Abstract
As a major organ of intermediary metabolism, the liver is exposed to a variety of metabolic insults due to diseases and xenobiotics viz., insulin resistance (IR) drugs, toxins, microbial products, etc. One of the consequences of these metabolic insults including obesity and type 2 diabetes mellitus is the development of non-alcoholic fatty liver disease (NAFLD). The recent alarming increase in the prevalence of NAFLD compels the need to develop an appropriate animal model of the disease so as to evolve effective interventions. In this study, we have developed, in the rat, a new model of NAFLD showing several key features akin to the disease in humans. Male Wistar rats were challenged with 30% high fat diet (HFD) – butter, for 2 weeks to induce NAFLD. A hydroalcoholic extract of Picrorhiza kurroa was administered to study the possible reversal of fatty changes in the liver. The extract was given in two doses viz., 200mg/kg and 400 mg/kg b.i.d., p.o. for a period of 4 weeks. There were three control groups (n = 6/group) – vehicle with a regular diet, vehicle with HFD, and HFD with silymarin – a known hepatoprotective.
Histopathology showed that the P. kurroa extract brought about a reversal of the fatty infiltration of the liver (mg/g) and a lowering of the quantity of hepatic lipids (mg/g) compared to that in the HFD control group (38.33 ± 5.35 for 200mg/kg; 29.44 ± 8.49 for 400mg/kg of P. kurroa vs.130.07 ± 6.36mg/g of liver tissue in the HFD control group; P<0.001). Compared to the standard dose of the known hepatoprotective silymarin, P. kurroa reduced the lipid content (mg/g) of the liver more significantly at the dose of 400mg/kg (57.71 ± 12.45mg/kg vs. 29.44 ± 8.49 for the silymarin group vs. 400mg/kg of P. kurroa, P<0.001). In view of the increasing prevalence of metabolic syndrome and NAFLD, P. kurroa should be investigated by the reverse pharmacology path as a potential drug for the treatment of NAFLD, and essential safety studies and preformulation research for concentration of the putative actives should be carried out.
Keywords: Hepatoprotective, high fat diet, insulin resistance, metabolic syndrome, non-alcoholic fatty liver, Picrorhiza kurroa reverse pharmacology
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has now emerged as a global health challenge. In 1980, Ludwig et al, noticed fatty infiltration in the liver biopsy of nonalcoholic patients.[1] Later, NAFLD was observed in diabetes mellitus (DM) and obesity.[2,3] In type 2 diabetes, NAFLD occurs in up to 75% of patients.[4] In obesity, NAFLD and hepatic fibrosis are prevalent.[5] In fact, DM is an independent risk factor for liver-related deaths in NAFLD.[6] Obesity and DM are often accompanied by insulin dysregulation of glucose and lipid metabolism. Potentially hepatotoxic fatty acids in hyperinsulinemic states can lead to NAFLD. Obesity per se is a risk factor of NAFLD: visceral fat is a predictor of hepatic steatosis.[7] Reduction in visceral fat mass has also been shown to decrease hepatic insulin resistance. Lipid-laden hepatocytes can store nonpolar hepatotoxic agents. The ensuing susceptibility to oxidant damage leads to lipid peroxides and unsaturated keto-aldehydes. The latter can cause hepatic necrosis and enhanced fibrogenesis. Starting from a simple steatosis NAFLD, can proceed to steatohepatitis and fibrosis. Cirrhosis and/or hepatic carcinoma can be terminal complications.[8]
In India, the approximately 32 million diabetics in the year 2000 is predicted to jump to 69.9 million by 2025. In these circumstances, NAFLD will assume alarming proportions.[4] Metabolic syndrome has NAFLD as a component in addition to visceral obesity, hypertension, dysglycemia, and dyslipidemia.[5–7] Underlying insulin resistance (IR), oxidative stress, and proinflammatory cytokines are implicated as causes of both steatohepatosis and steatohepatitis.[5,9,10] It is now recognized that IR is present even in NAFLD patients with euglycemia who are of normal weight.
The spectrum of NAFL and its complications being wide, management of the disease is challenging. Weight reduction, ursodeoxycholic acid, clofibrate, gemfibrozil, vitamin E, metformin, and betaine have been recommended. But none of these are conclusively effective, and well-controlled clinical trials are called for.[11] The effect on hepatic morphology of treatment of obesity with fasting, reducing diets, and small bowel bypass has been studied.[12]
In India, hepatoprotective medicinal plants and their formulations have been traditionally used in Ayurveda for the prevention and treatment of diverse liver diseases. Picrorhiza kurroa has been commonly used and well investigated for the treatment of jaundice.[13] The plant has also been shown to be hepatoprotective in various animal models of hepatotoxicity like carbon tetrachloride, d-galactosamine, paracetamol, and thioacetamide.[14–17] It plant has been shown to be hydrocholerectic in a biliary fistula model in dogs and humans.[18] In a double-blind trial in patients with viral hepatitis, P. kurroa rhizome powder was shown to be hepatoprotective by Vaidya et al.[19] Earlier, a commonly used Ayurvedic formulation – Arogyavardhini – containing 50% P. kurroa was also found effective in a double-blind trial in viral hepatitis.[20] It was therfore considered desirable to study P. kurroa in an animal model of NAFLD. Silybum marianum is the most well-researched plant in the treatment of liver diseases. Silymarin has been shown to have significant anti-inflammatory effects on hepatic tissue. Several studies have demonstrated a variety of anti-inflammatory effects, including mast cell stabilization, inhibition of neutrophil migration, and Kupffer cell inhibition.[21–25] It was used as a control in the present study. Significant effort was put into creating an animal model of NAFLD.
