Abstract
Regulatory T cells (Treg) are critical regulators of immune tolerance. Both IL-2 and CD28-CD80/CD86 signaling are critical for CD4+CD25+FOXP3+ Treg survival in mice. Yet, both belatacept (a second-generation CTLA-4Ig) and basiliximab (an anti-CD25 monoclonal antibody) are among the arsenal of current immunotherapies being used in kidney transplant patients. In this study, we explored the direct effect of basiliximab and belatacept on the Tregs in peripheral blood both in the short term and long term and in kidney biopsies of patients with acute rejection. We report that the combined belatacept/basiliximab therapy has no long-term effect on circulating Tregs when compared to a calcineurin inhibitor (CNI)-treated group. Moreover, belatacept-treated patients had a significantly greater number of FOXP3+ T cells in graft biopsies during acute rejection as compared to CNI-treated patients. Finally, it appears that the basiliximab caused a transient loss of both FOXP3+ and FOXP3− CD25+ T cells in the circulation in both treatment groups raising important questions about the use of this therapy in tolerance promoting therapeutic protocols.
Keywords: Basiliximab, belatacept, FOXP3, Kidney transplantation, Treg
Introduction
Over the past decade, there have been tremendous advances in our understanding of the basic processes that control immune tolerance. The identification of CD4+CD25+ regulatory T cells (Treg) as an important component of self-tolerance has opened a major area of investigation in immunology, and numerous studies have demonstrated the potent influence of Tregs in suppressing pathologic immune responses in autoimmune diseases, transplantation and graft-versus-host disease (reviewed in References 1–6). Absence of Tregs due to mutations in the forkhead transcription factor, FOXP3, which is essential for Treg development, leads to multiorgan autoimmunity in mice and humans (7–10). The vital function of FOXP3+ T cells in maintaining normal immune homeostasis is further demonstrated by the fatal consequence of depletion of these cells in normal adult mice (11). Moreover, adoptive transfer of Tregs in rodent models of allogeneic transplantation can lead to enhanced graft acceptance (12). A study by Muthukumar et al. showed that increased expression of FOXP3 mRNA in the urine of patients undergoing rejection is associated with better resolution of rejection and improved long-term outcome (13).
The blockade of either the CD28-CD80/CD86 or the IL-2-CD25 pathways results in T-cell anergy and tolerance in experimental organ transplantation (14,15). However, recent studies in mouse models suggest that drugs that target these pathways can have a deleterious effect on Treg survival and function. Both IL-2 and CD28 costimulation have been shown to be an essential survival factors for Tregs (16,17).
A phase II clinical trial combining short-term induction with basiliximab and maintenance therapy with belatacept, a second-generation CTLA-4Ig with increased binding affinity to CD80 (twofold) and CD86 (fourfold) (18), was shown to result in effective immunosuppression, superior renal function and reduced incidence of chronic allograft nephropathy than in patients treated with cyclosporin A (19). In this study, we analyzed patients in a completed phase II and an ongoing phase III renal transplantation trial comparing basiliximab/belatacept combined therapy to a basiliximab/CNI-based regimen. Evaluation of peripheral blood mononuclear cells by flow cytometric analysis and in vitro functional Treg assays suggested that significant, but incomplete, costimulation blockade does not interfere with Treg homeostasis. In contrast, anti-CD25 monoclonal antibodies (mAb) therapy resulted in a transient reduction of both CD25+ Tregs and non-Treg populations, suggesting that the efficacy of this therapy may be compromised in individuals where CD25+ Tregs are essential for the control of allograft rejection. Concomitantly, we observed a significant increase in FOXP3+ T cells in rejecting kidney allograft biopsies in the belatacept-treated group. Therefore, the combination of minimal long-term effects of the basiliximab/belatacept combination therapy on Tregs in the peripheral blood, combined with the short-term preferential effects of both immunosuppression protocols, most likely due to the shared anti-CD25 induction therapy, on non-Tregs, may lead to better resolution of graft rejection episodes and potentially promote tolerance.
Materials and Methods
Kidney transplant patients
Patients receiving a primary renal transplant from a living or deceased donor in the phase II and phase III clinical trials of belatacept were enrolled in both the Treg study as well as the CD86 saturation analyses. The protocol of the phase II study is in Figure 1. Details of the study and outcome of the phase II trial have been published (19). The phase III trial had a similar protocol as the phase II trial but differed in two ways: the low-intensity belatacept group had an additional belatacept infusion at day 4 and the maintenance administration of belatacept was 5 mg/kg every 4 weeks in all patients.
Figure 1. Clinical trial protocol and dosing regimen.
Patients were randomized to receive an intensive regimen of belatacept, a less-intensive regimen of belatacept, or cyclosporine for maintenance immunosuppression. Both belatacept regimens included an early phase (10 mg per kilogram of body weight), which was longer and more frequent in the intensive regimen, and a late phase (5 mg per kilogram of body weight at 4-week or 8-week intervals). All patients received induction therapy with basiliximab, mycophenolate mofetil and corticosteroid-taper.
Phase II patient recruitment and treatment timeline
Twenty-two patients in the phase III trial of belatacept randomized to either belatacept or cyclosporine A had blood samples collected prospectively at different time points. Patient demographics and renal function are shown in Table 1. Patients in the phase II trial of belatacept who have been on therapy for 3–5 years and a similar group treated with CNI were enrolled in the study to analyze the long-term effects of the immunosuppression on Treg cells (Table 2).
Table 1.
