Abstract
Objectives:
Epidemiologic evidence suggests the natural history of refractory mesial temporal lobe epilepsy is complicated, yet little is known about the hippocampus from the nontertiary center perspective.
Methods:
In a community-based cohort, individuals with nonsyndromic focal epilepsy with onset <16 years and controls had research MRI scans. Hippocampal (HC) volumes were manually measured, corrected for total brain volume, and converted to Z scores (ZHC) based on the controls' values. Volumes in cases and controls were compared.
Results:
Average volumes were not significantly different in cases with unknown cause (n = 117) relative to controls (n = 63). The group with structural and other conditions (n = 23) had significantly smaller volumes. Asymmetry (larger/smaller HC) did not vary among the 3 groups. Hippocampal variances were significantly larger in each epilepsy group relative to controls. In the unknown cause group, 25 (21%) had extreme values: 15 (13%) with ZHC >1.96; 10 (9%) with ZHC <−1.96. By contrast, 2/63 (3%) controls had extreme values (p = 0.001). Within the unknown cause group, temporal lobe epilepsy (TLE) cases were more likely to have extreme hippocampal volumes than non-TLE (31% vs 15%, p = 0.03). Extreme volumes were generally interpreted as normal visually. These anomalies were not associated with seizure remission or pharmacoresistance.
Conclusions:
Classic mesial TLE with hippocampal sclerosis is an uncommon finding in the general population. Volume anomalies, both large and small, are often bilateral. The significance of these findings is unclear; however, speculations regarding preexisting hippocampal pathology (e.g., dysplasia) as a factor in TLE and other neocortical epilepsies have been made by others.
Half or more of epilepsy is of unknown cause with focal features.1–3 The hippocampus has been the focus of research because of its role in mesial temporal lobe epilepsy (MTLE). Hippocampal sclerosis (HS) is the most common target for epilepsy surgery.4–6 The natural history of MTLE is complex, most adult patients having onset of epilepsy in childhood and delay to surgery averaging ≥20 years.7
Clinically, MTLE is not always recognized at initial onset, and HS is rarely seen in new-onset patients.8–10
The origins of HS and its role in epilepsy are poorly understood. HS may arise secondary to a single episode of status epilepticus11,12 or limbic encephalitis13 or be a progressive lesion worsening over time.10,14,15 Other pathology often occurs in conjunction with HS (e.g., migrational disorders in temporal cortex16–20 or within the hippocampus21), suggesting a preexisting pathologic substrate facilitates its development.
Although HS is the lesion of interest for surgery, evidence from various studies indicates that other hippocampal anomalies are present in a wider range of neocortical epilepsies.22–25 This raises questions regarding the nature of the association between hippocampal abnormalities and epilepsy and the involvement of the hippocampus in focal epilepsies.
Little is known about hippocampal abnormalities in the population, especially in childhood-onset epilepsy, where most temporal lobectomy cases arise. We examined hippocampal volumes in a community-based cohort with childhood-onset epilepsy to identify evidence of HS and other potential hippocampal abnormalities.
METHODS
Recruitment and follow-up of the cohort has been extensively described in previous publications1,26 (see appendix e-1 on the Neurology® Web site at www.neurology.org). At 8–9 years after initial diagnosis of epilepsy (2002–2006), 298 participants had a research MRI scan (see appendix e-1).
Healthy control subjects (n = 63) with no history of any seizures or neurologic impairments were recruited. Controls were selected to have the same gender distribution as cases and so as to cover the age range of the case group at the time of the research MRI scan. Cases who required sedation were not offered a research scan. Consequently, individuals with more severe intellectual disabilities were excluded.27
Hippocampal volumetry.
Hippocampal volumes were manually delineated on successive coronal slices using a modified protocol based on previously published methods.28 Total brain volume was estimated automatically using the software program BET, provided as part of the FSL software package.29 Hippocampal volumes were adjusted for brain volume using a covariance method that is commonly used for this purpose. Further details of the hippocampal volumetry, brain volume measurements, and hippocampal volume correction for head size are provided in appendix e-1. For ease of interpretation, corrected left and right hippocampal volumes were then transformed to Z scores based upon the control group's left and right hippocampal volume means and standard deviations. Automated volume measures were used as a secondary method. Further details are presented in appendix e-1.
Clinical characterization of MTLE.