MATERIALS AND METHODS
Chemicals
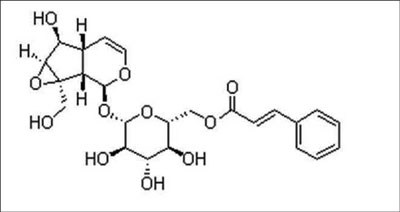
Picroside I [Figure 1] was kindly supplied by the Central Drug Research Institute (CDRI), Lucknow. Silymarin was purchased from Sigma Chemicals Co. USA. All other chemicals used were of analytical grade. Biochemical kits for cholesterol (CHO), triglyceride (TG), high-density lipoprotein (HDL), alanine aminotransferase (ALT), alkaline phosphatase (ALP) for lipid assays, and liver tests used for the study were purchased locally from Raichem, Merck and Ranbaxy, India. Erba Chempro Biochem Analyzer was used for the estimation of all biochemical parameters.
Figure 1.
Picroside I
Plant material
The medicinal plant P. kurroa was identified and its rhizomes collected from the Himalayan region. It was authenticated and standardized at the Zandu Pharmaceutical Works Pvt. Ltd., Mumbai, who have been using the plant for many decades. The pharmacognosy, markers, and other characteristics were as per the WHO guidelines. The voucher specimen was deposited in their herbarium.
Preparation of plant extract
After proper cleaning, the P. kurroa rhizomes were subjected to soxhlet extraction by the ethyl alcohol–water (50:50) method. Each extraction was done thrice for 4 hours each. The total filtrate of the extract was put up for distillation. The filtrate was finally spray-dried to yield a powder form. The yield of the extract was 33.95% w/w. This hydroalcoholic extract (HAE) of P. kurroa was then stored in a vacuum dessicator. During the experiment, an appropriate aliquot of the crude extract was diluted with carboxy methyl cellulose (CMC) before administration to the animals.
Experimental animals
Male Wistar rats, inbred at M/s. Bharat Serum and Vaccine Pvt. Ltd., Mumbai, India, were procured after obtaining clearance for the experiment from the Institutional Animal Ethics Committee of SNDT University. Animals weighing 200–250 g were reared on a balanced laboratory diet and given clean water ad libitum. They were kept at 20 ± 2°C, 65–70% humidity, and day/night cycle (12 h/12 h). “Amul” butter fresh batch available in the market was the HFD used for the study.
Experimental design
Methods for establishing NAFLD model and assessing the effect of HAE P. kurroa
The animals were fasted overnight for 16 hours with free access to water. At the end of the fasting period, blood was collected from the retro-orbital plexus under mild anesthesia to evaluate basal values for the biochemical estimations – TG, CHO, HDL, ALT and ALP. The animals were then randomized into five groups (n = 6/group).
Group 1: The animals were fed ad libitum for 2 weeks. After 2 weeks, 0.5ml CMC, the vehicle was given orally along the same diet for 4 weeks.
Group 2: The animals were fed on 30% HFD orally for 6 weeks.
Group 3–5: The animals were fed on 30% HFD for 2 weeks. After 2 weeks, along with 30% HFD, Silymarin 50 mg/kg (Group 3), HAE P. kurroa 200 mg/kg (Group 4), or HAE P. kurroa 400mg/kg (Group 5) were given p.o. for 4 weeks.
Body weight of the animals was taken on a weekly basis. Retro-orbital bleeding was again conducted at the end of 2nd and 6th week after overnight fasting for the biochemical tests. At the end of the treatment period, the animals were sacrificed by cervical decapitation, and the liver assessed for histopathology and lipid content.
Assessment of the biochemical and histopathological criteria
The liver was dissected and immediately rinsed in ice cold saline. The liver was weighed and gross examination was carried out. It was then stored in 10% formalin and later processed for histopathological studies by hematoxylin and eosin (H and E) staining.
Damage produced in the liver structure in the form of degeneration, necrosis, and fibrosis was graded as follows:
Degeneration
Gr 0, no degeneration; Gr 1, few fat cells per field; Gr 2, more than 100 fat cells per field;
Gr 3, one or two rows of fat cells per lesion; Gr 4, extensive centrilobular and midzonal fatty change with ballooning degeneration.
Necrosis
Gr 0, no necrosis; Gr 1, centrilobular necrosis or 1 or 2 cells per lesion; Gr 2, centro-centri bridging necrosis; Gr 3, massive centrilobular necrosis; Gr 4, massive centrilobular necrosis with necrotic tissue bridging the central veins.
Fibrosis
Gr 0, no fibrosis; Gr 1, central necrosis, mild periportal fibrosis; Gr 2, presence of fibrous tissue in portal tract area only, along with other change mentioned for Grade 1. Gr 3, fibrous tissue insinuating surrounding hepatic parenchyma; Gr 4, formation of pseudolobule by this insinuated fibrous tissue.
Lipid content of the liver was assessed by using Folch et al. method.[26] Liver function tests - TG, CHO, HDL, ALT, ALP were conducted spectrophotometrically using standard kits.
Statistical analysis
Statistical analysis was carried out using one way analysis of variance (ANOVA) test to assess the statistical significance between control, HFD and intervention groups. The difference between the mean ± standard error of the biochemical values was tested for significance.
RESULTS
Phytochemical analysis of the raw plant material and crude extract of HAE P. kurroa
The total ash content of raw material of P. kurroa was estimated to be 4.53%. Bitter principles (picrosides) were dominant in the crude P. kurroa material (20%), while HAE P. kurroa showed 6.54% bitters.[27]
Microbial analysis by colony forming units (CFU/10 g) was carried out for Escherichia coli, Staphylococcus, Streptococcus, Salmonella, Moulds, and Yeast. The extract used for intervention was free of microbes. Standardization of the selected P. kurroa for quality and purity was confirmed prior to the study as per WHO criteria.
Effect of the HAE P. kurroa on the rat model of NAFLD with high fat (30%) diet
Gross examination of the liver of the vehicle control group showed normal reddish colour compared to the pale colour in the HFD group. Histopathology of the liver showed normal architecture in the vehicle control group, while fatty infiltration with granular degeneration and mild multifocal biliary hyperplasia was observed in the diet control group. It is of interest to note that the group treated with P. kurroa, like the silymarin group, showed minimal hepatic damage and a distinct preservation of structure and architecture of liver tissue [Figures 2a–d].
