Abstract
Antioxidant and antifungal activity were determined for the essential oil of Alpinia calcarata Roscoe (Zingiberaceae) rhizomes. Its antioxidant properties were investigated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and thiobarbituric acid reactive substances (TBARS) assay. Butylated hydroxy toluene (BHT) and vitamin E served as positive controls. Antifungal activities were investigated against crop pathogens Curvularia spp. and Colletorichum spp. using the agar plate method. Fifty percent effective concentration (EC50) and % antioxidant index of the essential oil were 45 ± 0.4 and 16.1 ± 0.2 for DPPH and TBARS assays, respectively. The degree of, the essential oil’s inhibition of the growth of crop pathogens Curvularia spp. and Colletorichum spp. varied with time period its effects were higher than greater than for the positive control, daconil. In conclusion, the essential oil of A. calcarata rhizomes possess moderate antioxidant property and promising antifungal activity.
Keywords: Alpinia calcarata, antifungal activity, antioxidant power, essential oil
INTRODUCTION
Alpinia calcarata Roscoe (Zingiberaceae) is a rhizomatous perennial herb which is commonly used in traditional medicinal systems in Sri Lanka. A. calcarata is cultivated in tropical countries including Sri Lanka, India, and Malaysia.[1,2] In Sri Lankan, rhizomes of A. calcarata, are recommended as an aphrodisiac, and the decoction is widely used in the treatment of bronchitis, cough, respiratory ailments, diabetics, asthma, and arthritis.[2–4] Some 18 volatile constituents have been identified in the essential oil (EO) of Sri Lankan grown A. calcarata rhizomes, of which 1,8-cineol (33.3%) is the major constituent. Apart from 1,8-cineol, α-pinene (3.1%), camphene (4.1%), β-pinene (9.3%), p-cymene (1.4%), and limonene (4.0%) are also present.[5]
Research has shown antibacterial,[6] anthelmintic,[7] antifungal,[8] antinociceptive,[9] antioxidant,[10] gastroprotective,[11,12] aphrodisiac,[13] and antidiabetic[14] activities in aqueous and ethanolic extracts of A. calcarata rhizomes. However, no attempt has been made to investigate the bioactivities of their EO. Therefore, this study was carried out to investigate its antioxidant power using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay, and the thiobarbituric acid reactive substances (TBARS) assay, and antifungal activity against Curvularia spp. and Colletorichum spp.
MATERIALS AND METHODS
Plant material
Fresh A. calcarata rhizomes were collected from home gardens in Western province, Sri Lanka. The plant material was identified and authenticated by the Curator of the National Herbarium, Royal Botanical Gardens, Peradeniya, Sri Lanka. A voucher specimen (AS 01) was deposited in the Industrial Technology Institute, Colombo 7, Sri Lanka.
Preparation of the essential oil
Fresh A. calcarata rhizomes were sterilized with 1% NaOCl, rinsed with sterile distilled water and hydrodistilled for 4 h using a Clevenger arm. The EO was trapped in a mixture of n-pentane and ether (1:1 v/v), collected and weighed after evaporating the organic layer (yield 1.5 % w/w dry weight basis).
Standardization of the essential oil
Gas chromatographic profile was used to standardize the EO.
Details of the gas chromatograph operating conditions
Chromatograph: HewlettPackard 5890 Series II
Detector: Flame ionization detector
Column: DB-5 MS capillary column (30 m × 0.25 mm id, 0.25 μm film)
Initial oven temperature: 40° C
Final oven temperature: 280° C
Program rate: 10° C/min
Peaks were identified using retention time data, peak enhancement method using authentic compounds, and by comparing their mass spectra with spectra in the data bank. NMR data were obtained wherever possible.