Because the clinical profiles of the cohort members were not comparable to those seen in patients with refractory MTLE evaluated in epilepsy centers, an adult epileptologist specializing in adult epilepsy surgery evaluations (S.U.S.) reviewed all clinical and research records, but not volumetry results, from initial onset through the last contact. For each case, a determination was made whether the clinical profile was possibly or probably consistent with temporal localization (TLE) or whether it was probably or definitely not (not-TLE).
Pharmacoresistance was defined as the failure of 2 appropriate drugs used in adequate trials30 with the second drug failure having been prior to the research scan. Remission was defined as being ≥5 years seizure and aura-free at the time of the scan.
All research scans had been reviewed previously by 2 neuroradiologists who had visually assessed the hippocampal formations for atrophy and signal change and the entire brain for other abnormalities including signs of subtle focal cortical dysplasia.27 Because of interest in the hippocampus and the evolution of MTLE, only cohort members whose epilepsy could be characterized as nonsyndromic with focal features were included for these analyses. The group with unknown underlying cause was the primary focus as this eliminated the heterogeneity associated with the wide variety of known lesions.
Standard statistical techniques (t tests and χ2 tests) were used for testing differences in means and proportions (SAS).
Standard protocol approvals, registrations, and patient consents.
All procedures used in this study were approved by the Institutional Review Boards of the participating institutions. Written informed consent and assent were obtained as appropriate.
RESULTS
A total of 298 cases and 63 controls had research scans. Thirty case research scans could not be used for hippocampal volume measures due to artifacts (n = 14), the scan was postsurgical (n = 10), or other considerations (n = 7). The mean age at the time of the scan was 15.4 years (SD 4.3, range 8–30 years) in cases and 17 years (SD 5.0, range 10–30 years) in controls. Forty-nine percent of each group was female.
Of 268 included cases, 117 had nonsyndromic epilepsy with focal features and no identifiable cause or feature to suggest a lesion or other cause (NSE-UNK). Another 23 had similar epilepsies but with identified lesions or other evidence (e.g., hemiparesis despite normal scan) of an underlying structural lesion (NSE-structural).31 The remaining scans were performed in cases with other forms of epilepsy. Figure e-1 provides a flow diagram documenting inclusion and exclusion of cases from this analysis on the basis of type of epilepsy and imaging artifacts.
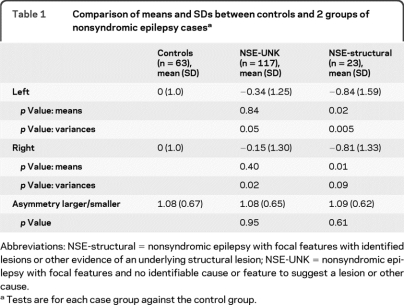
Mean hippocampal volumes were not significantly different in the NSE-UNK epilepsy group relative to controls. The NSE-structural group did have significantly smaller hippocampi. Indices of asymmetry (larger/smaller hippocampus [HC]) did not vary among the 3 groups. The standard deviations of the HC, however, were significantly larger in both of the epilepsy groups relative to controls (table 1).
Table 1.
Comparison of means and SDs between controls and 2 groups of nonsyndromic epilepsy casesa
Abbreviations: NSE-structural = nonsyndromic epilepsy with focal features with identified lesions or other evidence of an underlying structural lesion; NSE-UNK = nonsyndromic epilepsy with focal features and no identifiable cause or feature to suggest a lesion or other cause.
Tests are for each case group against the control group.
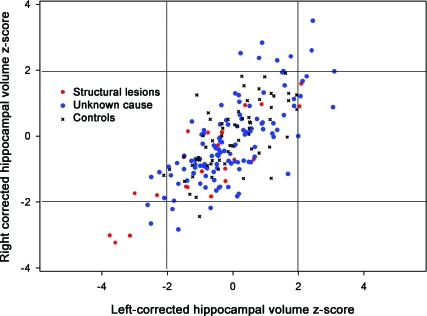
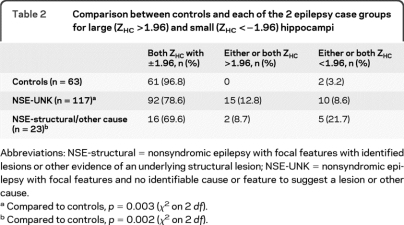
The Z scores for the corrected HC volumes (ZHC) were plotted for the 3 groups (figure 1). Relative to controls and consistent with the findings of a larger SD in the case groups, there were substantially more cases with extreme ZHC values, both high and low. Furthermore and also consistent with the lack of findings for asymmetry indices, the findings for both large and small hippocampi tended to be bilateral (see figure 2 for examples). Relative to controls, both case groups were more likely to have “extreme” hippocampi (one or both hippocampi outside the 5% confidence interval for controls, table 2).