Figure 2.
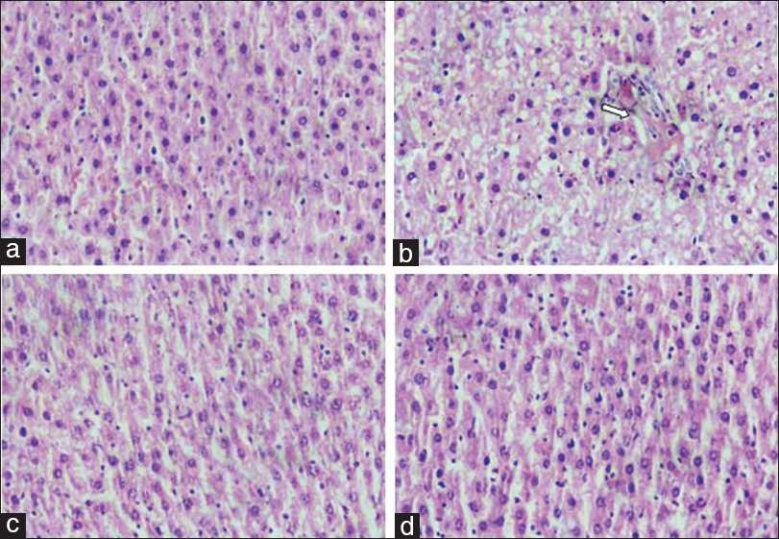
(a) Normal architecture of rat hepatic lobule (×40) H & E stained. (b) Fatty liver tissue with marked diffuse granular degeneration and mild multifocal biliary hyperplasia (×40). (c) Histology of liver tissue treated with standard silymarin showing normal architecture (×40) H & E stained. (d) Histology of liver tissue treated with P. kurroa 400 mg/kg showing normal architecture (×40) H & E stained.
Neither body weight nor the weight of the liver showed significant difference between groups during the course of the study. Despite lack of change in liver weights, there was a marked rise in lipid content of liver tissue [Figure 3] in the HFD model group (130.07± 2.6 mg/g) compared to the standard silymarin group (57.71 ± 5.08mg/g). The vehicle control without any HFD had lipid content of 69.2 ± 3.98mg/g. Both the P.kurroa-treated groups showed a decrease in lipid content in their liver tissue. The group treated with 400mg/kg P. kurroa (29.44 ± 3.47 mg/g) showed greather reduction in lipid content than the silymarin group (P<0.001) though the change was not significant compared to the 200 mg/kg P. kurroa group.
Figure 3.
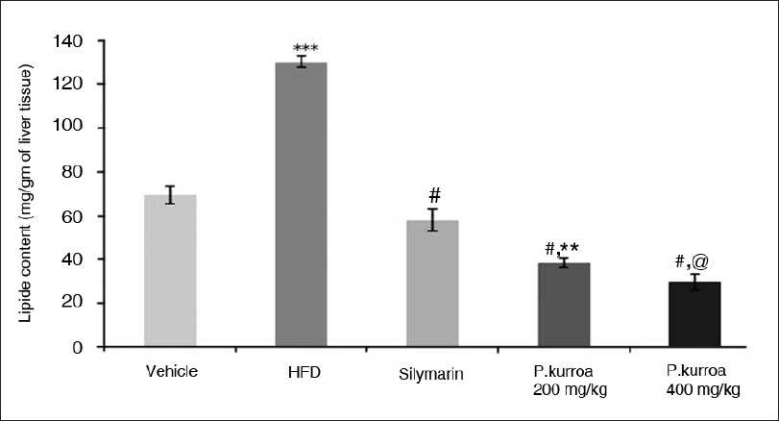
Lipid content in mg/gm of liver tissue. Vehicle vs HFD *** P<0.001; HFD vs Silymarin, P.kurroa 200 mg/kg and P.kurroa 400 mg/kg # P<0.001; Silymarin vs P.kurroa 200 mg/kg ** P<0.01; Silymarin vs P. kurroa 400 mg/kg @ P<0.001.
Serum cholesterol levels in all groups are shown in Figure 4. There was an age-related progressive increase in serum cholesterol in the vehicle control group as shown in Figure 3. In the HFD group, the age-related increase in serum cholesterol was further amplified. Compared to base values, there was a significant increase in the cholesterol values at the 2nd and 6th weeks (38.3 ± 1.75, 65.13 ± 2.04, 84.97 ± 2.4mg/dl, respectively). In the silymarin group, there was a significant increase in cholestrol levels at the 2nd week (61.45 ± 2.62mg/dl) compared to the base value (34.82 ± 2.61mg/dl). However, by the 6th week the silymarin group’s age-related rise in serum cholesterol levels had been attenuated (53.9 ± 1.62mg/dl) compared to those in the HFD group. Similarly the values of the group treated with HAE P. kurroa 200 mg/kg were further reduced by the end of the 6th week (49.08 ± 1.87mg/dl) compared to their 2nd week value (63.21 ± 8.39mg/dl). However, in the group treated with HAE P. kurroa 400mg/kg, attenuation in the value of serum cholesterol was not observed (59.52 ± 2.7 mg/dl).
Figure 4.
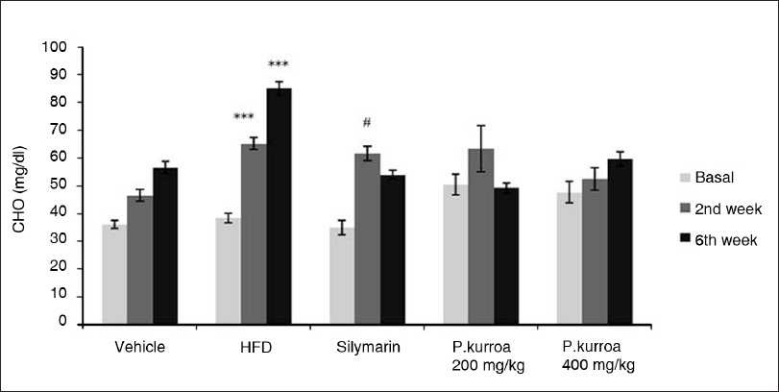
Comparison of serum cholesterol levels. HFD basal vs HFD 2nd and HFD 6th week values *** P<0.001; Silymarin basal vs Silymarin 2nd week values # P<0.001.