Demographic and clinical data of transplant patients in the phase III trial
| CNI (n = 11) | Belatacept (n = 11) | |
|---|---|---|
| Age (years) | 49 ± 13 | 53 ± 13 |
| Male sex (%) | 82% | 54% |
| Type of donor DD: LUR:LRD | 5:03:03 | 5:03:03 |
| Creatinine 1 month posttransplant | 1.4 ± 0.4 | 1.2 ± 0.2 |
| Creatinine 3 months posttransplant | 1.4 ± 0.4 | 1.2 ± 0.2 |
| Graft loss (%) | 0% | 0% |
DD = deceased donor; LUR = living unrelated donor; LRD = living related donor.
Table 2.
Demographic and clinical data of transplant patients in the phase II trial
| CNI (n = 4) | Belatacept (n = 8) | |
|---|---|---|
| Age (years) | 47 ± 8 | 36 ± 14 |
| Male sex (%) | 75% | 75% |
| Type of donor: DD:LUR:LRD | 3:00:01 | 1:03:04 |
| Postoperative (years) | 3.9 ± 0.5 | 4.6 ± 0.6 |
| Creatinine 1 month posttransplant | 1.2 ± 0.3 | 1.5 ± 0.6 |
| Creatinine 6 months posttransplant | 1.1 ± 0.1 | 1.5 ± 0.5 |
| Creatinine 1 year posttransplant | 1.2 ± 0.2 | 1.4 ± 0.5 |
| Last creatinine posttransplant | 1.3 ± 0.2 | 1.3 ± 0.3 |
| Graft loss (%) | 0% | 0% |
DD = deceased donor; LUR = living unrelated donor; LRD = living related donor.
Kidney biopsies during acute rejection were obtained from 18 patients in the phase II belatacept trial and transplant recipients on CNI-based immunosuppression regimens Patient demographics, renal function and type of acute rejection are shown in Table 3. All biopsy specimens were examined by a single histopathologist (ZL) according to Banff 97 criteria in a blinded fashion.
Table 3.
Demographic and clinical data of transplant patients with acute rejection
| CNI (n = 10) | Belatacept (n = 8) | |
|---|---|---|
| Age (year) | 43 + 11 | 43 + 15 |
| Male sex (%) | 60% | 75% |
| Type of donor: DD:LUR:LRD | 10:00:00 | 2:04:02 |
| Creatinine 1 month posttransplant | 1.5 + 0.6 | 2.1 + 1.8 |
| Creatinine 6 months posttransplant | 2.2 + 1.2 | 1.5 + 0.4 |
| Creatinine 1 year posttransplant | 2.0 + 0.7 | 1.4 + 0.4 |
| Time to biopsy (days) | 137 + 29 | 188 + 188 |
| Creatinine at rejection diagnosis | 2.5 + 1.0 | 3.6 + 3.6 |
| Creatinine 1 month after treatment for rejection | 2.0 + 1.1 | 2.1 + 1.8 |
| Time to last follow-up (months) | 38 + 27 | 44 + 16 |
| Graft loss (%) | 60% | 13% |
| Type of acute cellular rejection | 30% | 25% |
| borderline | borderline | |
| 60% 1 | 50% 1 | |
| 10% 2 | 25% 2 |
DD = deceased donor; LUR = living unrelated donor; LRD = living related donor.
All patients in the Treg and CD86 saturation analyses signed an informed consent. The Committee on Human Research approved the histologic study for FOXP3 and CD3 of biopsies during acute rejection (no consent was required).
Reagents
PE-conjugated anti-CD127, Alexa647-conjugated anti-CD127, APC-conjugated anti-CD25, PE-conjugated anti-CD25, APC-conjugated anti-CD8, PerCP-conjugated anti-CD4 and mouse IgG1 (isotype control) used for staining and in sorting was provided by Becton-Dickenson (BD Pharmingen, San Diego, CA). Alexa488-conjugated anti-FOXP3 was purchased from BioLegend (San Diego, CA) and intracellular staining was performed according to manufacturer’s instructions as modified as described previously (20). Carboxy fluoroscein succinimidyl ester (CFSE) was purchase from Invitrogen-Molecular Probe (Carlsbad, CA). DNAse I and human IgG were purchased from Sigma-Aldrich (St. Louis, MO)
Cell collection, antibody staining and cell sorting
Peripheral blood mononucleated cells (PBMC) were isolated from recipient blood samples and stained with CD4, CD127, CD25 and FOXP3 antibody as previously described (20). Data were then analyzed using FlowJo software by Tree Star, Inc. (Ashland, OR). For sorting, 100–120 × 106 PBMC were washed once, counted and resuspended in sorting buffer (PBS, 0.1% BSA, and 1 mM EDTA) at 100 × 106 per mL in a 15-mL conical tube. After addition of 1μL/1 million cells volume PerCP-conjugated anti-CD4, 1 μL/1 million cells PE-conjugated anti-CD127 and 0.7 μL/million cells APC-conjugated anti-CD25 antibodies, the cell suspension was mixed gently and incubated at 4°C for 30 min. Cold fluorescence-activated cell sorting (FACS) buffer was added up to a volume of 15 mL, T cells were pelleted and resuspended at 20 × 106 per mL. Labeled T cells were sorted using an Aria high-speed cell sorter (19). The sort gates for the various T-cell subsets were set to include only those events exhibiting the CD4-specific fluorescence that were also within the lowest density region of a scatter plot. This amounted to between 11.4% and 33.9% (mean 20.87%) (n = 22) of the total number of events for the CD4+ T cells. Based on the CD4 gate, cells were then further gated based on CD127 and/or CD25 expression (CD4+CD127+/−CD25+/− and CD4+CD127+/− alone independent of CD25 as well as CD25hi conventional Tregs). Cells were collected into 100% human AB serum (Cambrex, Walkersville, MD) and washed once with media (RPMI/5% human serum) until ready to be plated in suppression assay. Sorted Treg were 95–98% CD4+CD25hi with a typical yield of 5–12 × 105 T cells per sort whereas CD4+CD127+/−CD25+ cells had a typical yield of 0.9–1.2 × 106 and 98% purity.