Figure 1. Normalized bilateral hippocampal volume distributions in cases and controls.
Left and right hippocampal Z scores for controls and for cases with nonsyndromic epilepsies.
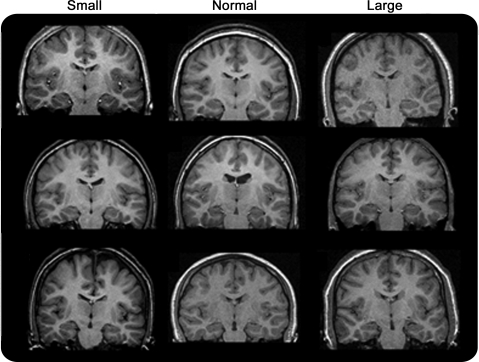
Figure 2. Examples of small, normal, and large hippocampi cases.
Examples of scans in which the hippocampi were either unusually large, ZHC 1.96, or unusually small, ZHC <−1.96, and other case brains for which −1.96 < ZHC <1.96.
Table 2.
Comparison between controls and each of the 2 epilepsy case groups for large (ZHC >1.96) and small (ZHC <−1.96) hippocampi
Abbreviations: NSE-structural = nonsyndromic epilepsy with focal features with identified lesions or other evidence of an underlying structural lesion; NSE-UNK = nonsyndromic epilepsy with focal features and no identifiable cause or feature to suggest a lesion or other cause.
Compared to controls, p = 0.003 (χ2 on 2 df).
Compared to controls, p = 0.002 (χ2 on 2 df).
The uncorrected (raw) volumes and ZHC for all controls and cases with extreme Z scores are provided in table e-1.
The NSE-structural group had a variety of defined lesions and other causes or evidence of brain dysfunction despite apparently “normal” imaging (table e-2). Two had a ZHC >1.96, and 5 were <−1.96 (4 cases with one or both ZHC <−3.0). Because the findings were mostly bilateral, they were not appreciated on visual interpretation and were assessed as normal or in one case with some asymmetry, as equivocal (right ZHC = −1.7, left ZHC = −3.0).
In the NSE-UNK group (n = 117), cases with TLE were more likely to have extreme hippocampal volumes relative to controls (15/49 [31%] vs 2/63 [3%], p < 0.0001) and relative to cases with not-TLE (31% vs 10/68 [15%], p = 0.04).
In comparing the variances of the 3 groups (controls, TLE, and not-TLE), the right and left hippocampal variances were not significantly different for controls vs the not-TLE groups but both groups differed significantly from the TLE group (table 3). Automated hippocampal measurements, when they could be performed, confirmed these findings (table e-3.)
Table 3.
Comparison of means and variances of hippocampal volumes in controls, TLE, and non-TLE cases
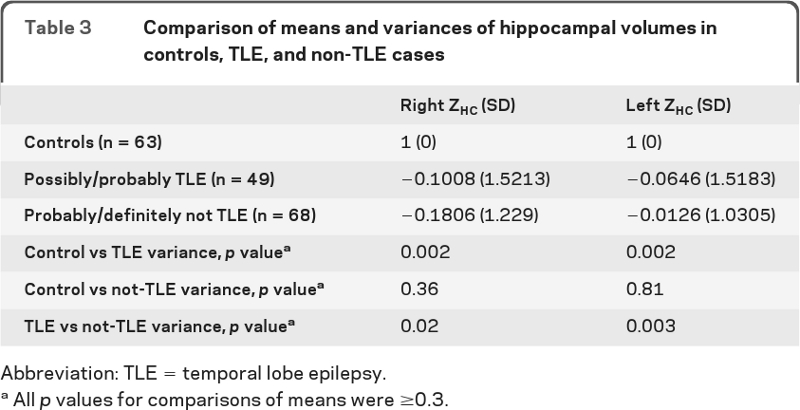
Abbreviation: TLE = temporal lobe epilepsy.
All p values for comparisons of means were ≥0.3.