Serum triglyceride levels are given for all groups in Figure 5. An age-related increase in serum TG was seen in the vehicle control group from basal to the 2nd and 6th weeks (41.28 ± 5.03, 82.07 ± 7.24, 71.1 ± 5.62 mg/dl). In the HFD group, there was a significant amplification in the age-related increase in levels of TG in the 2nd week – 393.99 ± 56.6 mg/dl and in the 6th week – 487.75 ± 70.94mg/dl compared to the basal value – 56.55 ± 4.3mg/dl. However, in the silymarin group, the 6th week value showed attenuation in the age-related rise in TG levels (basal – 42.46 ± 2.63mg/dl, 2nd week – 223.82 ± 22.63mg/dl, 6th week – 133.52 ± 14.7mg/dl). In the P. kurroa-treated groups, the values of the 6th week analysis showed a decrease compared to the 2nd week value though the effect was not significant.
Figure 5.
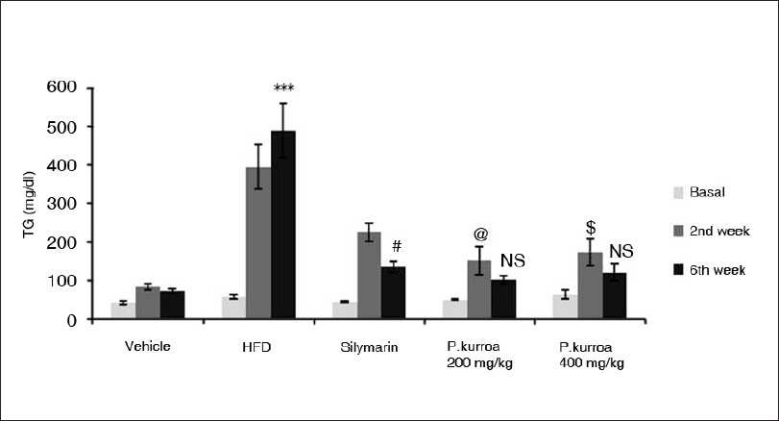
Comparison of serum triglyceride levels. HFD basal vs HFD 6th week values ***P<0.001; Silymarin 2nd week vs Silymarin 6th week values # P<0.01; P.kurroa 200 mg/kg basal vs P.kurroa 2nd week values @ P<0.05; P.kurroa 200 mg/kg 2nd week vs 6th week values - No significance (NS); P.kurroa 400 mg/kg basal vs P.kurroa 2nd week values $ P<0.05; Pk 400 mg/kg 2nd week vs P.kurroa 6th week values – NS.
The HDL levels are shown for the groups in Figure 6. In the vehicle control group, the values of HDL in the 2nd and 6th week of the analysis were 37 ± 1.86, 42.5 ± 3.38mg/dl, respectively. However, a marked decrease in the HDL levels was observed in the HFD group by the 6th week (26.5 ± 1.1 mg/dl). In the silymarin group and in the P. kurroa 200mg/kg, there was no remarkable change in the HDL levels by the 6th week of the intervention. However, it was of interest to observe that the HDL levels in the HAE P. kurroa 400mg/kg did not show a decline in the 6th week, but was comparable to that seen in the vehicle control group.
Figure 6.
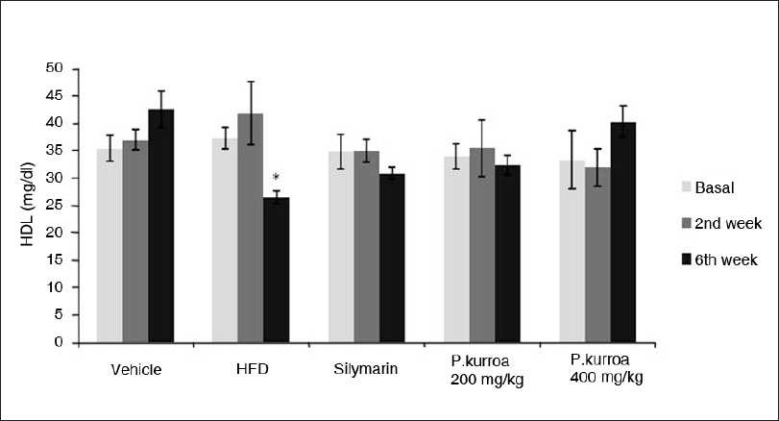
Comparison of serum high density lipoprotein levels. HFD 2nd week vs HFD 6th week values * P < 0.05.
After the two week induction period, a marked rise in ALT levels was observed in the HFD group and also in the group treated with silymarin. In the HFD model and silymarin group, there was a significant increase in ALT levels at the 2nd week of the study (103.43 ± 16.04 U/L; 102.65 ± 19.45 U/L) compared to the vehicle control (46.95 ± 3.14 U/L). At 6 weeks, the HFD group showed a further increase in ALT values (126.65 ± 14.98 U/L), whereas in the silymarin group the level was reduced (73.65 ± 3.89 U/L). HAE P. kurroa 200 and 400 mg/kg showed dose-dependent results (51.81 ± 2.13 and 42.86 ± 2.35 U/L, respectively) [Figure 7].
Figure 7.