Suppression assay using CSFE dilution
Frozen PBMC cells from patients treated with belatacept or CNI were thawed, counted, stained with anti-CD4 and anti-CD127 antibody and sorted on the BD FACSAria based on CD4+CD127 lo/− expression (20). Frozen PBMC from a previously tested donor were thawed, counted and plated as responder in the presence of varying titrations of Tregs. Fifty-thousand CFSE-labeled responders were plated with Tregs in ratios (Treg:Tconv) of 1:1 and 1:2 in the presence of anti-CD3 and anti-CD28 for 4 days. Cells were stained with CD8 in order to distinguish between responders that had completely gone into cycle versus unlabeled Tregs. Data were then analyzed using Modfit software by Verity.
Receptor competition assay-CD86-binding competition
One hundred μL of blood was dispensed into 12 × 75-mm polystyrene tubes and 20 μg of murine IgG (Sigma catalog number: I 5381) was added to each tube for 10 min on ice to block Fc receptors. For identification of monocytes, FITC-labeled anti-CD14 was added to all tubes. Labeled anti-CD86 mAbs, HA5.2B7-PE and 2D4-APCy, were added and samples were incubated for 30 min on ice. To assess nonspecific binding of the labeled anti-CD86 antibodies, a five-fold excess of unlabeled purified anti-CD86 mAbs were added to replicate tubes prior to the addition of the corresponding labeled anti-CD86 mAb and incubated for 15 min on ice. Following final incubations, red blood cells (RBCs) were lysed, and samples were fixed and analyzed on a flow cytometer (BD FACSCanto). The relative level of CD86 present on the surface of CD14+ monocytes was measured and expressed as median fluorescence intensity (MFI).
To determine levels of specific binding, the following calculation was used: MFIT − MFINS = ΔMFI, where MFIT is the total median fluorescence of the PE- or APCy-labeled anti-CD86 mAb; MFINS is the nonspecific median fluorescence of the replicates preincubated with excess unlabeled anti-CD86 mAb, then stained with the PE- or APCy-labeled anti-CD86 mAb; and ΔMFI is the difference between these two values, representing a relative measurement of specific binding.
Immunohistochemistry
Immunohistochemical stains for the CD3 and FOXP3 markers were performed using standardized streptavidin-peroxidase methodology. For antigen retrieval, deparaffinized 3-μm-thick sections of formalin-fixed paraffin-embedded tissues were heat-treated in boiling sodium citrate buffer, at pH 6.0, for 15 min. Endogenous peroxidase activity was blocked with 1.25% H2O2 in methanol for 30 min. After preincubation with 10% normal horse serum, consecutive serially cut sections from each case were incubated with monoclonal anti-CD3 (Dako, Carpinteria, CA) and anti-FOXP3 (BioLegend, Clone 206D, San Diego, CA) antibodies, respectively, for 1 h. This was followed by sequential incubations with biotinylated horse antimouse antibodies (Vector Laboratories, Burlingame, CA) and streptavidin-biotin per-oxidase complex (Vector Laboratories) for 20 min each. All incubations were carried out at room temperature. The reactions were developed with diamino-benzidine (DAB), under microscopic visualization. For six cases, double immunohistochemical stains were also performed with anti-CD3 and anti-FOXP3 antibodies, in a sequential fashion. First, staining was carried out with FOXP3 antibodies and the reaction was developed with DAB, as described above. Next, the slides were incubated with anti-CD3 antibodies followed by sequential incubations with biotinylated horse antimouse antibodies (Vector Laboratories) and streptavidin-biotin alkaline phosphatase complex (Vector Laboratories). The staining was completed using Fast Red (Dako) as chromogen.
Quantitation of CD3 and FOXP3-positive cells in biopsies
The CD3+ and FOXP3+ lymphocytes in the renal biopsies were quantitated in a blinded fashion by one investigator (ZGL). The cells were counted manually in five corresponding cortical fields of the CD3 and FOXP3-stained sections, at 40× magnification, for each biopsy. Only cortical areas away from the immediate subcapsular regions without glomeruli were considered; interstitial and intratubular lymphocytes were counted together. Periglomerular, perivascular and corticomedullary lymphoid aggregates were not included in the quantitation. The average numbers of CD3 and FOXP3+ cells per square millimeter were calculated for each case and each group. The ratio of the FOXP3+ cells to CD3-positive cells was also calculated for each individual case and each group.