We performed an exploratory analysis of clinical factors that might be correlated with the extreme hippocampal sizes by performing a series of comparisons in which subjects with average hippocampal volumes (±1 SD of control means) were compared to subjects with extreme (large or small), large only, and small only volumes. Consistent with the results above, TLE was more common in the extreme hippocampal group than in the average group (p = 0.02). In addition, age at onset appeared to be younger in subjects with smaller than in those with larger hippocampi (p = 0.02 for trend). Average age at onset was 4.2 years (SD = 2.6) in the small HC group and 7.3 years (SD = 3.6) in the large HC group (p = 0.03). Table e-4 provides a summary of the other findings.
Hippocampal sclerosis in the entire cohort.
A total of 518/613 (85%) study subjects had either research or clinical MRI scans. Hippocampal sclerosis or volume loss was visually identified in patients with nonsyndromic epilepsy on 13 (7 clinical and 6 research scans). Nineteen patients in the entire cohort have had surgery to date. Only 2 had hippocampal sclerosis according to the pathology reports.27 Both had diffuse processes, multiple surgeries, and were not typical temporal lobectomy patients although one initially presented with febrile status epilepticus and developed HS shortly after that event.
Only 5 of the research scans were read as showing findings consistent with hippocampal sclerosis based on volume, signal, or both. Interestingly, none of these hippocampi, when assessed quantitatively, fell below −1.96 on the Z scores although most did have a moderate degree of asymmetry between the 2 sides, ranging from 0.66 to 1.07 SD difference.
There was only one patient with visually appreciable evidence of hippocampal sclerosis, who was previously considered to have TLE but for this review was not and whose seizures were pharmacoresistant. In this community-based cohort, which has now been followed a median of 15 years, there were few cases who might fit the profile of a typical adult temporal lobectomy patient with childhood onset.
DISCUSSION
Most of what is known about the hippocampus in epilepsy has come from patients evaluated for surgery, most of whom have had epilepsy for many years. Studies of newly diagnosed epilepsy fail to find many patients with clear HS at onset. When it is found, it tends to be unilateral.8–10 While there is evidence from adult studies or studies in chronic epilepsy patients that hippocampal atrophy may be a progressive lesion, there is little information regarding hippocampal abnormalities early in the course of epilepsy in patients, particularly children, who are representative of those arising in the general population. In fact, relative to adults, there are relatively few instances of children with MTLE and HA going to surgery.20,32
Our cohort, while identified at onset, was not scanned for research purposes until 8–9 years after onset. Consistent with the literature's emphasis on unilateral sclerosis, we had expected to find some evidence of early hippocampal atrophy in the form visually discernable unilateral atrophy and signal change. In fact, visual analysis yielded little if any evidence of HA, and for that reason, we turned to quantitative techniques.
These methods yielded evidence of hippocampal abnormalities that were unexpected based on the visual assessments. This was because the findings were bilateral and also because our results suggested that some individuals have not just smaller but also larger hippocampi than would be expected by chance alone and based on a similarly aged control sample scanned at the same institutions and with the same protocols as the cases. This is not something that is appreciated from the surgical literature which is heavily focused on unilateral pathology.
At this point, the significance of bilaterally large or small hippocampus is unclear. Both large and small extreme volumes were modestly associated with a clinical assessment of whether the individual's epilepsy was likely to be TLE. Admittedly, this designation is error-prone simply because the quality and extent of information was often limited. Conversely, the results from several studies suggest that hippocampal anomalies are not limited to patients with clear unilateral temporal lobe epilepsy.20,23,25 For this reason and because of dwindling subgroup sample sizes, we did not further stratify on the TLE designation.
Relative to subjects with average sized hippocampi, subjects with extreme hippocampal volumes were not clearly more likely to have poor seizure control or a history of status epilepticus or generalized tonic-clonic seizures. The size of the subsamples is limited, however, and some of these associations may bear re-examination in an independent sample. Within the group with unknown cause, the small and large hippocampi likely represent different processes, damage (small) and possibly dysplasia (large). In the group with other structural lesions and related conditions, small hippocampi may reflect damaged hippocampi related to the insult.
As most of the participants in this study had well-controlled epilepsy, few have been thoroughly evaluated at tertiary epilepsy centers. Ten participants whose research scans were postsurgical had to be excluded. In the entire cohort, however, there were only 2 (one with a research scan) with evidence of hippocampal sclerosis on pathology; both had multiple surgeries and diffuse processes, not typical MTLE.