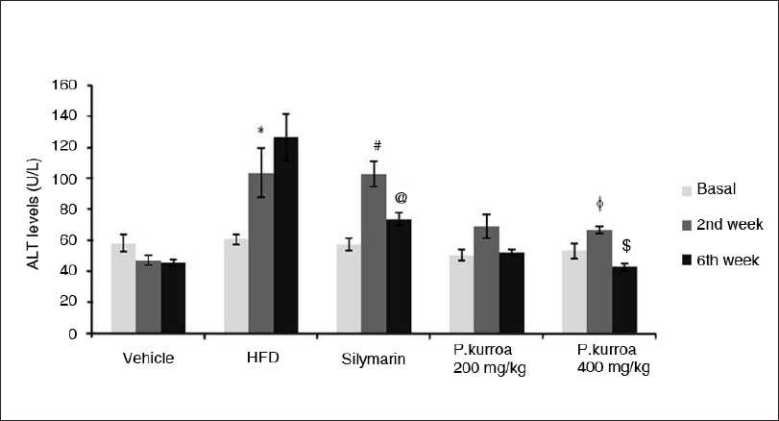
Comparison of serum Alanine Amino Transferase levels. HFD basal vs HFD 6th week values *P<0.05; Silymarin basal vs Silymarin 2nd week values # P < 0.001; Silymarin 2nd week vs Silymarin 6th week values @ P < 0.001; P.kurroa 400 mg/kg basal vs P.kurroa 2nd week values Φ P<0.05; P.kurroa 400 mg/kg 2nd week vs P.kurroa 6th week values $ P<0.001.
The ALP levels in HFD, silymarin, P. kurroa 200 and 400 mg/kg groups were significantly higher in the 2nd week (1408.08 ± 233.74, 1563.5 ± 189.23 U/L, 1417.10 ± 253.58, 1192.05 ± 718.18 U/L, respectively) compared to the vehicle control group (402.23 ± 22.01 U/L; P<0.001). At the end of the study, the ALP values showed a marked rise in the HFD group compared to the 2nd week (2136.17 ± 41.01 U/L). Although a reduction in ALP levels was observed by the 6th week of treatment with silymarin, the values had not normalized. However, in both the P. kurroa-treated groups ALP levels not only reduced but had returned to normal values [Figure 8].
Figure 8.
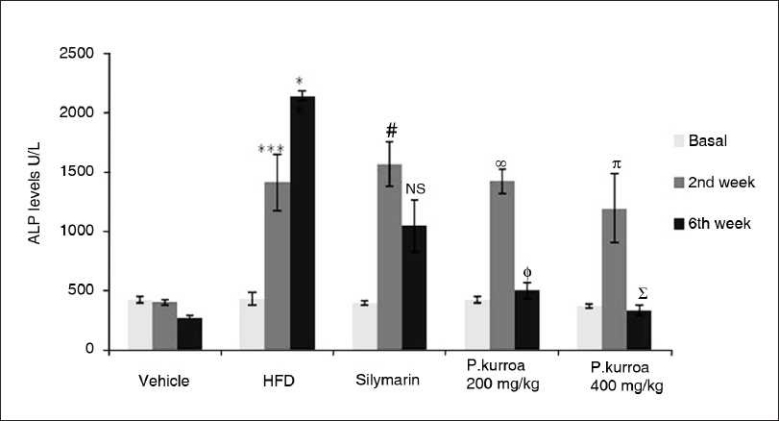
Comparison of serum Alkaline Phosphatase levels. HFD basal vs HFD 2nd week ***P<0.001; HFD 2nd week vs HFD 6th week values *P<0.05; Silymarin basal vs Silymarin 2nd week values # P< 0.001; Silymarin 2nd week vs Silymarin 6th week values - NS; P.kurroa 200 mg/kg basal vs P.kurroa 2nd week values @ P<0.001; P.kurroa 200 mg/kg 2nd week vs P.kurroa 6th week values Φ P<0.001; P.kurroa 400 mg/kg basal vs P.kurroa 2nd week values π P<0.05; P.kurroa 400 mg/kg 2nd week vs P.kurroa 6th week values Σ P<0.01.
DISCUSSION
NAFLD is a component of metabolic syndrome which comprises central obesity, hypertension, impaired fasting glucose (IFG), impaired glucose tolerance (IGT), hypertriglyceridemia, and low HDL.[28] Metabolic syndrome is a risk factor for type II diabetes mellitus, coronary artery disease, and cerebro-vascular disease.[29] NAFLD has also been implicated in the occurrence of insulin resistance and diabetes mellitus.[30–32] The latter conditions have reached globally epidemic proportions. India has the world’s highest incidence of diabetes mellitus. However, insufficient epidemiological data exist on the prevalence of NAFLD in the subcontinent. But clinical impressions and some studies suggest it has a high prevalence.[33,34] The spectrum of NAFLD is wide, ranging from simple and reversible fat accumulation in hepatocytes, eventually leading to irreversible fibrosis, and even terminal cirrhosis or cancer. There are thus, challenges and opportunities for the management and prevention of the disease. Although weight reduction, ursodeoxycholic acid, clofibrate, gemfibrozil, vitamin E, metformin, and betaine have been recommended as modalities of therapy, none of them are very effective.[35–40] Ancient Ayurvedic literature and current basic and clinical research have identified several Indian medicinal plants / formulations with hepatoprotective effects.[14,20,41] The purpose of the present study was to investigate the hepatoprotective potential of a standardized extract of P. kurroa in an HFD-induced animal model of NAFLD to demonstrate a reversal of hepatic lesions, lipid, and enzyme changes. Silymarin, a flavolignan extracted from the seeds and fruit of Silybum marianum, was used as a positive control.
Extracts of roots and rhizomes of P. kurroa have shown hepatoprotective activity in diverse models of liver toxicity.[42–44] P. kurroa contains iridoid glycosides (including picroside I, II, III, pikuroside, kutkoside and 6-feruloyl catalpol), cucurbitacin glycosides, androsin, apocynin, and other organic acids such as vanillic and cinnamic acids. It is pertinent to note its ayurvedic properties of tikta rasa, laguruksha guna, and katuvipaka. Based on these properties, one may anticipate its pharmacodynamic activity on lipids specifically related to lipid disorders.[45] Picroside I has earlier been shown to be active in several models of liver toxicity.[43]
In the present study markers and pharmacologically active compounds of the selected medicinal plants were demonstrated by chromatographic methods. The finger print pattern of the HAE P. kurroa was carried out by TLC, HPTLC, and HPLC for the standard compound, Picroside I, at CDRI, Lucknow. The chromatographic identity of the experimental extracts with pure standards validated the extraction methods, and gave relevance to the interventional studies, with the putative actives in the extracts. Picroside I was detected by HPLC in the standardized extracts (data not shown).