Results
Short-term effects of belatacept, CNI and basiliximab treatment of FOXP3+ Tregs
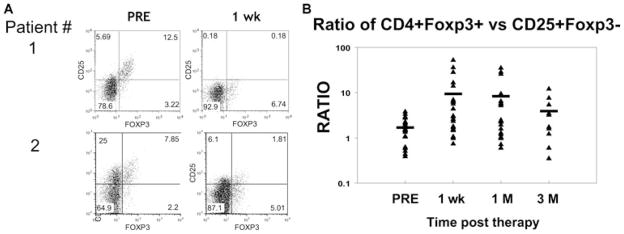
Initially studies were carried out to examine the effects of either a combination of belatacept/basiliximab/mycophenolate mofetil (MMF)/corticosteroids versus CNI/basiliximab/MMF/corticosteroids in the number of circulating CD4+FOXP3+ Tregs in kidney transplant recipients. Previous studies had shown that the majority of Tregs in humans express high levels of CD25 (IL-2 receptor) but low levels of CD127 (IL-7 receptor) (19). Treatment with either regimen resulted in loss of circulating CD4+CD25+FOXP3+ Tregs. In one example, the percentage of CD25+ Tregs dropped from just over 5% pretreatment by more than half after either therapy (supplemental Figure S1). Aggregate data, (Figure 2) showed that the number of Tregs continued to decrease at 30 days but began to recover by 90 days posttherapy while under the cover of either belatacept or CNI therapy. Interestingly, although the CD4+CD25+FOXP3+ Tregs decreased, the percentage of CD25− Tregs remained the same or even went up during this period (supplemental Figure S2; Figure 2B–D). These results were confirmed by staining with a competing versus a noncompeting anti-CD25 mAb (Figure 2A) or the CD127 marker in conjunction with FOXP3 (data not shown) demonstrating that basiliximab was not just coating CD25 on the cell surface.
Figure 2. Short-term effects of belatacept and basiliximab treatment of FOXP3+ Tregs.
Human PBMCs were cell surface stained using a combination of anti-CD4, anti-CD25 and anti-CD127 mAbs. Once fixed, the cells were stained additionally with anti-FOXP3 mAb. (A) This panel represents the changes of FOXP3+ Tregs in the whole cohort of patients before treatment and at different time points posttreatment in either the Belatacept or CNI arms. The cells are gated on CD4+ and percentage of CD25+ cells were analyzed. Data are representative of 10–22 independent individuals from either Belatacept group or CNI group, shown as Mean ± SD with both competing (black bar) and noncompeting (grey bar) anti-CD25 mAbs. (B) This panel represents the ratio of CD25-FOXP3+ versus CD25+FOXP3+ with competing anti-CD25 mAb. Each solid black triangle represents an independent individual, the solid bar represents average percentage among the 10–22 individuals. (C) This panel represents the quantitative changes in CD4+FoxP3+ cells before treatment and at different time points posttreatment in both the belatacept group and CNI groups. Each solid black diamond represents an individual, the solid bar represents the average percentage among the individuals examined. (D) This panel represents the percentage of CD4+CD127 lo/− cells in the whole cohort of patients before treatment and at different time points posttreatment. Each solid black diamond represents an independent individual, the solid bar represents the average percentage among the individuals from both belatacept and CNI groups.
The observation that the decrease in CD4+CD25+FOXP3+ Tregs occurred in both treatment groups, combined with the absolute reduction in the number of FOXP3+ cells, suggest that anti-IL-2R caused a transient reduction of Tregs during the therapy. In fact, in some patients, the ratio of CD4+CD25−FOXP3+ Tregs to CD4+CD25+FOXP3+ Tregs increased as much as 10-fold resulting in a relative increase in the number of CD25− Tregs. However, the total number of FOXP3+ T cells remained relatively stable in the circulation suggesting that there may be proliferation occurring of the remaining Tregs. Finally, the data suggest that neither belatacept nor CNI had a direct effect on Tregs. It remains possible that the generalized effects of organ transplantation and immunosuppression caused the reduction of FOXP3+ cells, however, the fact that treatment regimens selectively resulted in loss of only the CD25+ subset of FOXP3+ cells combined with the observation that the depletion was transient in spite of continuous immunosuppressive therapy, support the conclusion that the reduction was a direct consequence of the basiliximab therapy. Further analysis of all the transplant recipients (a combination of the belatacept- and CNI-treated groups) showed that although the therapy resulted in a significant decrease in CD4+CD25+FOXP3+, there was even a more dramatic decrease in CD4+CD25+FOXP3− (presumably, effector T cells) (Figure 3A). This observation, coupled with the maintenance of CD4+CD25−FOXP3+ Tregs, led to selective increase in Tregs as compared to non-Treg CD4+CD25+ T effector cells (Figure 3B). In fact, overall the relative ratio of CD4+ Tregs to CD4+CD25+ T cells was maintained even at 90 days when the CD4+CD25+FOXP3+ Tregs began to return.
Figure 3. Anti-CD25 therapy results in a selective loss of CD25+ non-Tregs in the short term.
For analysis, the PBMCs were gated on lymphocytes (based on forward and side light scatter) and CD4+ cells and analyzed for CD25 and FOXP3 expression. (A) This panel represents two individual patients comparing CD25+ Treg versus CD25+ non-Tregs. The numbers in the dot plot indicate the percentage of gated cells expressing the relevant marker. (B) This panel represents the ratio of CD4+FOXP3+ versus CD25+FOXP3- cells. Data are representative of 10–22 independent individuals (pooled from both the Belatacept plus Basiliximab and CsA plus Basiliximab groups) at different time point pre- and post-treatment.
Short-term effects of belatacept and CNI plus basiliximab treatment in Treg suppressor activity
Given the loss of CD25 expression on Tregs, it was impossible to use this cell surface marker to select Tregs for in vivo Treg function. Last year, we reported that human CD4+FOXP3+ Tregs express low levels of CD127 that distinguishes these cells from memory cells (20). In fact, the FOXP3+CD127 lo/− T cells are anergic and suppress T-cell responses in vitro as well or better than CD4+CD25hi T cells (20). Therefore, we examined the ability of CD4+CD127lo/− T cells from treated patients to suppress anti-CD3- and anti-CD28-stimulated T cells.