Determination of antioxidant property of essential oil by DPPH scavenging assay
The antioxidant property was determined by measuring the remaining concentration of DPPH as described by Singh and coworkers.[15] For this assay, known concentrations of (0–100 μg/mL) EO and butylated hydroxy toluene (BHT) were placed in different test tubes. The volume was adjusted to 1 mL by adding methanol (MeOH). Five milliliters of methanolic solution of DPPH (2 mg/100 mL MeOH) were added to these tubes and shaken vigorously. The tubes were allowed to stand at room temperature for 20 min and the absorbance was measured at λ 517 nm (UV-160, Shimadzu, Japan). A control was prepared as above by adding MeOH instead of test solutions. BHT served as the positive control. This experiment was done twice, each time in triplicate.
The DPPH concentration in the reaction medium was calculated from a calibration curve analyzed by linear regression. The percentage of remaining DPPH (%DPPHREM) of each concentration was calculated as follows:
where T is the experimental duration time 20 min and C is the control. The mean effective scavenging concentrations (EC50) were calculated by plotting the %DPPH REMconcentrations versus the concentrations of extract used.
Determination of antioxidant property of essential oil by TBARS assay
The antioxidant property was determined by measuring the oxidation of egg yolk lipids as described by Dorman and coworkers.[16] In this assay, the egg yolk (10%, v/v) solution was prepared in KCl (1.15%, w/v). It was homogenized for 30 sec and ultrasonicated for 5 min and stored at 4 °C until use. The EO, vitamin E, and BHT at 0.01% (w/v) concentrations were prepared using 8.1 % (w/v) sodium dodecyl sulphate (SDS) solution. A solution of 0.8% (w/v) thiobarbituric acid (TBA) was prepared in 1.1% (w/v) of SDS solution. Test solutions (0.1 mL) were added to tubes containing 0.5 mL egg yolk homogenate. After adding 1.5 mL of acetic acid (20%, v/v), the pH value was adjusted to 3.5 with 1 Mol NaOH. Then, 1.5 mL of 0.8% TBA was added and the final volume adjusted to 4 mL with deionized water. Samples were vortexed and left in a 95°C water bath for 60 min. When they had cooled, 5 mL of n -butanol was added, vortexed, centrifuged, and the absorbance of butanol layer was measured at λ 532 nm (UV-160, Shimadsu, Japan) against an n-butanol blank. The above procedure was followed for the control by using 0.1 mL of 8.1 % (w/v) SDS instead of the test solution. Both vitamin E and BHT served as positive controls. The experiment was done twice, each time in triplicate.
Antioxidant index percentage (AI %) was calculated using the following formula:
where, T = the absorbance of the test sample
C = the absorbance of the fully oxidized control
Determination of antifungal activity of essential oil by agar plate method
In this assay, 1000, 1500, 2000, and 2500 ppm of A. calcarata EO were tested against the activity of crop pathogens Curvularia spp. and Colletorichum spp. Daconil was used as the positive control. The authentication of Curvularia spp. and Colletorichum spp. were done by polymerase chain reaction (PCR) technique using molecular detection of these fungi. Stock solution of EO was prepared with 95% ethanol. Test solutions were added to potato dextrose agar (PDA) medium, with the temperature of the sterilized medium at 45° C. Circular fungi culture discs were kept in the center of the each plate and the diameter of the fungal culture was measured after 3, 4, 5, 6 and 7 days. Untreated PDA medium served as the control and each experiment was done in triplicate. Growth inhibition percentage was calculated by the equation (C – T/C) × 100 where C is hyphal extension (mm) of controls and T is hyphal extension (mm) of EO-treated plates.
Statistical analysis
Statistical comparisons were made using a one-way ANOVA test.