Development of an animal model of NAFLD was a formidable challenge. For interventional studies with the selected medicinal plant, the model had to be simple, suitable, and economical. Studies have demonstrated that monounsaturated and saturated fatty acids (MUFA and SFA) favor adiposity by increasing lipogenesis.[46] Hence, the commonly used form of animal fat – butter – which is rich in saturated fatty acids was selected. An HFD comprising various concentrations of butter was used to induce NAFLD in the rat model and 30% of high fat was found suitable for the study. The current animal model has displayed a close resemblance to the clinical entity of NAFLD in terms of histopathological and biochemical changes. Earlier attempts to develop NAFLD animal models with other hepatotoxic agents met with less success (unpublished data).
In the present study, we have shown that 30% HFD significantly increased the lipid content of the liver tissue without affecting the body weight or liver weight. Raised serum lipid levels were also observed. A rise in serum ALT and ALP – an important feature of NAFLD – was observed in this model. Pan et al, have demonstrated that HFD can induce oxidative stress with extensive liver steatosis in rats.[47]
Histological findings in NAFLD range from steatosis, hepatocyte ballooning, mild-lobular inflammation, and perisinusoidal collagen deposition.[48] In the rat HFD model (30%) used in the present study, the histopathology of the liver sections showed fatty changes. As this model generated hepatic lesions and blood chemistry changes similar to those in NAFLD it was adopted to study interventional effects of extracts of the selected medicinal plant.
Changes in body weight in all groups showed age-related increases, but differences were not significant between groups. Despite decreases in weight gain, there was no statistical significance in body weight values obtained with plants compared to the vehicle control or the diet model. Liver weight expressed as g/100g of body weight did not show any significant difference between the control, diet model, and plant intervention groups.
A twofold increase in the liver-lipid content was observed with HFD justifying the suitability of the model. The group on silymarin showed a significant reduction in the lipid content of the liver compared to the diet model group. The P. kurroa intervention group showed a reduction in lipid content with dose response directionality.
The histopathological studies corroborated the above statements of reduction in lipid content by a parallel and significant morphological normalization of liver architecture and a marked reduction in fatty infiltration in hepatocytes. Both P. kurroa and its active principles have been shown to have hydrocholeretic activity and to increase bile production.[18] Currently therapy-induced reduction in serum cholesterol and fatty liver are mainly ascribed to bile salt sequestrants such as the resin – cholestyramine. Resin bound bile salts do not enter the enterohepatic circulation, and this assists further biliary excretion of bile salts, the breakdown products of cholesterol. The mechanism of lipid-lowering effects of the active principles of P. kurroa needs to be explored. The plant’s anti-inflammatory effects may be an additional benefit when steatosis evolves into steatohepatitis.[49]
Both silymarin and HAE P. kurroa showed a significant hypocholesterolemic effect compared to the diet model. The distinct rise of cholesterol in the second week was not reflected in the HDL change. This finding suggests that the specific lipoprotein under the influence of 30% HFD should be characterized. By the 6th week, the HFD model showed a significant (P<0.001) reduction in HDL as compared to vehicle control. However it was interesting to note that P. kurroa at 400 mg/kg showed an HDL value of 40.27 ± 2.85mg/dl, a significant rise compared to the value for the HFD group (26.50 ± 1.1mg/dl). The value with P. kurroa almost approached the 6th week value of the vehicle control. These findings need to be pursued in-depth with other models of hyperlipidemia and atherosclerosis as elevation of HDL levels is considered quite difficult to achieve. Low HDL is an important risk factor for coronary heart disease.[50]
Both intervention doses and silymarin reduced TG levels significantly compared to the HFD group. The elevation of transaminase reflects necrosis of hepatocytes.[51] ALT in particular is a more specific index of hepatic necrosis. With silymarin, ALT levels were reduced significantly compared to HFD. However, the effect observed with HAE P. kurroa was more effective, bringing down ALT levels to values comparable to vehicle control at the higher 400mg/kg dose. This finding needs to be pursued further in in vitro hepatocyte cultures, and other models of mild to moderate hepatic injury. The anti-inflammatory activity of this plant should also be evaluated vis-à-vis the degree of necrosis-induced inflammation in vivo. In particular, first-phase proteins and release of proinflammatory cytokines, which occur as a reaction to cell death, should be studied.[52]
ALP reflects cholestatic damage that may be induced by hepatotoxic agents.[53,54] Although intrahepatic biliary stasis was observed in the liver biopsies of HFD group, it was attenuated in the treated groups. This clearing of cholestasis along with low ALP levels in the P. kurroa-treated groups confirms the plant’s hepatoprotective activity. In both the P. kurroa dose groups, 6th week serum ALP levels were significantly lower compared to those observed in the silymarin group. P. kurroa showed highly significant reduction in ALP at both dose levels. The values almost approached basal values of ALP and showed dose–response relationship. Further studies with cholestatic models should be carried out.
CONCLUSIONS
The present study has evolved an appropriate animal model with several features of NAFLD, correlating with those seen in humans. Intervention with standardized plant extracts of P. kurroa regressed several features of NAFLD like lipid content of the liver tissue, morphological regression of fatty infiltration, hypolipidemic activity, and reduction of cholestatis. The present study should be pursued further for drug development based on reverse pharmacology for management of NAFLD.
Acknowledgments
We thank J Pathak, Research Director, Zandu Pharmaceuticals Works Pvt. Ltd., India for conducting a detailed taxonomical study for the correct botanical identity for P. kurroa. We thank Sri Dhiru Mehta, President, Kasturba Health Society, and Gabhe, Principal C.U.Shah College of Pharmacy for support to research. We thank Ratnaparkhi for guidance in histopathology. The Lady Tata Memorial Trust is thankfully acknowledged for the fellowship to Sapna N. Shetty.
