Abstract
Benthic invertebrates may be exposed to metals in porewater, overlying water, ingested sediments, and other food particles. Rates and routes of metal exposure have important implications for predicting toxicity and interpreting toxicity test results. For the standard test amphipod Leptocheirus plumulosus, radiotracer techniques were used to quantify rates of Cd, As, Hg(II), and CH3Hg bioaccumulation from water and from suspension-feeding on labeled microalgae. Measured parameters were incorporated into a bioaccumulation model to predict steady-state metal concentrations in L. plumulosus and to evaluate the relative importance of aqueous and dietary uptake pathways across a range of ingested particle types and ingestion rates. Results indicate that ingested particles contribute strongly to metal bioaccumulation, and that feeding plasticity could strongly influence metal exposure. As L. plumulosus switches from suspension-feeding to deposit feeding or selectively feeds on particles for which it has a high assimilation efficiency, metal exposure and body burden will increase. At ingestion rates previously reported for deposit feeding (3 g/g/d), dietary metal sources dominate metal bioaccumulation and can be responsible for greater than 90% of metal bioaccumulated, regardless of metal partitioning or ingested particle type. Theses results suggest that more research on L. plumulosus feeding behavior is needed to produce a more complete mechanistic understanding of metal bioaccumulation.
Keywords: Leptocheirus plumulosus, Bioaccumulation, Sediment toxicity, Biokinetic model
INTRODUCTION
Estuarine benthic invertebrates can accumulate toxic metals from dissolved and particulate sources [1]. Dissolved phase exposure occurs through direct adsorption of metals dissolved in porewater, burrow water or overlying water, whereas particulate phase exposure occurs through metals associated with ingested food or sediments. The contribution of each exposure route to bioaccumulation may be affected by sediment-porewater metal partitioning [2], metal bioavailability [2], food quality [3], and feeding behavior [2,4]. For organisms used in standard sediment toxicity and bioaccumulation tests, delineating exposure pathways and their relative importance is critical for predicting metal bioaccumulation [5] and interpreting toxicity test results [2]. Quantifying how factors such as metal partitioning and feeding behavior affect rates and routes of metal exposure can help provide a mechanistic understanding of metal bioaccumulation in test organisms.
The estuarine benthic amphipod Leptocheirus plumulosus is a standard test animal used in acute [6] and chronic [7] sediment toxicity tests. In addition, L. plumulosus is widely distributed throughout the eastern United States [8], can reach densities greater than 2.5×104 individuals/m2 ([8],[9], http://web.vims.edu/GreyLit/MDNR/pprp-ltb-est-88-1-2.pdf) and may be an important seasonal prey item for benthic-feeding organisms. Variation in exposure route and feeding behavior may be especially important to L. plumulosus metal bioaccumulation. Leptocheirus plumulosus selectively feeds on high quality food items such as algae [4]. Moreover, in whole-sediment toxicity tests, feeding amphipods a combination of Isochrysis galbana and TetraMin® (Tetrawerke, Melle, Germany) flakes, rather than TetraMin flakes alone, increases the probability of classifying test sediment as non-toxic [10]. In estuaries, L. plumulosus secondary production is correlated with sedimenting chlorophyll a concentrations [11], suggesting L. plumulosus can feed on pelagic food sources that may not be in equilibrium with sediments. In addition, L. plumulosus can ingest food through either suspension feeding or deposit feeding. Ingestion rates for deposit feeding have been reported at 3 g/g/d [12], but ingestion rates for suspension feeding have not previously been reported. If ingestion rates vary substantially between feeding modes, then metal exposure, bioaccumulation, and toxicity may vary as well.
The present study investigates bioaccumulation of four non-essential metals which are common in contaminated estuarine sediments, but have different biogeochemistries and affinities for protein [13]: Cd, As(V), Hg(II), and CH3Hg. As(V), the dominant form of As in estuarine systems [14] is anionic, whereas Cd, Hg(II), and CH3Hg are particle reactive, complex with chloride in seawater, and are cationic [15–16]. For Hg(II) and especially CH3Hg dietary bioaccumulation is important and can affect metal biomagnification [4]. Radiotracer techniques are used to quantify L. plumulosus uptake and efflux rates for dissolved Cd, As, Hg(II) and CH3Hg, and metal assimilation efficiencies and efflux rates for suspension-feeding on metal-enriched algae. Measured parameters are then used in a biokinetic model to predict routes of metal exposure and metal body burden for each metal across a realistic range of water concentrations, particle types, ingestion rates, and water-particle Kd values. Results are discussed in terms of implications for L. plumulosus metal bioaccumulation in toxicity tests and in nature.