RESULTS AND DISCUSSION
As shown in Figure 1, the EO of A. calcarata rhizomes was standardized using a gas chromatograph. Similar to the previous studies,[5] 1,8-cineol was found to be the major component in the EO of A. calcarata rhizomes. Antioxidant property of cold ethanolic extract (CEE), hot ethanolic extract (HEE), and hot water extract (HWE) of A. calcarata rhizomes have been investigated in previous studies. Further, antioxidant property of CEE was comparable to BHT, the synthetic antioxidant.[10] However, in the present investigation, EO of A. calcarata rhizomes showed a moderate antioxidant property (as judged by DPPH scavenging assay [Table 1] and TBARS assay [Table 2]) when compared to the antioxidant properties of CEE, HEE, and HWE. Antioxidants react with the free radical DPPH and convert it to 1,1-diphenyl-2-picrylhydrazine by donating a hydrogen.[17] The change in absorbance produced by this reaction was used to test the ability of EO to act as a free radical scavenger. Therefore, this indicates that DPPH radical scavenging activity of EO is due to its hydrogen-donating ability. Lipid peroxidation, which is widely recognized as a primary toxicological event, is caused by the generation of free radicals from a variety of sources including organic hydroperoxides, redox cycling compounds, and iron-containing compounds. The TBARS assay has been used to measure the degree of lipid peroxidation. TBA reacts specifically with malondialdehyde (MDA), a secondary product of lipid peroxidation. to give a red chromogen, which may then be determined spectrophotometrically.[18] The degree of the EO’s inhibition of the growth of the crop pathogens Curvularia spp. and Colletorichum spp. varied with time period [Table 3]. Its antifungal activity appears to be more pronounced against Curvularia spp. and the effect was better than that of positive control, daconil. Therefore, A. calcarata essential oil can be used to prepare a natural fungicide. In conclusion, this study revealed moderate antioxidant property and promising antifungal activity of A. calcarata rhizomes.
Figure 1.
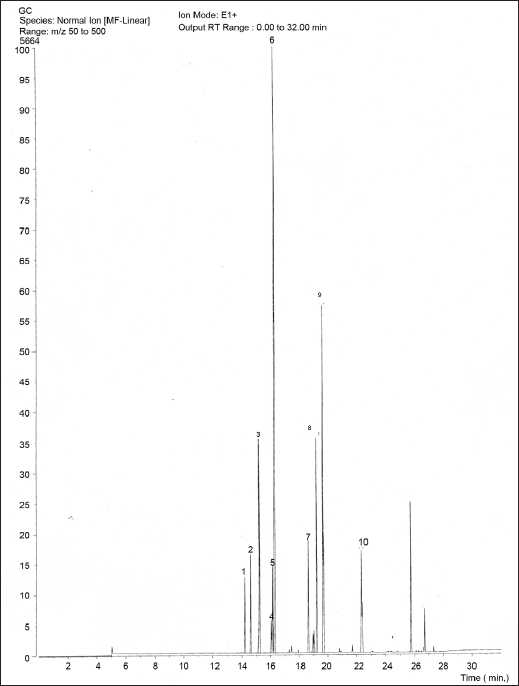
Gas chromatograph of essential oil (EO) of Alpinia calcarata rhizomes
P eak no 1: α-pinene, peak no 6: 1,8-cineol, peak no 2: camphene, peak no 7: camphor, peak no 3: β-pinene, peak no 8: γ-muurolene, peak no 4: ρ-cymene, peak no 9: caratol, peak no 5: limonene, peak no 10: α-eudesmol
Table 1.
Mean scavenging concentrations of (EC50) of essential oil of Alpinia calcarata rhizomes in DPPH free radical scavenging assay
| Sample | EC50 (μg/mL) |
|---|---|
| EO | 45.8 ± 0.4a |
| BHT | 8.4 ± 0.2b |
Values are expressed as mean ± SE. The values marked with the different letters (a and b) are significantly (P ≤ 0.05) different from each other. BHT, butylated hydroxyl toluene; SE, standard error.
Table 2.
Antioxidant index percentage of essential oil Alpinia calcarata rhizomes in TBARS assay
| Sample | AI (%) |
|---|---|
| EO | 16.1 ± 0.2a |
| BHT | 34.7 ± 0.2b |
| Vitamin | E 32.0 ± 0.2b |
Values are expressed as mean ± SE. The values marked with the different letters (a and b) are significantly (P ≤ 0.05) different from each other. BHT, butylated hydroxyl toluene; SE, standard error.
Table 3.