There are some important drawbacks to our study. Detailed hippocampal imaging was not performed from the outset of this study which began recruiting patients in 1993. Research scans were done 8–9 years after initial diagnosis in 2002–2006. We can, however, account for patients who did and did not have research scans. This provides some indication of potential biases in who was scanned, the main ones being severe intellectual impairment as well as exclusion of subjects who had already had surgery. In addition, we did not include T2 relaxometry as the intention was to perform visual analysis only. We resorted to quantitative analysis once it became clear that yield of visual analysis was extremely low. Because we had planned originally for visual analysis only, and because it was out of our control, changes occurred in the scanners used for research scans. The types of measurements that were made are relatively robust to scanner differences and should not have introduced any errors or biases into our data.33 Further, the basic findings were confirmed using an independent automated measure although the manual approach is generally superior.34,35
Particular strengths of our study come from the fact that we have studied a group of young people with childhood-onset epilepsy who are representative of the population from which they came. We know the history of the study participants who were not scanned including the results of surgical evaluations and surgeries for anyone in the cohort who was evaluated and can thus reconstruct the overall experience of this representative cohort with respect to developing MTLE with HS, a lesion which is very rare even though this is the age group from which more than half of adult temporal lobectomy patients arise. This suggests either that the hippocampal pathology has not yet progressed enough and cases will develop HS and pharmacoresistance in the future or that the phenomenon of MTLE with HS is actually very rare in the general population of people with epilepsy.
By contrast, bilateral hippocampal anomalies are moderately common. The connection between hippocampal abnormalities seen in our cohort and intractable MTLE is not obvious at this time. In fact, we have been unable to document much MTLE either through research or clinical scans and despite close monitoring of the cohort through direct contact every 3–4 months and review all accumulated neurologic records. Evidence of bilaterally hypertrophic hippocampi may represent the milder end of a spectrum of dysplasia with hippocampal involvement. In our cohort, this may not have immediate implications for treatment; indeed, most of the patients in our group were seizure-free for many years. Conversely, studies demonstrating dual pathology with HS raise the question of whether it is such cases who, given the right provocation, are the ones to develop HS later on. A final consideration is that brain malformations often have a genetic basis,36 and hippocampal malformations have been suggested in familial cases of febrile seizures.37 If our findings can be replicated in other studies, they could have implications for understanding the causes of the formerly “cryptogenic” epilepsies, may provide new targets for studying the genetic contributions to brain malformations, and could possibly be useful in the future for targeting specific treatments to specific types of lesions.
Supplementary Material
- HC
- hippocampal
- HS
- hippocampal sclerosis
- MTLE
- mesial temporal lobe epilepsy
- NSE-structural
- nonsyndromic epilepsy with focal features with identified lesions or other evidence of an underlying structural lesion
- NSE-UNK
- nonsyndromic epilepsy with focal features and no identifiable cause or feature to suggest a lesion or other cause
- TLE
- temporal lobe epilepsy
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Berg and Dr. Pardoe. Dr. Berg is the PI of the study, designed the original study, conducted the statistical analyses described herein, and drafted the original manuscript. Dr. Pardoe performed all of the hippocampal and whole brain volumetric analyses, participated in the analysis and interpretation of the data, and has contributed to drafting the manuscript. Dr. Fulbright has overseen the acquisition of research scans, reviewed all study scans, participated in the interpretation of the data, and contributed to the drafting of the manuscript. Dr. Schuele reviewed the clinical histories of cases described in this report and classified them, and contributed to drafting the manuscript. Dr. Jackson has overseen the volumetry component of this study, has participated in the analysis and interpretation of the data, and has participated in drafting the manuscript.
COINVESTIGATORS
Susan R. Levy, MD (Yale University School of Medicine, New Haven, CT, participated in recruiting and characterizing original cohort), Francine M. Testa, MD (Yale University School of Medicine, New Haven, CT, participated in recruiting and characterizing original cohort), Shlomo Shinnar, MD (Albert Einstein College of Medicine, Bronx, NY, participated in characterizing original cohort), Francis DiMario, MD (Connecticut Children's Medical Center, Hartford, CT, site coordinator 2002–2006), Richard Bronen, MD (Yale University School of Medicine, New Haven, CT, oversaw collection of MRI scans at site). Contributors: Christina Rios (Yale University School of Medicine, New Haven, CT, study coordinator); Charles Hurst (Yale University School of Medicine, New Haven, CT, research associate).