METHODS
General
Radiotracer methods were used to describe L. plumulosus metal bioaccumulation from water and from suspension-feeding on the prymnesiophyte Isochrysis galbana. Separate experiments were conducted for each metal (Cd, As, Hg(II), and CH3Hg) and uptake pathway. Suspension feeding on I. galbana was evaluated because this approach has been used successfully previously [3], and because preliminary experiments where L. plumulosus were allowed to burrow into sediment containing algae proved infeasible. Adult L. plumulosus (>3 mm) were obtained from a commercial supplier and were held in 20 ppt Instant Ocean® (Aquarium Systems, USA) without food overnight prior to use in experiments. Each exposure used five replicates (n=5) and followed the same general protocol. In each exposure replicate, ten L. plumulosus were placed in 100 ml of radiolabeled filtered water for a 13 (Cd), 24 (Hg(II), CH3Hg), or 48 h (As) uptake period or placed in an algal suspension for a 45 min uptake period. After uptake, all ten amphipods were pipetted out of their radiolabeled liquid, thoroughly rinsed with unlabeled water, pipetted into a plastic container with 30 ml of unlabeled water, and counted for radioactivity. The amphipods were then pipetted into clean, unlabeled water for a 72 to 96 h depuration period, during which they were placed in an 18 °C incubation chamber with a 14:10 light:dark cycle. For each replicate, all ten amphipods were periodically removed from their depuration water, pipetted into a plastic container together with 30 ml of unlabeled water, and assayed for radioactivity. During uptake and depuration, amphipods were held in glass containers for Hg(II) and CH3Hg experiments, and in polycarbonate containers for Cd and As experiments. Past research, has indicated that mercury, unlike Cd and As, absorbs into plastic, but sorption to glass containers is typically minimal. At the end of each exposure, amphipods were dried at 60°C for at least 12 h to obtain dry weights for each replicate. Dry weight comparisons indicated amphipods used in the CH3Hg dietary uptake exposure weighed significantly more than amphipods used in the Cd aqueous exposure (p<0.05) but no other significant differences were evident.
Sterile 0.2 μm filtered 20 ppt seawater collected from 1m depth in the Elizabeth River (Norfolk, VA USA) (384 μM dissolved organic carbon (DOC)) was used during radioisotope uptake and depuration (hereafter: experimental water). Stocks of 109Cd (t1/2 = 462.3 d) and 73As (As (V), t1/2 = 80.3 d) were obtained from the U.S. Department of Energy (Los Alamos National Laboratory, Los Alamos, NM). 203Hg(II) (t1/2 = 46.6 d) was obtained from Georgia State University (Atlanta, GA, USA) and was methylated [17–19] for use in CH3Hg bioaccumulation experiments. Because all radioisotope stocks were in HCl, a small volume of 1M sodium hydroxide was added along with stock to prevent changes in experimental water pH during radiolabeling.
Uptake from the dissolved phase
For aqueous exposures, experimental water was injected with radioisotope and allowed to equilibrate for 12 h. Specific activities of radioisotope stocks were 0.52 kBq/μg (109Cd), 1.72 kBq/μg (73As), 0.03 kBq/μg (203Hg(II)) and 0.09 kBq/μg (CH3203Hg). Metal concentrations and radioisotope activities in experimental water were: 1 nM and 77.74 kBq/L (109Cd), 0.5 nM and 86.30 kBq/L (73As), 1.69 nM and 14.06 kBq/L (203Hg(II)), and 0.54 nM and 3.195 kBq/L (CH3203Hg). During uptake, amphipods were placed in radiolabeled water for 13 (Cd), 24 (Hg(II), CH3Hg), or 48 h (As). These time periods were chosen in order to produce animals sufficiently well-labeled for depuration measurements while minimizing the potential for efflux during the uptake period. One ml samples of water from each replicate were taken at the beginning and end of uptake and assayed for radioactivity. During depuration, amphipods were assayed for radioactivity at 0, 4, 12, 24, 48, and 72 h, and 3×106 unlabeled I. galbana cells were added to each depuration container each day to prevent starvation.
Uptake from Isochrysis galbana
To generate radiolabeled algae, I. galbana from a culture in late exponential growth phase was added to radiolabeled culture media to create 500 ml of algae at roughly 5×105 cells/ml. For 109Cd, 203Hg(II), and CH3203Hg, culture media was f/2 [20] without Zn, Cu, or ethylenediaminetetraacetic acid (EDTA). For 73As, culture media also contained f/200 phosphate to avoid preferential uptake of phosphate over 73As [21]. Specific activities of radioisotope stocks used for spiking were 0.46 kBq/μg (109Cd), 0.878 kBq/μg (73As), 0.07 kBq/μg (203Hg(II)) and 0.09 kBq/μg (CH3203Hg). After combining algae and radiolabeled water, metal concentrations and activities in the media were: 0.54 nM and 37.31 kBq/L (109Cd), 1.71 nM and 148.80 kBq/L and (73As), 0.73 nM and 14.05 kBq/L (203Hg(II)), and 0.68 nM and 16.61 kBq/L (CH3203Hg). After a 4 d incubation period, algae were collected on a 0.2 μm polycarbonate filter and resuspended into 500 ml of 20 ppt seawater. Isochrysis galbana cell densities (cells/ml) after incubation and resuspension were 6.64 × 105 for Hg(II) and Cd, 5.02 × 105 for As, and 8.65 × 105 for CH3Hg. The fraction of added radioactive metal associated with filtered algae was 22% for Cd, 17% for As, 72% for Hg(II), and 65% for CH3Hg.
Amphipods used in experiments were starved overnight prior to use in pulse feeding. Based on observations of a 65 to 90 min gut passage time for L. plumulosus feeding on I. galbana at 105 cells/ml [22], a 45 min feeding time was used. After uptake, amphipods were pipetted into 100 ml of unlabeled I. galbana at the same density for depuration. Depuration algae were changed every 24 h to keep the density of I. galbana approximately constant. During depuration, amphipod radioactivity was assayed at 0, 45 min, 2 h, 4 h, 12 h, 24 h, 48 h, 72 h, and 96 h. Amphipod feces were collected by pipette at each depuration time point and counted for radioactivity separately from amphipods.