Antifungal activity of essential oil of Alpinia calcarata rhizome
| Fungi | Sample | Concentration (ppm) | Growth rate (diameter of colony in mm) |
||||
|---|---|---|---|---|---|---|---|
| 3 days | 4 days | 5 days | 6 days | 7 days | |||
| Curvularia spp. | Essential oil | 1000 | 9.00±0.47* (73.0) | 10.50±0.41* (73.0) | 11.17±0.27* (76.5) | 12.33±0.14* (77.4) | 13.33±0.14* (79.1) |
| 1500 | 6.83±0.14* (79.5) | 8.17±0.14* (80.0) | 9.33±0.14* (80.3) | 10.67±0.14* (80.4) | 12.17±0.14* (80.9) | ||
| 2000 | 6.00* (82.0) | 6.00* (85.2) | 6.00* (87.4) | 6.00* (89.0) | 6.00* (90.6) | ||
| 2500 | 6.00* (82.0) | 6.00* (85.2) | 6.00* (87.4) | 6.00* (89.0) | 6.00* (90.6) | ||
| Daconil | 1000 | 15.67±0.27* (53.0) | 18.83±0.36* (53.5) | 23.00±0.41* (51.6) | 25.67±0.27* (52.8) | 28.67±0.27* (55.0) | |
| 1500 | 14.83±0.14* (55.5) | 17.67±0.14* (56.4) | 21.17±0.14* (55.4) | 24.17±0.14* (55.6) | 27.33±0.27* (57.1) | ||
| 2000 | 14.17±0.14* (57.5) | 17.17±0.14* (57.6) | 20.83±0.14* (56.1) | 23.50±0.24* (56.9) | 26.83±0.14* (58.9) | ||
| 2500 | 13.83±0.14* (58.5) | 16.67±0.14* (58.8) | 19.83±0.14* (58.2) | 23.17±0.14* (57.5) | 26.17±0.14* (58.9) | ||
| Control | 33.33±0.27 | 40.50±0.24 | 47.50±0.41 | 54.50±0.24 | 63.67±0.72 | ||
| Blank | 36.67±0.27 | 44.83±0.36 | 51.67±0.27 | 58.50±0.24 | 67.67±0.27 | ||
| Colletotrichum spp. | Essential oil | 1000 | 20.50±0.24* (42.0) | 24.83±0.14* (44.0) | 29.33±0.14* (46.0) | 33.83±0.14* (46.9) | 38.67±0.27* (46.8) |
| 1500 | 17.67±0.14* (50.0) | 21.33±0.27* (51.9) | 26.17±0.14* (51.8) | 30.17±0.14* (52.6) | 34.17±0.14* (53.0) | ||
| 2000 | 12.33±0.27* (65.1) | 15.50±0.24* (65.0) | 20.17±0.14* (62.9) | 23.67±0.14* (62.8) | 27.33±0.27* (62.4) | ||
| 2500 | 9.50±0.24* (73.1) | 11.67±0.14* (73.7) | 14.17±0.14* (73.9) | 16.83±0.14* (73.6) | 19.67±0.14* (72.9) | ||
| Daconil | 1000 | 17.67±0.27* (50.0) | 20.83±0.36* (50.0) | 24.83±0.36* (54.3) | 29.00±0.41* (54.4) | 33.67±0.27* (53.7) | |
| 1500 | 16.17±0.14* (54.2) | 19.17±0.14* (54.2) | 22.17±0.14* (59.2) | 25.17±0.14* (60.5) | 29.67±0.27* (59.2) | ||
| 2000 | 14.83±0.14* (58.0) | 17.83±0.14* (58.0) | 20.50±0.24* (62.3) | 22.83±0.14* (64.1) | 26.50±0.24* (63.5) | ||
| 2500 | 12.67±0.14* (64.1) | 16.33±0.27* (64.1) | 18.50±0.24* (65.9) | 20.83±0.14* (67.3) | 23.83±0.14* (67.2) | ||
| Control | 35.33±0.27 | 44.33±0.27 | 54.33±0.27 | 63.67±0.27 | 72.67±0.27 | ||
| Blank | 38.67±0.27 | 49.67±0.27 | 59.00±0.47 | 69.67±0.27 | 78.67±0.72 | ||
Significant at P ≤ 0.05 level with the control or blank; diameter of circular fungi culture disc = 6.0 mm.