Footnotes
Source of Support: Sri Dhiru Mehta, President, Kasturba Health Society, and Dr. Gabhe, Principal C.U.Shah College of Pharmacy for support to research
Conflict of Interest: None declared.
REFERENCES
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 2.Rodrigues-Hernandez H, Gonzalez JL, Marquez-Ramirez MD, Flores-Hernandez M, Rodrizues-Morian M, Guerrero-Romero F. Risk factors associated with nonalcoholic fatty liver disease and its relationship with the hepatic histological changes. Eur J Gastroenterol Hepatol. 2008;20:399–403. doi: 10.1097/MEG.0b013e3282f448af. [DOI] [PubMed] [Google Scholar]
- 3.Verrijken A, Francque S, Van Gaal L. The metabolic syndrome and the liver. Acta Gastroenterol Belg. 2008;71:48–59. [PubMed] [Google Scholar]
- 4.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of Type 2 diabetes : Indian Scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 5.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–60. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association, National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 7.Stern M, Williams K, Gonzalez-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of Type 2 diabetes or cardiovascular disease? Diabetes Care. 2004;27:2676–81. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 8.Gray J, Chattopadhyay D, Beale GS, Patman GL, Miele L, King BP, et al. A proteomic strategy to identify novel serum biomarkers for liver cirrhosis and hepatocellular cancer in individuals with fatty liver disease. BMC Cancer. 2009;9:271. doi: 10.1186/1471-2407-9-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson PJ, Critchley JA, Chan JC, Cockram CS, Lee ZS, Thomas GN, et al. Factor analysis of the metabolic syndrome: Obesity vs insulin resistance as the central abnormality. Int J Obes. 2001;25:1782. doi: 10.1038/sj.ijo.0801837. [DOI] [PubMed] [Google Scholar]
- 10.Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh K Acad Geneeskd Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- 11.Paul A, Keith DL. Treatment of nonalcoholic fatty liver: Present and emerging therapies. Semin Liver Dis. 2001;21:81–8. doi: 10.1055/s-2001-12931. [DOI] [PubMed] [Google Scholar]
- 12.Drenick EJ, Simmons F, Murphy J. Effect on hepatic morphology of treatment of obesity by fasting, reducing diets and small bowel bypass. N Engl J Med. 1970;282:829–34. doi: 10.1056/NEJM197004092821502. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya AB, Sirsat MS, Doshi JC, Antarkar DS. Selected medicinal plants and formulations as hepato-biliary drugs: An Over-view. Clin Pharmacol Ther. 1996;14:7–11. [Google Scholar]
- 14.Dwivedi Y, Rastogi R, Ramesh C, Sharma SK, Kapoor NK, Garg NK, et al. Hepatoprotective activity of Picroliv against carbon tetrachloride induced liver damage in rats. Indian J Med Res. 1990;92b:195–200. [PubMed] [Google Scholar]
- 15.Visen PK, Shukla B, Patnaik GK, Dhawan BN. Prevention of galactosamine-induced hepatic damage by Picroliv: Study on bile flow and isolated hepatocytes. Planta Med. 1993;59:37–41. doi: 10.1055/s-2006-959600. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi Y, Rastogi R, Garg NK, Dhawan BN. Prevention of paracetamol-induced hepatic damage in rats by Picroliv, the standardized active fraction from Picrorhiza kurroa. Phytother Res. 1991;5:115–9. [Google Scholar]
- 17.Dwivedi Y, Rastogi R, Sharma SK, Garg NK, Dhawan BB. Picroliv affords protection against thioacetamide-induced hepatic damage in rats. Plant Med. 1991;57:25–8. doi: 10.1055/s-2006-960009. [DOI] [PubMed] [Google Scholar]
- 18.Pandey VN, Chaturvedi GN. Effect of indigenous drug kutaki on bile after producing biliary fistula in dogs. Indian J Med Res. 1970;5:1–24. [Google Scholar]
- 19.Vaidya AB, Antarkar DS, Doshi JC, Bhat AD, Ramesh V, Vora PV, et al. Picrorhiza kurroa (Kutaki) Royle ex Benth as a hepatoprotective agent - experimental and clinical studies. J Postgrad Med. 1996;42:105–8. [PubMed] [Google Scholar]
- 20.Antarkar DS, Tathed PS, Vaidya AB. A pilot phase II trial with Arogyawardhini and Punarnavadi kwath in viral hepatitis. Panminerva Med. 1978;20:157–63. [PubMed] [Google Scholar]
- 21.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: From bench to bed side. Anticancer Res. 2006;26:4457–98. [PubMed] [Google Scholar]
- 22.Giese LA. Milk thistle and the treatment of hepatitis. Gastroenterol Nurs. 2001;24:95–7. doi: 10.1097/00001610-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Jeong DH, Lee GP, Jeong WI, Do SH, Yang HJ, Yuan DW, et al. Alterations of mast cells and TGF-beta1 on the silymarin treatment for CCl(4)-induced hepatic fibrosis. World J Gastroenterol. 2005;11:1141–8. doi: 10.3748/wjg.v11.i8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zielińska-Przyjemska M, Wiktorowicz K. An in vitro study of the protective effect of the flavonoid silydianin against reactive oxygen species. Phytother Res. 2006;20:115–9. doi: 10.1002/ptr.1812. [DOI] [PubMed] [Google Scholar]
- 25.Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–54. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Soane GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Rastogi RP, Dhawan BN. Research on medicinal plants at the Central Drug Research Institute, Lucknow (India) Indian J Med Res. 1982;76:27–45. [PubMed] [Google Scholar]
- 28.Cankurtaran M, Tayfur O, Yavuz BB, Gevik S, Akhan O, Arslan S. Insulin resistance and metabolic syndrome in patients with NAFLD but without diabetes: effect of a 6 month regime intervention. Acta Gastroenterol Belg. 2007;70:253–9. [PubMed] [Google Scholar]
