 ):243–9. doi: 10.1054/arth.2003.50058. [DOI] [PubMed] [Google Scholar]
- 3.Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: results of a meta-analysis of randomized controlled trials. Transfusion. 2005;45(8 ):1302–7. doi: 10.1111/j.1537-2995.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 4.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3 ):434–40. [PubMed] [Google Scholar]
- 5.Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84(4 ):839–44. doi: 10.1097/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt PE, Llewelyn CA, Mackenzie J, Will RG. Creutzfeldt-Jakob disease and blood transfusion: results of the UK Transfusion Medicine Epidemiological Review study. Vox Sang. 2006;91(3 ):221–30. doi: 10.1111/j.1423-0410.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 7.Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br. 2008;90(9 ):1128–36. doi: 10.1302/0301-620X.90B9.21115. [DOI] [PubMed] [Google Scholar]
- 8.Chiaroni J, Legrand D, Dettori I, Ferrera V. Analysis of ABO discrepancies occurring in 35 French hospitals. Transfusion. 2004;44(6 ):860–4. doi: 10.1111/j.1537-2995.2004.03337.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43(4 ):459–69. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 10.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1 ):2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Nelson CL, Bowen WS. Total hip arthroplasty in Jehovah's Witnesses without blood transfusion. J Bone Joint Surg Am. 1986;68(3 ):350–3. [PubMed] [Google Scholar]
- 12.Camarasa MA, Olle G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5 ):576–82. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 13.Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10 ):1206–11. doi: 10.1034/j.1399-6576.2002.461007.x. [DOI] [PubMed] [Google Scholar]
- 14.Zohar E, Ellis M, Ifrach N, Stern A, Sapir O, Fredman B. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg. 2004;99(6 ):1679–83. doi: 10.1213/01.ANE.0000136770.75805.19. [DOI] [PubMed] [Google Scholar]
- 15.Faris PM, Ritter MA, Abels RI The American Erythropoietin Study Group. The effects of recombinant human erythropoietin on perioperative transfusion requirements in patients having a major orthopaedic operation. J Bone Joint Surg Am. 1996;78(1 ):62–72. doi: 10.2106/00004623-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Feagan BG, Wong CJ, Kirkley A, et al. Erythropoietin with iron supplementation to prevent allogeneic blood transfusion in total hip joint arthroplasty. A randomized, controlled trial. Ann Intern Med. 2000;133(11 ):845–54. doi: 10.7326/0003-4819-133-11-200012050-00008. [DOI] [PubMed] [Google Scholar]
- 17.Friederichs MG, Mariani EM, Bourne MH. Perioperative blood salvage as an alternative to predonating blood for primary total knee and hip arthroplasty. J Arthroplasty. 2002;17(3 ):298–303. doi: 10.1054/arth.2002.30409. [DOI] [PubMed] [Google Scholar]
- 18.Goodnough LT, Despotis GJ, Merkel K, Monk TG. A randomized trial comparing acute normovolemic hemodilution and preoperative autologous blood donation in total hip arthroplasty. Transfusion. 2000;40(9 ):1054–7. doi: 10.1046/j.1537-2995.2000.40091054.x. [DOI] [PubMed] [Google Scholar]
- 19.Hersekli MA, Akpinar S, Ozkoc G, et al. The timing of tourniquet release and its influence on blood loss after total knee arthroplasty. Int Orthop. 2004;28(3 ):138–41. doi: 10.1007/s00264-004-0550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rama KR, Apsingi S, Poovali S, Jetti A. Timing of tourniquet release in knee arthroplasty. Meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89(4 ):699–705. doi: 10.2106/JBJS.F.00497. [DOI] [PubMed] [Google Scholar]
- 21.Widman J, Isacson J. Surgical hemostasis after tourniquet release does not reduce blood loss in knee replacement. A prospective randomized study of 81 patients. Acta Orthop Scand. 1999;70(3 ):268–70. doi: 10.3109/17453679908997805. [DOI] [PubMed] [Google Scholar]
- 22.Tria AJ., Jr Advancements in minimally invasive total knee arthroplasty. Orthopedics. 2003;26(8 Suppl):s859–63. doi: 10.3928/0147-7447-20030802-07. [DOI] [PubMed] [Google Scholar]
- 23.Tria AJ, Jr, Coon TM. Minimal incision total knee arthroplasty: early experience. Clin Orthop Relat Res. 2003;416:185–90. doi: 10.1097/01.blo.0000093030.56370.d9. [DOI] [PubMed] [Google Scholar]
- 24.Kumar N, Saleh J, Gardiner E, Devadoss VG, Howell FR. Plugging the intramedullary canal of the femur in total knee arthroplasty: reduction in postoperative blood loss. J Arthroplasty. 2000;15(7 ):947–9. doi: 10.1054/arth.2000.8592. [DOI] [PubMed] [Google Scholar]
- 25.Gasparini G, Papaleo P, Pola P, Cerciello S, Pola E, Fabbriciani C. Local infusion of norepinephrine reduces blood losses and need of transfusion in total knee arthroplasty. Int Orthop. 2006;30(4 ):253–6. doi: 10.1007/s00264-005-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006;88(2 ):282–9. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]
- 27.Mylod AG, Jr, France MP, Muser DE, Parsons JR. Perioperative blood loss associated with total knee arthroplasty. A comparison of procedures performed with and without cementing. J Bone Joint Surg Am. 1990;72(7 ):1010–2. [PubMed] [Google Scholar]
- 28.Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86-A(6 ):1146–52. doi: 10.2106/00004623-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. (CD001825).Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD001825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74(5 ):534–7. doi: 10.1093/bja/74.5.534. [DOI] [PubMed] [Google Scholar]
- 31.Jansen AJ, Andreica S, Claeys M, D'Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4 ):596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 32.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2 ):106–10. doi: 10.1016/j.knee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Ralley FE, Berta D, Binns V, Howard J, Naudie DD. One intraoperative dose of tranexamic Acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7 ):1905–11. doi: 10.1007/s11999-009-1217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5 ):687–96. doi: 10.1016/j.thromres.2008.09.015. [DOI] [PubMed] [Google Scholar]