Acknowledgments
The authors express their gratitude to the National Science Foundation for the research grant (SIDA (1L) 2000/BT/03).
Footnotes
Source of Support: National Science Foundation, Sri Lanka
Conflict of Interest: None declared
REFERENCES
- 1.Dassanayake MD, Fosberg FR. Revised Hand Book to the flora of Ceylon. Amreind, New Delhi, India: 1981. pp. 517–8. [Google Scholar]
- 2.Jayaweera DM. Colombo: National Science Council of Sri Lanka; 1982. Medicinal Plants Used in Ceylon; p. 213. [Google Scholar]
- 3.Ramanayake L. Publication of Department of Ayurveda. Colombo, Sri Lanka: 1994. Osu Visithuru; pp. 68–71. [Google Scholar]
- 4.Arambewela LS, Basnayake CS, Serasinghe P, Tissera MS, Dias S, Weerasekara DR. Colombo, Sri Lanka: 1995. Traditional treatment in Sri Lanka for chronic Arthritis.NARESA Printing Unit. [Google Scholar]
- 5.Arambewela LS, Kumaratunga A, Arawwawala M, Owen NL, Du L. Volatile oils of Alpinia calcarata grown in Sri Lanka. J Essent Oil Res. 2005;17:124–5. [Google Scholar]
- 6.George M, Pandalai KM. Investigations on plant antibiotics. Ind J Med Res. 1949;37:169–81. [Google Scholar]
- 7.Kaleysa RR. Screening of indigenous plants for anthelmintic action against human Ascaris lumbricoides. Indian J Physiol Pharmacol. 1975;19:47–9. [PubMed] [Google Scholar]
- 8.Pushpangadan P, Atal CK. Ethno – medico – botanical investigations in Kerala. J Ethnopharmacol. 1984;111:59–77. doi: 10.1016/0378-8741(84)90096-5. [DOI] [PubMed] [Google Scholar]
- 9.Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD. Antinociceptive activities of aqueous and etahnolic extracts of Alpinia calcarata rhizomes in rats. J Ethnopharmacol. 2004;95:311–6. doi: 10.1016/j.jep.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Arambewela LSR, Arawwawala LDAM. Antioxidant activities of ethanolic and hot aqueous extracts of Alpinia calcarata rhizomes. Aust J Med Herbalism. 2005;17:91–4. [Google Scholar]
- 11.Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD. Gastroprotective activity of hot ethanolic extract of Alpinia calcarata rhizomes in rats. Ceylon J Med Sci. 2005;48:1–11. [Google Scholar]
- 12.Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD. 2009.Effect of Alpinia calcarata rhizomes on ethanol - induced gastric ulcers in rats. Phcog Mag. 2009;4:226–31. [Google Scholar]
- 13.Ratnasooriya WD, Jayakody JR. Effects of aqueous extract of Alpinia calcarata rhizomes on reproductive competence of male rats. Acta Biol Hung. 2006;57:23–35. doi: 10.1556/ABiol.57.2006.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD. Hypoglycemic and antihyperglycemic activities of the aqueous and the ethanolic extracts of Alpinia calcarata rhizomes in rats. Phcog Mag. 2009;5:412–8. [Google Scholar]
- 15.Singh RP, Murthy KN, Jayaprakasha GK. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using in Vitro Models. J Agric Food Chem. 2002;50:81–6. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 16.Dorman HJ, Deans SG, Noble RC. Evaluation In Vitro of Plant Essential Oils as Natural Antioxidants. J Essent Oil Res. 1995;7:645–51. [Google Scholar]
- 17.Son S, Lewis BA. Free Radical Scavenging and Antioxidative Activity of Caffeic Acid Amide and Ester Analogues; Structure - Activity Relationship. J Agric Food Chem. 2002;50:468–72. doi: 10.1021/jf010830b. [DOI] [PubMed] [Google Scholar]
- 18.Coppen PP. Allen JC, Hamilton RJ. Rancidity in foods. New York, USA: 1983. The Use of Antioxidants. [Google Scholar]