Modeling
To evaluate the relative importance of aqueous and dietary uptake pathways for metal bioaccumulation, parameters measured during radiotracer experiments were incorporated into a biokinetic model [1,5]. Steady-state metal concentration in an organism (Css) can be calculated using the first-order equation:
| (1) |
where ku is the metal uptake rate constant from the dissolved phase (L/g/d), Cw is the metal concentration in water (μg/L), kew is the metal efflux rate constant following uptake from the dissolved phase (1/d), g is the animal’s growth rate constant (1/d), AE is the metal assimilation efficiency from ingested particles, IR is the animal‘s ingestion rate (mg/g/d), Cf is the metal concentration in ingested particles (μg/mg), calculated as kdalgae · Cw, and kef is the metal efflux rate constant following uptake from food (1/d). The fraction of metal uptake from food (Rf) and water (Rw) can be calculated as:
| (2) |
| (3) |
The kew values were calculated from metal retention data in amphipods between 12 to 72 h of the depuration period. To calculate AE and kef, the % metal retained was plotted against depuration time during the second compartment of depuration (24–96 h) and an exponential regression line was fitted to the data. Assimilation efficiency was calculated as the y-intercept and kef was calculated as the regression line slope [23]. Measured growth rate constants for L. plumulosus range from 0.04 to 0.1/d [4, 24], so a growth rate constant of 0.07/d was used here. Ingestion rates were calculated as IR = If/(AE · Cf), where If is the metal influx, calculated using the g of metal in amphipods after 45 min feeding and Cf is the concentration of metal in spiked, filtered and resuspended algae. To calculate Cf, water concentrations were multiplied by kdalgae that was calculated as:
| (4) |
where Pradioactivity is the proportion of radioactivity in filtered cells/the proportion of radioactivity in water after filtering, and I. galbana weighs 16×10−12 g/cell [25].
Using equations 1 to 3, the effect of variation in four different parameters on Rf and Css was modeled. All simulations conducted here assume metal bioavailability from water is the same as that observed for experimental water used here. The Rf may be greater than predicted here in estuarine waters with higher salinities (for Cd) or DOC concentrations (for Hg(II) and CH3Hg), where dissolved metals are less bioavailable.
Variation in AE and IR
The effect of variation in AE and IR was examined by parameterizing Equation 1 one using a range of AE values, and either the suspension feeding IR value measured here or the 3/g/g/d IR reported by Schlekat et al. [12] for deposit feeding, and then calculating Rf values. For Cd, AE was parameterized using measurements available in the literature for a range of algal and sediment particle types (2.9–35.8%) [3,12,22]. For As, Hg(II) and CH3Hg, it was assumed that variation in AE is similar to that reported for Cd. Specifically, it was assumed that AE varied up to 10% above and 20% below the value measured here. Variation in Cd AE may not be representative of other metals, but to our knowledge no other As, Hg(II), or CH3Hg AE measurements are available for marine amphipods. For modeling Cw values were assumed to be the same as used in experiments here for Cd (1 nM) and As (0.5 nM). These concentrations represent the low end of realistic porewater concentrations. For Hg(II) and especially CH3Hg, the water concentrations used in experiments (Hg(II) : 1.69 nM, CH3Hg: 0.5 nM) are higher than in most natural waters due to the specific activity of the radioisotopes available. Therefore, modeling used maximum porewater concentrations measured in a New England estuary (Kittery, ME, USA; Chen et al., unpublished data): 0.2 nM (Hg(II) ) and 0.046 nM (CH3Hg). The Kd values measured here for I. galbana were assumed constant across variation in AE associated with particle type. As a result, Cf values (Cw · kd) were assumed to be 3.19 μg/g (Cd), 0.79 μg/g (As), 9.68 μg/g (Hg(II) ), and 1.34 μg/g (CH3Hg) for all particle types. Thus, this modeling exercise estimates the effect of realistic variation in L. plumulosus AE and IR on exposure pathways assuming that all available particle types have the same metal concentration and seawater has a 20 ppt salinity.
Variation in particle Kd
To examine effects of variation in food concentration or sediment-porewater metal partitioning (when sediment is the primary food source), Rf values were calculated for a range of Kd values (104–106). The same Cw values as in the AE and IR simulation were used, and it was assumed that other parameter values in Table 1 are unaffected by variation in Kd. Across variation in Kd, Rf values were calculated using the IR measured here for suspension feeding and the 3 g/g/d previously reported for deposit feeding [12].
Table 1.
Mean ± standard error measured biokinetic parameters and predicted Css and Rf for Leptocheirus plumulosus
| Aqueous | Dietary | |||
|---|---|---|---|---|
| Ku (L g−1 d−1) | Kew (d−1) | AE(%) | Kef (d−1) | |
| Cd | 0.012 ± 0.002 | 0.021 ± 0.009 | 24 ± 4 | 0.31 ± 0.0003 |
| As | 0.028 ± 0.003 | 0.091 ± 0.006 | 11 ± 2 | 0.58 ± 0.005 |
| Hg | 1.14 ± 0.10 | 0.005 ± 0.06 | 6 ± 1 | 0.089 ± 0.0003 |
| CH3Hg | 1.59 ± 0.1 | 3.0 × 10−5 ± 0.8 × 10−5 | 80 ± 1 | 0.052 ± 0.001 |
AE=assimilation efficiency.
Radioactivity measurements and calculations
A Canberra (Meriden, CT, USA) deep-well NaI(Tl) γ-detector was used to quantify radioactivity in live amphipods (5 min counts to obtain a propagated counting error ≤ 5%). A LKB Pharmacia-Wallac 1282 CompuGamma CS gamma counter (Turku, Finland) was used to quantify radioactivity in water as well as in amphipod feces. γ emissions were measured at 22 keV for 109Cd (X-rays from 109Cd’s daughter 109Ag), 53 keV for 73As, and 279 keV for 203Hg(II) and CH3203Hg. All radioactivity measurements were blank adjusted and decay corrected.