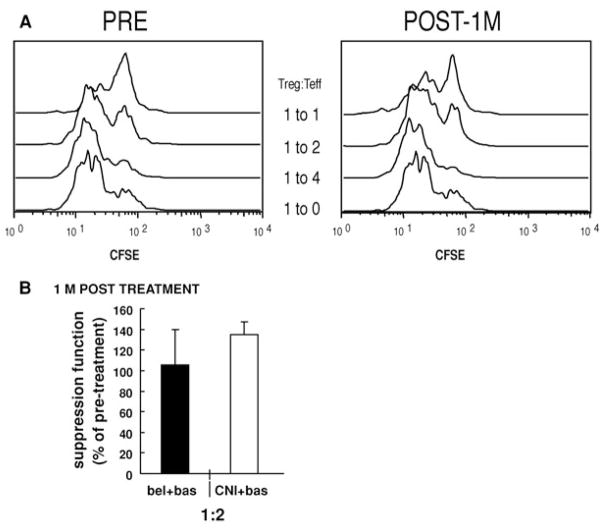
CD4+CD127lo/− T cells from baseline and 1-month post-treatment samples were added to cultures of conventional T cells (Tconv) stimulated with anti-CD3 plus anti-CD28 and compared for their ability to suppress T-cell proliferation. The CD4+CD127lo/− T cell subset suppressed the Tconv proliferation greater then 80% at the 1:2 Treg:Tconv ratio and all the CD4+CD127 lo/− populations were anergic as previously reported (data not shown). As there is variability in suppressor cell activity among different individuals, we compared the ability of CD4+CD127 lo/− T cells from baseline samples to CD4+CD127 lo/− T cells isolated 1 month after transplant. The CD4+CD127 lo/− T cell subset suppressed the proliferation of the Tconv 1 month after therapy as efficiently as the cells isolated prior to transplantation or initiation of treatment (Figure 4). In some cases, the suppression was increased at the later time point. However, the increased suppressor T-cell activity may have been due to loss of effector T cells as a consequence of anti-CD25 therapy and not enhanced Treg activity. These results suggest that Treg function is maintained during the basiliximab induction phase due to the presence of CD25−FOXP3+ T cells.
Figure 4. Suppressive activity of CD4+CD127lo/− cells on a per cell basis.
PBMCs were isolated using Ficoll-plaque from human peripheral blood and stained with anti-CD4 and anti-CD127. CD4+CD127 lo/− were sorted using FACSAria and cultured with CFSE-labeled responder cells (PBMC from an unrelated individual) at different Tregs:Tconv ratios, along with anti-CD3/anti-CD28. Cultured cells were analyzed by gating on CD8+ cells using FACSCalibur and cell division determined using the Modfit software program. (A) Suppression in the original CFSE histograms from one representative patient is illustrated. Data are shown as Treg:Tconv at 1:1 ratio and 1:2 ratios. (B) This panel represents the suppressive function of Tregs in the whole cohort of patients before treatment and at 1 month posttreatment in either the belatacept or CNI arms. Percentage of suppression was normalized to the pretreatment level of each individual (percentage of pretreatment = percentage of suppression of posttreatment/percentage of suppression of pre-treatment × 100). Data were shown as Treg:Tconv at a 1:2 ratio. Black bars represent six individuals of belatacept group, Mean ± SD. Grey bars represent four individuals of CNI group, Mean ± SD.
No long-term effect of belatacept treatment of FOXP3+ Tregs in peripheral blood
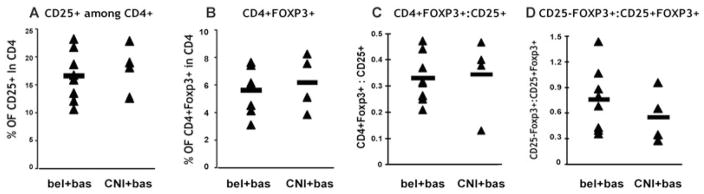
Flow cytometric analyses suggested that the number of Tregs in all treated patients returned to normal numbers by 3 months posttransplantation. However, the patients were continued on a belatacept or CNI inhibitor regimen in the long term. Thus, it was essential to determine if the continued treatment led to long-term changes in Treg numbers or function after the patients had been off of anti-CD25 therapy 3–5 years posttransplant. Treatment with either regimen had no long-term effect on circulating CD4+CD25+FOXP3+ Tregs. The percentage of CD4+FOXP3+ Tregs in both belatacept/basiliximab-treated and CNI/basiliximab-treated patients remained within normal ranges and was no different from the pretransplant levels (Figure 5). In addition, there was no significant difference in the relative percentage of CD4+FOXP3+CD25+ versus CD4+FOXP3+CD25− T cells when compared within groups or to the overall pretransplant levels observed (Figure 5). These data suggest that long-term treatment with either regimen did not affect the number of circulating FOXP3+. To determine whether the long-term therapies had any effect on Treg function, both CD4+CD127 lo/− as well as CD4+CD25+CD127 lo/− cells were purified from each treatment group and added to an in vitro suppression assay as described above. As seen in Figure 6, there was no significant effect of either treatment regimen (belatacept or CNI) on long-term Treg function when analyzing the CD4+CD127 lo/− population. Interestingly, there was a small reduction in the ability of the CD4+CD25+CD127 lo/− to suppress Tconv responses when compared to healthy controls, although, these small differences were not statistically different. These effects were not related to the belatacept or CNI treatment as it was seen in both treatment groups and thus, more likely reflects long-term changes due to the anti-CD25 induction therapy.