- 29.Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in Nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1941–51. doi: 10.2174/138161210791208875. [DOI] [PubMed] [Google Scholar]
- 30.Stein LL, Dong MH, Loomba R. Insulin sensitizers in nonalcoholic fatty liver disease and steatohepatitis : Current status. Adv Ther. 2009;26:893–907. doi: 10.1007/s12325-009-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H, Zeng L, Liang B, Shu X, Xie D. High prevalence of coronary heart disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Arch Med Res. 2009;40:571–5. doi: 10.1016/j.arcmed.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Vanni E, Bugianesi E, Kotronen A, Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320–30. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Singh SP, Nayak S, Swain M, Rout N, Mallik RN, Agrawal O, et al. Prevalence of nonalcoholic fatty liver disease in coastal eastern India: A preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–9. [PubMed] [Google Scholar]
- 34.Madan K, Batra Y, Acharya SK. Non-alcoholic fatty liver disease may not be a severe disease at presentation among Asian Indians. World J Gastroenterol. 2006;12:3400–5. doi: 10.3748/wjg.v12.i21.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel AA, Torres DM, Harrison SA. Effect of weight loss on non-alcoholic fatty liver disease. J Clin Gastroenterol. 2009;43:970–4. doi: 10.1097/MCG.0b013e3181b57475. [DOI] [PubMed] [Google Scholar]
- 36.Musso G, Gambino R, Cassader M. Non-alcoholic fatty liver disease from pathogenesis to management: An update. Obes Rev. 2010;11:430–45. doi: 10.1111/j.1467-789X.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- 37.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, et al. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–60. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 38.Uzun MA, Koksal N, Aktas S, Gunerhan Y, Kadiogly H, Dursun N, et al. The effect of ursodeoxycholic acid on liver regeneration after partial hepatectomy in rats with non-alcoholic fatty liver disease. Hepatol Res. 2009;39:814–21. doi: 10.1111/j.1872-034X.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 39.Angulo P. Treatment of non-alcoholic fatty liver disease. Ann Hepatol. 2002;1:12–9. [PubMed] [Google Scholar]
- 40.Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Betaine improved adipose tissue function in mice fed high-fat diet: A mechanism for hepatoprotective effect of betaine in non-alcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2010;298:G634–42. doi: 10.1152/ajpgi.00249.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panchabhai TS, Ambarkhane SV, Joshi AS, Samant BD, Rege NN. Protective effect of Tinospora cordifolia, Phyllanthus emblica and their combination against antitubercular drugs induced hepatic damage: An experimental study. Phytother Res. 2008;22:646–50. doi: 10.1002/ptr.2356. [DOI] [PubMed] [Google Scholar]
- 42.Pilankar PD. A study of hepatoprotective effects of some indigenous plants in experimental animals. Ph.D. Thesis. University of Mumbai. 1981 [Google Scholar]
- 43.Singh B, Rastogi RP. Chemical examination of Picrorhiza kurroa Part VI: Re-investigation of Kutkin. Indian J Chem. 1972;10:29–31. [Google Scholar]
- 44.Sharma PV. Chaukhambha Visvabharati. Vol. 69. Varanasi, India: 1996. Classical uses of medicinal plants. [Google Scholar]
- 45.Koolman AH, Bloks VW, Oosterveer MH, Jonas I, Kuipers F, Sauer PJ, et al. Metabolic responses to long-term pharmacological inhibition of CB1-receptor activity in mice in relation to dietary fat composition. Int J Obes (Lond) 2010;34:374–84. doi: 10.1038/ijo.2009.219. [DOI] [PubMed] [Google Scholar]
- 46.Brunt EM. Nonalcoholic steatohepatitis: Definition and Pathology. Semin Liver Disease. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 47.Pan M, Song YL, Xu JM, Gan HZ. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J Pineal Res. 2006;41:79. doi: 10.1111/j.1600-079X.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 48.Pandey BL, Das PK. Immunopharmacological studies on Picrorhiza kurroa Royle-Ex-Benth. Part V: Anti-inflammatory action : Relation with cell types involved in inflammation. Indian J Physiol Pharmacol. 1988;32:289–92. [PubMed] [Google Scholar]
- 49.Alsheikh-Ali AA, Lin JL, Abourjaily P, Ahearn D, Kuvin JT, Karas RH. Extent to which accepted serum lipid goals are achieved in a contemporary general medical population with coronary heart disease risk equivalents. Am J Cardiol. 2006;98:1231–3. doi: 10.1016/j.amjcard.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 50.Dunbar RL, Rader DJ. Current drug options for raising HDL cholesterol. Curr Treat Options Cardiovasc Med. 2005;7:15–23. doi: 10.1007/s11936-005-0002-6. [DOI] [PubMed] [Google Scholar]
- 51.Liu R, Pan X, Whitington PF. Increased hepatic expression is a major determinant of serum alanine aminotransferase elevation in mice with nonalcoholic steatohepatitis. Liver Int. 2009;29:337–43. doi: 10.1111/j.1478-3231.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- 52.Budd RC. Death receptors couple to both cell proliferation and apoptosis. J Clin Invest. 2002;109:437–42. doi: 10.1172/JCI15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon NK, Angulo P, Lindor KD. Severe cholestatic hepatitis from troglitazone in a patient with nonalcoholic steatohepatitis and diabetes mellitus. Am J Gastroenterol. 2001;96:1631. doi: 10.1111/j.1572-0241.2001.03809.x. [DOI] [PubMed] [Google Scholar]
- 54.Sorrentino P, Tarantino G, Perrella A, Micheli P, Perella O, Conca P. A clinical-morphological study on cholestatic presentation of nonalcoholic fatty liver disease. Dig Dis Sci. 2005;50:1130–5. doi: 10.1007/s10620-005-2719-1. [DOI] [PubMed] [Google Scholar]