RESULTS
Uptake from the dissolved phase
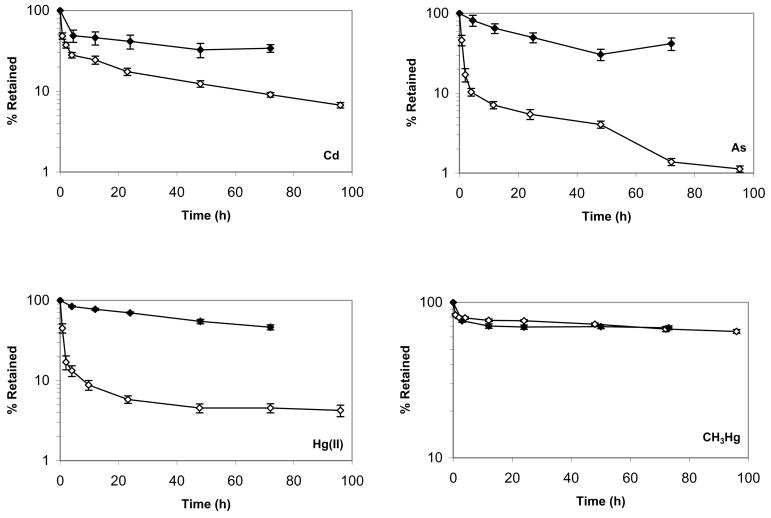
Biokinetic parameters measured for L. plumulosus are presented in Table 1 and depuration curves are presented in Figure 1. Mean metal influx rate constants from the dissolved phase (L/g/d) were 0.012 (Cd), 0.028 (As), 1.14 (Hg(II)), and 1.59 (CH3Hg). During depuration, amphipods showed an initial rapid loss within the first 4 h, followed by a slower loss rate through the remaining 68 h for all metals (Fig. 1). Mean metal efflux rate constants (1/d) were 3.0×10−5 (CH3Hg), 0.0053 (Hg(II)), 0.021 (Cd), and 0.091 (As), (Table 1).
Figure 1.
Leptocheirus plumulosus depuration after uptake from food (Isochrysis galbana) (◇)and water (◆) for Cd, As, inorganic Hg(II) and CH3Hg. Error bars indicate standard error.
Uptake from I. galbana
When suspension-feeding on I. galbana, the mean AE (%) in L. plumulosus was 6 (Hg(II)), 11 (As), 24 (Cd), and 80 (CH3Hg) (Table 1). Efflux followed a two-compartment pattern, an initial rapid loss (0–24 h), due primarily to egestion of unassimilated metals in feces, followed by a slower loss rate (24–96 h) reflecting physiological turnover of assimilated metal (Fig. 1). After 24 h of depuration, the radioactivity of collected feces could not be detected for all metals. Measured efflux rate constants (1/d) were 0.052 (CH3Hg) 0.089 (Hg(II)) 0.31 (Cd) and 0.58 (As). Mean calculated ingestion rates (mg/g/d) were 49.4 (Cd), 104 (Hg(II)), 140 (As), and 151 (CH3Hg), respectively, somewhat lower than the range (150–250 mg/g/d) reported for the estuarine amphipod Melita plumulosa [26]. Variation in algal cell densities and amphipod weights between exposures were small and did not appear related to differences in ingestion rates between metals. The distribution of metal in algal cells may differ for each metal, which may affect L. plumulosus AE and therefore IR calculations.
Modeling
Calculated kdalgae values were 2.65×104 (Cd), 2.65×104 (As), 2.42×105 (Hg(II)), and 1.35×105 (CH3Hg). Using water concentrations and biokinetic parameter values measured experimentally, predicted Rf values were 0.86 (Cd), 0.77 (As), 0.39 (Hg(II)), and 0.85 (CH3Hg) for suspension feeding, and were 0.99 (Cd), 0.99 (As), 0.94 (Hg(II)), and 0.99 (CH3Hg) at a 3 g/g/d IR.