Figure 5. No long-term effect of belatacept or CNI treatment on FOXP3+ Treg numbers.
PBMCs were isolated and stained with CD4, CD25 and CD127. Once fixed and permeablized, the cells were stained with anti-FOXP3 mAb. PBMCs were gated on lymphocytes and CD4+ before CD25 and FOXP3 analysis. (A) This panel represents the percentage of CD25+ in the CD4 gate for each individual. (B) This panel represents the percentage of CD4+FOXP3+ cells. (C) This panel shows the ratio of CD4+FOXP3+ versus CD25+ cells for each individual. (D) This panel shows the ratio of CD25−FOXP3+ versus CD25+FOXP3+ cells. Each solid black triangle represents an independent individual, the solid bar represents an average number of 4–8 individuals.
Figure 6. Suppressive activity of CD4+CD127lo/− and CD4+CD25+CD127lo/− cells on a per cell basis.

PBMCs were isolated, stained and sorted for CD4+CD127lo/− and/or CD4+CD25+CD127 lo/− cells. The CFSE dilution assay was used to test suppression function. Percentage of suppression was normalized by the healthy control performed at the same time (normalization formula: percentage of healthy control = percentage of suppression of patient/percentage of suppression of healthy control × 100). Data were shown as Treg versus Tconv at 1:1 and 1:2 ratios. Each solid black triangle represents an independent individual, the solid bar represents an average number of 2–8 individuals.
Belatacept treatment does not fully saturate CD86
During the course of the mouse studies, we observed that although CD80/CD86 KO mice were Treg deficient, the CD80/CD86+/− heterozygotes had significant levels of Tregs despite a 50% reduction in CD80 and CD86 expression (data not shown). This raised the possibility that perhaps the belatacept treatment in humans was subsaturating resulting in substantial residual CD80/CD86 expression. It is important to indicate that these data are from patients receiving belatacept during the maintenance phase as the data from subjects just following the transplant may be different because the dosing is more frequent.
Therefore, we used a receptor competition assay to examine the residual CD86 expression on circulating cells. Analysis of the cells stained with the competing mAb showed that just prior to treatment 10–15% of the CD86 was ‘free’ on the surface of the monocytes (Figure 7). Comparable levels of CD86 were expressed on the cell surface in belatacept-treated patients when compared to CsA-treated renal transplant patients (Figure 7). Analysis of CD86 expression after belatacept treatment showed that virtually all of the remaining CD86 molecules were bound by belatacept with no modulation off the cell surface. Interestingly, in previous studies we observed that saturation of expression of cell surface molecules by mAb therapy is often most complete on circulating cells but far less complete on tissue resident cells (21). Thus, it seems likely that there is substantial free CD86 expression when using this therapeutic regimen that may account for the minimal long-term affects of drug treatment on Treg numbers during the maintenance phase.
Figure 7. CD86 receptor competition assay.
(A) This panel depicts the median fluorescence intensity of CD86 expression on monocytes from three treated patients (tested twice). This analysis utilized the noncompeting anti-CD86 mAb, 2D4. (B) In this panel, the ratio of the competing HA5 to2D4 staining is depicted for the same three individuals at two time points showing that a significant percentage of free CD86 was evident at time of trough levels of belatacept therapy.
Long-term effect of belatacept in transplanted tissues
In a previously published phase II study, belatacept-treated kidney transplant recipients had similar acute rejection rates and 1-year graft survival as compared to the CNI-treated group but significantly better glomerular filtration rate and reduced incidence of chronic allograft nephropathy (19). Previous studies have suggested that the presence of FOXP3 mRNA in cells harvested from the urine of transplant recipients was a predictor of long-term allograft survival (22). Therefore, kidney biopsy specimens from 18 patients receiving either belatacept or CNI, who demonstrated acute rejection, were stained for the presence of CD3+ and FOXP3+ T cells. A representative biopsy, illustrated in Figure 8, shows that both CD3+FOXP3+ and CD3+FOXP3− cells were observed in the kidney allografts of both the belatacept- and CNI-treated patients. Although, FOXP3 can be expressed in the majority of acutely activated T cells, the level of FOXP3 expression is significantly lower than in ‘bonafide’ Tregs and is not readily observed by immunohistochemistry (23). Thus, the majority of CD3+FOXP3+ in the graft biopsy likely represent Tregs, and the balance of the FOXP3+CD3+and FOXP3−CD3+ T cells likely reflects immune homeostasis in the graft (Figure 8). In fact, the ratio of FOXP3+ cells among total CD3+ T cells in the graft biopsies demonstrated that the relative percentage of FOXP3+ cells was significantly elevated in kidney allografts with rejection in the belatacept-treated patients when compared to CNI-treated patients (Figure 9). In fact, the change in ratio was a direct result in alteration of absolute numbers of the respective populations. As seen in Table 4, the relative number of FOXP3+ cells among the total CD3+ cells in the graft changes as a consequence of belatacept theraphy. There was a demonstrable decrease in the total number of CD3+ cells in the belatacept-treated group with a concomitant increase in the total number of FOXP3+CD3+ cells leading to a significant change in the CD3+T cells to FOXP3+ T-cell ratio (p = 0.44).
Figure 8. IHC of biopsies from patients undergoing acute rejection.

Representative microphotographs from the biopsies of calcineurin-inhibitor (A) and belatacept (B and C)-treated patients. Double immunohistochemical stains for CD3 (blue) and FOXP3 (brown) demonstrate a higher number of FOXP3-positive cells in the interstitial cellular infiltrates in the biopsy from the belatacept-treated patient. Note that FOXP3-positive cells are present not only in the interstitial areas, but also between the tubular epithelial cells in a tubule showing prominent tubulitis (C). Magnifications: A and B = 40×; C = 100×.