Variation in AE and IR
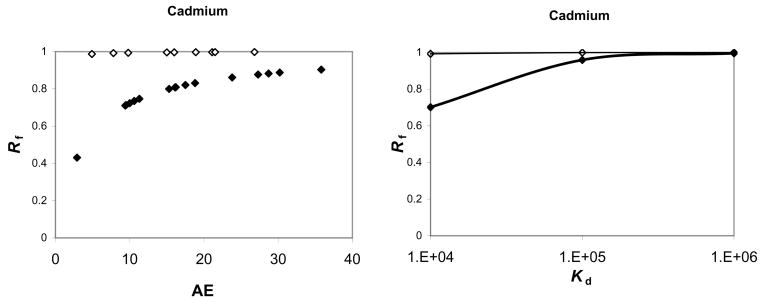
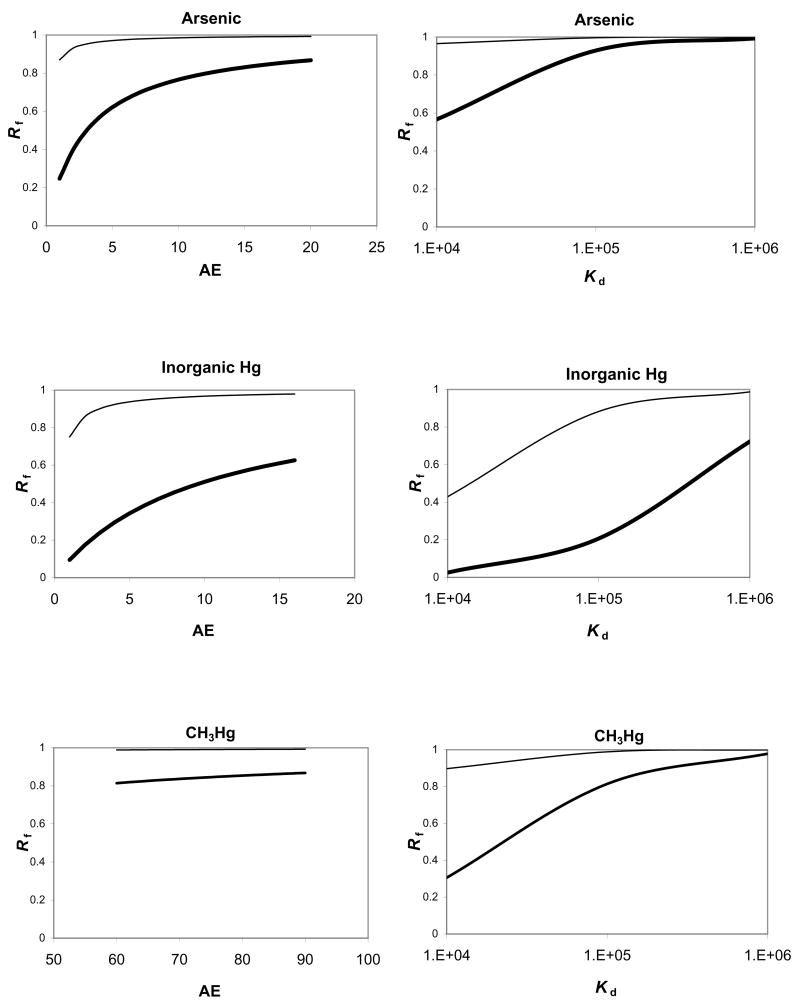
For Cd, calculated Rf values associated with suspension feeding ranged from 0.43 to 0.90 across the simulated range of AE values. At a 3 g/g/d IR [12], variation in AE was overwhelmed by high ingestion rates and the dietary bioaccumulation pathway dominates, with Rf values ranging from 0.989 to 0.998 (Fig. 2). For As and Hg(II), a similar pattern was observed; change in Rf associated with variation in AE was damped for deposit feeding compared to suspension feeding (Fig. 3). In contrast, for CH3Hg, Rf was >80% over the simulated AE range for both IR values. At suspension feeding ingestion rates, predicted Css (μg/g) values were 0.03 to 0.163 (Cd), 0.007 to 0.039 (As), 0.669 to 1.69 (Hg(II)) and 1.00 to 1.72 (CH3Hg). At deposit feeding ingestion rates, predicted Css (μg/g) values were 1.3 to 6.7 (Cd), 0.04 to 0.70 (As), 2.43 to 29.82 (Hg(II)) and 19.99 to 29.88 (CH3Hg).
Figure 2.
The relative contribution of ingested particles (Rf) to Cd bioaccumulation resulting from variation in assimilation efficiency (AE), Kd, and ingestion rate for Leptocheirus plumulosus. Variation in AE for Cd is derived from literature values; variation in Kd is assumed to vary around the value (2.65×104) measured for Isochrysis galbana. Open diamonds indicate deposit feeding and black diamonds indicate suspension feeding.
Figure 3.
The relative contribution of ingested particles (Rf) to metal (As, Hg, CH3Hg) bioaccumulation resulting from variation in assimilation efficiency (AE), Kd, and ingestion rate for Leptocheirus plumulosus. Variation in AE for As, Hg, and CH3Hg is assumed to follow a similar pattern as observed for Cd. Variation in Kd is assumed to vary around values measured here for Isochrysis galbana. Thin lines indicate deposit feeding and heavy lines indicate suspension feeding.
Variation in Kd
For Cd, Rf varied between 0.7 and 1 at the suspension feeding IR, but remained fixed above 0.99 at a 3 g/g/d IR when Kd varied between 104 and 106. A similar pattern was observed for As (Fig. 2) but for Hg(II) Rf variation was greater. For CH3Hg, Rf showed substantial variation at suspension feeding rates, but Rf values were consistently above 0.89 at deposit feeding ingestion rates, due to the much higher AE and low Kef measured for CH3Hg. The Css values increased linearly with Kd. At suspension-feeding ingestion rates, Css (μg/g) varied from 0.05–3.50 (Cd), 0.015–0.85 (As), 0.62–2.18 (Hg(II)), and 0.32–10.19 (CH3Hg). At deposit feeding ingestion rates, Css (μg/g) varied from 2.12 to 211.2 (Cd), 0.18 to 18.21 (As), 1.06 to 46.19 (Hg(II)), and 2.20 to 198 (CH3Hg).
DISCUSSION
In test sediments and in situ, L. plumulosus metal exposure and bioaccumulation will be affected by several important factors: metal partitioning between aqueous and particulate phases, which affects metal concentrations in water and in ingested particles, biogeochemistry affecting dissolved metal bioavailability (e.g. porewater salinity and dissolved organic carbon, sediment total organic carbon and sulfides), variation in L. plumulosus AE associated with different ingested particle types, and variation in L. plumulosus IR associated with different feeding modes. These factors will also affect the relative importance of aqueous and dietary bioaccumulation pathways. Here, Rf values calculated across a realistic range of assimilation efficiencies and particle Kd values suggest that diet contributes substantially to and, under some conditions, dominates metal bioaccumulation (Figs. 2,3). In addition, these results highlight the importance of L. plumulosus feeding behavior. At the 3 g/g/d IR reported for deposit feeding [13], ingested particles were responsible for over 80% of predicted metal bioaccumulation for all metals across all simulated conditions, except for Hg(II), where ingested particles contributed at least 40% (Figs. 2,3). This suggests variation in feeding mode could substantially affect the relative importance of exposure pathways and metal body burdens.
For Cd, similar patterns have been observed for the estuarine amphipod Melitia plumulosa [26]. Biokinetic modeling suggested that at 32 ppt salinity, aqueous Cd may only account for up to 30% of Cd bioaccumulated by M. plumulosa [26]. Similarly, a previous L. plumulosus bioaccumulation model developed using experimentally-measured bioaccumulation factors indicated that ingested particles may be responsible for 40 to 100% of CH3Hg bioaccumulated under conditions tested, depending on ingested particle type and metal concentration [4]. Leptocheirus plumulosus Cd AE has been measured previously for sediment and algal particles (2.9–35.8%) [3,12,22], and the Cd AE measured here is within this range. Cadmium assimilation efficiencies for suspension feeding on algae range from 2.9 to 35.8% [3,12] and increase as N content increases within an algal species [3]. For sediments, measured AE values are 9.8% for sediments from a diatom-encrusted mudflat, 18.9% for 63-μm washed, sieved, and autoclaved sediment, and 21.1% for sediments from within a stand of Spartina alterniflora [12]. Yu and Fleeger [3] measured Cd uptake and efflux rates for L. plumulosus suspension-feeding on I. galbana with differing N content using similar methods. Measured assimilation efficiencies were 10.0, 15.3, and 16.2 % for I. galbana with N enrichment of 0, 60, and 180 μmol/L, respectively [3]. Corresponding kef values (1/d) were 0.480, 0.504, and 0.384 [3]. Measured Cd AE (24%) and kef (0.31/d) values in the present study do differ slightly, although larger animals were used here (>3 mm) than by Yu and Fleeger [3] (1–2.0 mm). Larger animals have a longer gut, and generally longer gut transit times, which may increase assimilation efficiency and reduce efflux rates. Ingestion rate measurements for suspension feeding in the present study (0.049–0.151 g/g/d ) are similar to those reported for the estuarine amphipod Melita plumulosa (0.07–0.25 g/g/d), although M. plumulosa’s feeding mode was not specified [26]. In contrast, citing unpublished data (unpublished data in Schlekat et al. [12]), Schlekat et al. [12] reported that L. plumulosus may have ingestion rates of up to 3 g/g/d when deposit feeding.
Available published body burden data suggest steady-state metal concentrations predicted here fall within a realistic range for Cd and As. When exposed to uncontaminated Chesapeake Bay sediments for 42 d, L. plumulosus had body burdens of 0.085 μg/g (Cd) and 3.23 μg/g (As); when exposed to contaminated sediments, body burdens were 0.237 μg/g (Cd) and 5.23 μg/g (As), respectively, and Hg(II) body burdens were below detection limit [27]. These Cd body burdens overlap with the range of Css values predicted here across variation in AE and Kd for suspension feeding, and the As body burdens overlap with the range of Css values predicted here across variation in Kd for deposit feeding (Table 2). The CH3Hg body burdens measured in experiments where L. plumulosus fed on different combinations of enriched or unenriched I. galbana and sediments ranged from 0 to 5 μg/g wet weight, which corresponds to 0–0.75 μg/g (assuming dry wt is 15% of wet wt) [4], which overlaps wtih body burdens predicted here for suspension feeding (Table 2). In general, amphipod metal body burdens measured in the field are in the low to mid ppm range [2,5,28], which correspond to model predictions.
Table 2.
Predicted Css (μg/g dry wt) values for Leptocheirus plumulosus associated with variation in assimilation efficiency (AE), Kd and ingestion rate (IR). Simulations assume 20 psu salinity, Cw (nM) = 1 (Cd), 0.5 (As), 0.2 (Hg), 0.046(CH3Hg), suspension feeding IR is that measured here, and deposit feeding IR is 3 g/g/d. In simulations varying AE, Kd was assumed to be the same as measured for Isochrysis galbana.
| Variation in AE
|
Variation in Kd |
|||
|---|---|---|---|---|
| Suspension feeding | Deposit feeding | Suspension feeding | Deposit feeding | |
| Cd | 0.03–0.163 | 1.3–6.74 | 0.05–3.50 | 2.12–211.2 |
| As | 0.007–0.039 | 0.04–0.70 | 0.015–0.85 | 0.18–18.21 |
| Hg | 0.669–1.69 | 2.43–29.82 | 0.62–2.18 | 1.06–46.19 |
| CH3Hg | 0.995–1.72 | 19.99–29.88 | 0.32–10.19 | 2.20–198 |
Prior to the present study, L. plumulosus AEs had been quantified for Cd, Ag, and Zn for a range of algal and sediment particle types [3,12, 22]. However, Kef values were only available for Cd [3], and aqueous uptake or efflux parameters were not available for any metal. Aqueous Cd uptake and efflux rates measured for the estuarine amphipod M. plumulosa were 2 to 10 times higher than those measured here for L. plumulosus, suggesting that biokinetic parameters are species-specific for estuarine amphipods [26]. To our knowledge, comparable amphipod aqueous uptake rate measurements are not available for As Hg(II) or CH3Hg. In copepods, 15% or more of metal uptake in aqueous exposures may result from metal sorption to copepod exoskeletons [29]. Amphipods were thoroughly rinsed with unlabeled water prior to radioassaying animals, but did not quantify potential sorption effects. The length of depuration periods used to calculate aqueous efflux rates vary widely for marine crustaceans, from 24 h [26] to 7 d [29]. Calculated efflux values based on longer depuration periods may more accurately reflect efflux from slowly exchanging metal pools.