Figure 9. Ratio of FOXP3+ to CD3+ cells in biopsies.

The FOXP3/CD3 ratio is depicted as a mean and standard deviation for the group of individuals in each treatment group. A significantly higher percentage of Fopxp3+ T cells were observed in the belatacept biopsies (p< 0.05).
Table 4.
Summary of FOXP3 staining results in transplant kidneys
| Average CD3/mm2 | Average FOXP3/mm2 | Percentage (FOXP3/CD3) | |
|---|---|---|---|
| CNI (n = 10) | 1427 + 136.5 | 73 + 5.16 | 6.45 + 3.8 |
| Belatacept (n = 8) | 839 + 119.3 | 131 + 30.4 | 17.99 + 15.6 |
| p-value | 0.20 | 0.23 | 0.044 |
Discussion
Regulatory T cells have been broadly implicated in immune homeostasis across a wide range of immune disorders including autoimmune disorders, infectious immunity and, most important, allogeneic organ transplantation. Thus, with the array of new drugs that are being developed and implemented in the transplant setting to block both acute and chronic graft rejection, it is essential that care is taken to ensure that these therapies do not interfere in Treg development and/or function.
In this study, we have focused on two such drugs, belatacept and basiliximab, a second-generation CTLA-4Ig and anti-CD25, respectively. Both drugs have been shown in animal models to compromise Treg activity and promote immunity by eliminating Tregs. Taking advantage of an ongoing phase II and phase III trials conducted by University of California San Francisco (UCSF) in conjunction with Bristol–Myers Squibb, we were able to study the short-and long-term effects of these drugs in patients treated with either an immunosuppressive regimen that included basiliximab, MMF and corticosteroids in conjunction with either a CNI or belatacept. The results show that belatacept treatment has no short- or long-term effects on circulating Treg numbers or function when compared to the CNI arm. In contrast, it appears that the anti-CD25 monoclonal antibody therapy led to a profound, but transient, reduction in CD4+CD25+FOXP3+ Tregs within 7 days of treatment. It remains possible that reduction of CD25+FOXP3+ cells was due to the generalized effects of organ transplantation and/or immunosuppression. However, as noted above, we observed that immunosuppressive treatment resulted in the selective loss of only the CD25+ subset of FOXP3+ cells. Previous studies have shown that basiliximab treatment leads to transient depletion of effector T cells.
Most significantly, an increased number of Treg cells were observed in rejecting allografts in patients treated with belatacept when compared to the CNI treated. Costimulation blockade has proven to be a very effective therapy in both small and large animal models of allograft transplantation. Thus, it was a surprise, and a concern, when some animal studies showed that CD28 was an essential costimulatory pathway for the development and survival of Tregs (17). We have observed different results in our human studies—why? First, it is possible that human Tregs are not as sensitive to CD28 costimulation blockade as mouse Tregs. This might be due to a different balance of natural versus adaptive Tregs in the two settings (24). For instance, our studies in the autoimmune nonobese diabetic mouse model have shown that adaptive Tregs can be induced in the CD28-deficient setting (25). Thus, it is possible in humans that over time a larger number of adaptive Tregs may be created in response to the allotransplant and, thus, be more CD28 independent. Another possibility is that other costimulatory molecules may ‘substitute’ for the absence of CD28 blockade. In that regard, CD2 is not only highly expressed on human Tregs, but can also function as an extremely effective costimulatory pathway to promote Treg expansion and function (26). Finally, we have noted that disruption of either of the two CD28 ligands, CD80 or CD86, in isolation had only minimal effects on Treg numbers in the periphery (data not shown). Moreover, although the treatment of mice with high concentrations of CTLA-4Ig led to a precipitous decline in the number of circulating Tregs, the Tregs returned rapidly reaching near-normal levels by 1 month (data not shown). Thus, it seemed possible that the long-term dosing schedule in kidney transplant recipients was subsaturating. The data presented here clearly showed that, although >95% of the CD86 was blocked immediately postdosing (due mostly to belatacept coating), only 80% of the CD86 molecule was blocked on the cell surface at the trough of the circulating drug. In fact, this level of ‘free’ CD86 is likely to be an underestimate of the percentage of free CD86 in the tissues, including the kidney allowing Tregs to get sufficient CD28 signals to maintain their survival.
The second surprising observation was the selective early loss of CD4+CD25+ Tregs during basiliximab therapy. Anti-CD25 mAbs have been approved for the treatment of kidney transplant recipients since the late 1990s. The antibody has been thought to target activated T cells and thus eliminate potential alloreactive cells. Thus, the finding that elimination of CD25+ T cells in animal models results in enhanced immunity and autoimmunity raised significant concern in the transplant setting. These concerns intensified when it became clear that CD25 deficiency resulted in loss of Tregs. However, to date there have been no reports of Treg deficiency in patients treated with these CD25-specific drugs early after induction therapy. In this study, we observed that anti-CD25 therapy resulted in a profound short-term depletion of CD25+FOXP3+ Tregs. This effect was not due to modulation of the CD25 molecule but due to elimination of the CD25+FOXP3+ T-cell subset as intracellular staining with a noncompeting antibody, as well as the use of other markers showed that the cells were indeed gone from the circulation (data not shown). However, all the FOXP3+ cells were not eliminated. In fact, the percentage of CD25−FOXP3+ Tregs was equal or increased over time. A combination of CD4 and CD127 was used to purify these cells and show that they retained their suppressive activity, although the functional suppressor assay of proliferation showed a somewhat compromised suppressor cell activity of the CD4+CD127 lo cells presumably due to loss of CD25+ Treg subset. Finally, the cells returned by ~ 90 days posttreatment as did the suppressive function of the CD25+FOXP3+ Treg subset. The finding that the FOXP3+ returned to normal levels at 90 days is in contrast to the report of a protracted Treg decrease in basiliximab-and CNI-treated patients (27), which may either reflect the antio-CD25 or the long-term CNI therapy. Importantly, we observed that the basiliximab therapy led to a dramatic decrease in CD25+FOXP3−CD4+ Tconv cells. This population presumably includes potential allograft-specific effector T cells. Thus, in spite of the transient Treg depletion following anti-CD25 therapy the net result was a shift toward regulation.
Another interesting observation was the finding of a significant increase of FOXP3+ cells in the rejecting allografts of patients treated with belatacept. In the study by Muthukumar et al., patients with increased expression of FOXP3 mRNA in the urine had better resolution of their rejection (13). In our study, we had too few patients to evaluate the impact of FOXP3 cells on outcome, although in the overall phase II trial belatacept-treated patients had significantly better renal function and reduced incidence of chronic allograft nephropathy than the CNI-treated controls. The data suggest that belatacept may function to enhance Treg infiltration in renal allografts and facilitate recovery from rejection.
In summary, this study has shown that treatment with immunosuppressive drugs can have an inadvertent effect on Tregs. Whether the drug effects on Treg cells have an impact on the ultimate outcome of renal allografts remains unclear. However, in this study, we have demonstrated that belatacept therapy has no adverse impact on Treg cells and may in fact enhance their infiltration in the graft during rejection and counteract the effects from effector T cells.
Supplementary Material
Human PBMCs were cell surface stained using a combination of anti-CD4, anti-CD25 and anti-CD127 mAbs. Once fixed, the cells were stained additionally with anti-FOXP3 mAb. The individual panels represent the changes of FOXP3+ Tregs of two individuals (top row vs. bottom row) before treatment and posttreatment at different time points, one from belatacept group and one from CNI group. The number in the dot plot indicates the percentage of gated cells expressing the relevant marker.
PBMCs were isolated and stained with CD4, CD25 and CD127. Once fixed and permeablized, the cells were stained with anti-FOXP3 mAb. PBMCs were gated on lymphocytes and CD4+ before CD25 and FOXP3 analysis. The individual panels represent two individuals, one in the belatacept arm and the other in the CNI arm. The number in the dot plot indicates the percentage of gated cells expressing the relevant marker.
Acknowledgments
The authors would like to thank Todd Brusko, Michael Lee and Greg Szot for technical assistance and review of the manuscript. This study was supported by the following grants: NIH P30 DK63720, NIH R37 AI46643, JDRF 4–2005-1168, T32 HL07970–05 (DG). The authors with the exception of Dr. Vincenti have no conflicts of interests. Dr. Vincenti has received research grants from Bristol–Myers Squibb.
References
- 1.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto AM, Chernajovsky Y, Lepault F, et al. The activity of immunoregulatory T cells mediating active tolerance is potentiated in nonobese diabetic mice by an IL-4-based retroviral gene therapy. J Immunol. 2001;166:4973–4980. doi: 10.4049/jimmunol.166.8.4973. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 4.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 5.Curotto de Lafaille MA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–778. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for scurfin in CD4+CD25 +T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 10.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–538. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 12.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 14.Vincenti F. Costimulation blockade in autoimmunity and transplantation. J Allergy Clin Immunol. 2008;121:299–306. doi: 10.1016/j.jaci.2008.01.002. quiz 307–308. [DOI] [PubMed] [Google Scholar]
- 15.Bluestone JA. CTLA-4Ig is finally making it: A personal perspective. Am J Transplant. 2005;5:423–424. doi: 10.1111/j.1600-6143.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 16.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006;212:149–162. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 18.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 19.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellenhorn JD, Woodle ES, Ghobreal I, Thistlethwaite JR, Blue-stone JA. Activation of human T cells in vivo following treatment of transplant recipients with OKT3. Transplantation. 1990;50:608–612. doi: 10.1097/00007890-199010000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Strom TB, Suthanthiran M. Transcriptional profiling to assess the clinical status of kidney transplants. Nat Clin Pract Nephrol. 2006;2:116–117. doi: 10.1038/ncpneph0115. [DOI] [PubMed] [Google Scholar]
- 23.Veronese F, Rotman S, Smith RN, et al. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant. 2007;7:914–922. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 24.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 25.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci USA. 2007;104:6335–6340. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 27.Bloom DD, Chang Z, Fechner JH, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1 H. Am J Transplant. 2008;8:793–802. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human PBMCs were cell surface stained using a combination of anti-CD4, anti-CD25 and anti-CD127 mAbs. Once fixed, the cells were stained additionally with anti-FOXP3 mAb. The individual panels represent the changes of FOXP3+ Tregs of two individuals (top row vs. bottom row) before treatment and posttreatment at different time points, one from belatacept group and one from CNI group. The number in the dot plot indicates the percentage of gated cells expressing the relevant marker.
PBMCs were isolated and stained with CD4, CD25 and CD127. Once fixed and permeablized, the cells were stained with anti-FOXP3 mAb. PBMCs were gated on lymphocytes and CD4+ before CD25 and FOXP3 analysis. The individual panels represent two individuals, one in the belatacept arm and the other in the CNI arm. The number in the dot plot indicates the percentage of gated cells expressing the relevant marker.