Porewater is frequently assumed to be the primary route of exposure of Cd to benthic animals, but results presented here indicate ingested particles could be responsible for at least 40% of metal bioaccumulated at the salinity and dissolved metal concentrations tested here. As water concentrations increase, the model used here assumes that aqueous uptake rates increase linearly [1,5]. However, because bioavailability of Cd in porewater and Cd partitioning to organic matter decrease as salinity increases [22], diet may contribute more to metal bioaccumulation at lower salinities. Results for CH3Hg indicate that diet is the primary route of exposure, which corresponds with previous observations for L. plumulosus [4]. However, Hg(II) and CH3Hg metal bioavailability also decreases as DOC in water and organic carbon in sediments increases [4]. For As, no mechanistic information relevant to bioaccumulation for marine amphipods was previously available. In general, marine organisms have a limited ability to bioaccumulate inorganic arsenic from seawater but can bioaccumulate organic arsenic compounds from ingested particles [14]. Here, As(V) influx and efflux rates from water were higher than that observed for Cd, but results predict that relative importance of ingested particles is similar for both metals. Cd bioavailability from water decreases with increasing salinity [22], which may partly explain why ku values for As were higher than for Cd.
Results indicate that variation in L. plumulosus feeding mode and feeding selectivity greatly influences metal uptake. As L. plumulosus switches from suspension-feeding to deposit feeding or switches from feeding on sediments to a particle such as pelagic algae which results in a higher AE of ingested metal, modeling predicts that metal exposure and body burden will increase. When L. plumulosus deposit feeds, ingested particles dominate metal bioaccumulation, regardless of L. plumulosus AE or particle metal concentration, except for inorganic Hg(II) (Figs. 2,3). In addition, predicted Css increased 9 to 450 times during deposit feeding, depending on metal, simulated AE, and Kd (Table 2). Similarly, when L. plumulosus suspension-feeds, ingested particle type can cause Rf to increase by up to 60% (Figs. 2,3) and Css to increase 10 to 120 times, depending on metal (Table 2). Ingesting sediments after suspension-feeding on algae can cause L. plumulosus algal Cd AE to decrease by up to seven times by reducing algal gut residence time [3]. However, model results predict that ingested particles could still be responsible for 10 to 80% of metal bioaccumulation at the lowest AE values simulated here, and suggests switching from suspension feeding to deposit-feeding on sediments would rapidly increase metal bioaccumulation.
Variation in feeding behavior also has important implications for toxicity test methods. In the 10 d acute sediment toxicity test, test sediments are the only food source [5], and during the 28-d chronic test, amphipods are typically fed 20 to 40 mg of TetraMin® flakes 3 d a week [6]. Results here suggest that whether L. plumulosus selectively feeds on benthic algae, other highly organic material associated with sediments, or TetraMin® flakes will affect L. plumulosus AE and the amount of metal bioaccumulated. Further, if L. plumulosus primarily ingests recently deposited or suspended algae in estuaries, standardized toxicity tests may not accurately reflect L. plumulosus food sources or feeding behavior. More research on variation in L. plumulosus ingestion rate, feeding mode, and feeding selectivity is needed. This was the first study to quantify suspension feeding ingestion rates, but only used one algal type. The one deposit-feeding ingestion rate estimate available was originally cited by Schlekat et al. [12] as unpublished data, and is much higher than that measured for most other amphipods, although relatively few measurements exist. As a result, ingestion rates used in modeling here may represent an upper limit for L. plumulosus when deposit feeding. In addition, factors which cause L. plumulosus to switch between feeding modes or to feed selectively on certain particles are not known. Results presented here suggest feeding modes can have a large effect on metal bioaccumulation and may potentially affect toxicity. A more complete mechanistic understanding of metal bioaccumulation will require a more detailed knowledge of L. plumulosus feeding behavior.
Acknowledgments
This research was funded by the U.S. Department of Defense Strategic Environmental Research and Development Program (SERDP) Project ER-1503 and by National Institutes of Health Grant P42 ESO7373 from the National Institute of Environmental Health Sciences. J. Dutton was funded by National Science Foundation 0549370.
References
- 1.Wang WX, Fisher NS. Delineating metal accumulation pathways for marine invertebrates. Sci Total Environ. 1999;237/238:459–472. [Google Scholar]
- 2.Simpson SL, Batley GE. Predicting metal toxicity in sediments: a critique of current approaches. Integrated Environmental Assessment and Management. 2007;3:18–31. [PubMed] [Google Scholar]
- 3.Yu R, Fleeger JW. Effects of nutrient enrichment, depuration substrate, and body size on the trophic transfer of cadmium associated with microalgae to the benthic amphipod Leptocheirus plumulosus. Environ Toxicol Chem. 2006;25:3065–3072. doi: 10.1897/06-029r.1. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence AL, Mason RP. Factors controlling the bioaccumulation of mercury and methylmercury by the estuarine amphipod Leptocheirus plumulosus. Environ Pollut. 2001;111:217–231. doi: 10.1016/s0269-7491(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 5.Luoma SN, Rainbow PS. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Technol. 2005;39:1921–1931. doi: 10.1021/es048947e. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Environmental Protection Agency. Methods for assessing the toxicity of sediment-associated contaminants with estuarine and marine amphipods. EPA/600/R-94/025. Office of Research and Development; Washington, DC: 1994. [Google Scholar]
- 7.U.S. Environmental Protection Agency. Leptocheirus plumulosus. 1. Office of Research and Development; Washington, DC: 2001. Methods for assessing the chronic toxicity of marine and estuarine sediment-associated contaminants with the amphipod. EPA/600/R-01/020. [Google Scholar]
- 8.Bousfield EL. Shallow Water Gammeridean amphipoda of New England. Cornell University Press; Ithaca, NY, USA: 1973. [Google Scholar]
- 9.Holland AF, Shaughnessy AT, Scott LC, Dickens VA. Progress report: Long-term benthic monitoring and assessment program for the Maryland portion of the Chesapeake Bay (July 1986–Ocvtober 1987) Versar; Springfield VA, USA: 1988. PPRP-LTB/EST-88-1. [Google Scholar]
- 10.McGee BL, Fisher DJ, Wright DA, Yonkos LT, Ziegler GP, Turley SD, Farrar D, Moore DW, Bridges TS. A field test and comparison of acute and chronic sediment toxicity tests with the estuarine amphipod Leptocheirus plumulosus in Chesapeake Bay, USA. Environ Toxicol Chem. 2004;23:1751–1761. doi: 10.1897/03-326. [DOI] [PubMed] [Google Scholar]
- 11.Marsh AG, Tenore KR. The role of nutrition in regulating the population dynamics of opportunistic surface-deposit feeders in a mesohaline community. Limol Oceanogr. 1990;35:710–724. [Google Scholar]
- 12.Schlekat CE, Decho AW, Chandler TG. Bioavailability of particle-associated silver, cadmium, and zinc to the estuarine amphipod Leptocheirus plumulosus through dietary ingestion. Limnol Oceanog. 2000;45:11–21. [Google Scholar]
- 13.Nieboer E, Richardson DHS. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ Pollut Ser B. 1980;1:3–26. [Google Scholar]
- 14.Neff JM. Ecotoxicology of arsenic in the marine environment. Environ Toxicol Chem. 1997;16:917–927. [Google Scholar]
- 15.Bruland KW. Trace elements in sea-water. In: Riley JP, Chester R, editors. Chemical Oceanography. Vol. 8. Academic; London, UK: 1983. pp. 157–220. [Google Scholar]
- 16.Fisher NS, Reinfelder JR. The trophic transfer of metals in marine systems. In: Tessier A, Turner DR, editors. Metal Speciation and Bioavailability in Aquatic Systems. John Wiley & Sons; Chichester, UK: 1995. pp. 363–406. [Google Scholar]
- 17.Imura N, Sukegawa E, Pan S-K, Nagao K, Kim J-Y, Kwan T, Ukita T. Chemical methylation of inorganic mercury with methylcobalamin, a vitamin B12 analog. Science. 1971;172:1248–1249. doi: 10.1126/science.172.3989.1248. [DOI] [PubMed] [Google Scholar]
- 18.Rouleau C, Block M. Working methods paper—Fast and high-yield synthesis of radioactive (CH3Hg)-Hg203(II) Appl Organomet Chem. 1997;11:751–753. [Google Scholar]
- 19.Bancon-Montigny C, Yang L, Sturgeon RE, Colombini V, Mester Z. High-yield synthesis of milligram amounts of isotopically enriched methylmercury ((CH3HgCl)-Hg198) Appl Organomet Chem. 2004;18:57–64. [Google Scholar]
- 20.Guillard RRL, Ryther JH. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 21.Sanders JG, Windom H. The uptake and reduction of arsenic species by marine algae. Estuar Coast Mar Sci. 1980;10:555–567. [Google Scholar]
- 22.Schlekat CE, Decho AW, Chandler TG. Dietary assimilation of cadmium associated with bacterial exopolymer sediment coatings by the estuarine amphipod Leptocheirus plumulosus: effects of Cd concentration and salinity. Mar Ecol Prog Ser. 1999;183:205–216. [Google Scholar]
- 23.Wang WX, Fisher NS. Assimilation efficiencies of chemical contaminants in aquatic invertebrates: a synthesis. Environ Toxicol Chem. 1999;18:2034–2045. [Google Scholar]
- 24.Emery VL, Moore DW, Gray BR, Duke BM, Gibson AB, Wright RB, Farrar JD. Development of a chrinic sub-lethal bioassay using the estuarine amphipod Leptocheirus plumulosus (Shoemaker) Environ Toxicol Chem. 1997;16:1912–1920. [Google Scholar]
- 25.Reinfelder JR, Fisher NS. Retention of elements absorbed by juvenile fish (Menidia menidia, Menidia beryllina) from zooplankton prey. Limnol Oceanog. 1994;38:1783–1789. [Google Scholar]
- 26.King CK, Simpson SL, Smith SV, Stauber JL, Batley GE. Short-term accumulation of Cd and Cu from water, sediment and algae by the amphipod Melita plumulosa and the bivalve Tellina deltoidalis. Mar Ecol Prog Ser. 2005;287:177–188. [Google Scholar]
- 27.Manyin T, Rowe CL. Chronic exposure of Leptocheirus plumulosus to Baltimore Harbor sediment: Bioenergetic and population-level effects. Mar Environ Res. 2006;62:116–130. doi: 10.1016/j.marenvres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Rainbow PS. Trace-metal concentrations in aquatic invertebrates: Why and so what? Environ Pollut. 2002;120:497–507. doi: 10.1016/s0269-7491(02)00238-5. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Fisher NS. Accumulation of trace elements in a marine copepod. Limnol Oceanogr. 1998;43:273–283. [Google Scholar]