DISCLOSURE
Dr. Berg receives research support from the NIH/NINDS; has received funding for travel and speaker honoraria from Eisai Inc., the British Pediatric Neurological Association, and the Epilepsy Research Center (Melbourne); has received funding for travel from UCB, the American Epilepsy Society, and the International League Against Epilepsy; has received awards from the American Epilepsy Society and British Pediatric Neurological Association; has served as a consultant for Dow Agro Science; serves on the editorial boards of Epileptic Disorders and Epilepsy & Behavior; and is past Chair of the ILAE's Commission on Classification and Terminology, current Chair of the ILAE's Task Force on Classification-Diagnostic Manual, member of the ILAE's Pediatric Commission's Task Force on Autism, member of the AES's Commission on Nonepileptic Seizures, member ad hoc Task Force of the ILAE Commission on Therapeutic Strategies, member of the AES Suicidality Task Force, and steward for the NINDS Benchmarks in Epilepsy Research. Dr. Pardoe has received research support from NIH/NINDS. Dr. Fulbright has received research support from NIH/NINDS. Dr. Schuele has served on the scientific advisory board for Lundbeck Inc.; serves on the editorial board of Epileptic Disorders; serves on speakers' bureaus for GlaxoSmith Kline and UCB; and has received research support from NIH/NINDS. Dr. Jackson serves on a scientific advisory board for Neurosciences Victoria; receives royalties from the publication of Magnetic Resonance in Medicine, 2nd ed. (Elsevier 2005); and has received research support from NHMRC and NIH/NINDS.
REFERENCES
- 1. Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia 1999;40:445–452 [DOI] [PubMed] [Google Scholar]
- 2. Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Epilepsia 2001;42:464–475 [DOI] [PubMed] [Google Scholar]
- 3. Callenbach PM, Geerts AT, Arts WF, et al. Familial occurrence of epilepsy in children with newly diagnosed multiple seizures: Dutch Study of Epilepsy in Childhood. Epilepsia 1998;39:331–336 [DOI] [PubMed] [Google Scholar]
- 4. Van Paesschen W, Connelly A, King MD, Jackson GD, Duncan JS. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann Neurol 1997;41:41–51 [DOI] [PubMed] [Google Scholar]
- 5. Kuzniecky RI, Knowlton RC. Neuroimaging of epilepsy. Semin Neurol 2002;22:279–288 [DOI] [PubMed] [Google Scholar]
- 6. Blümcke I, Kistner I, Clusmann H, et al. Toward a clinico-pathological classification of granule cell dispersion in human mesial temporal lobe epilepsies. Acta Neuropathol 2009;117:535–544 [DOI] [PubMed] [Google Scholar]
- 7. Berg AT. The natural history of mesial temporal lobe epilepsy. Curr Opin Neurol 2008;21:173–178 [DOI] [PubMed] [Google Scholar]
- 8. Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics 2000;106:527–532 [DOI] [PubMed] [Google Scholar]
- 9. King MA, Newton MR, Graeme GD, et al. Epileptology of the first seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet 1998;352:1007–1011 [DOI] [PubMed] [Google Scholar]
- 10. Salmenpera T, Kononen M, Roberts N, Vanninen R, Pitkanen A, Kalviainen R. Hippocampal damage in newly diagnosed focal epilepsy: a prospective MRI study. Neurology 2005;64:62–68 [DOI] [PubMed] [Google Scholar]
- 11. Nohria V, Lee N, Tien RD, et al. Magnetic resonance imaging evidence of hippocampal sclerosis in progression: a case report. Epilepsia 1994;35:1332–1336 [DOI] [PubMed] [Google Scholar]
- 12. Provenzale JM, Barboriak DP, VanLandingham K, MacFall J, Delong D, Lewis DV. Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. AJR Am J Roentgenol 2008;190:976–983 [DOI] [PubMed] [Google Scholar]
- 13. Bien CG, Urbach H, Schramm J, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology 2007;69:1236–1244 [DOI] [PubMed] [Google Scholar]
- 14. Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology 2005;65:223–228 [DOI] [PubMed] [Google Scholar]
- 15. Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: A longitudinal volumetric MRI study. Ann Neurol 2003;53:413–416 [DOI] [PubMed] [Google Scholar]
- 16. Kuzniecky R, Ho SS, Martin R, et al. Temporal lobe developmental malformations and hippocampal sclerosis: epilepsy surgery outcome. Neurology 1999;52:479–484 [DOI] [PubMed] [Google Scholar]
- 17. Salanova V, Markland O, Worth R. Temporal lobe epilepsy: analysis of patients with dual pathology. Acta Neurol Scand 2004;109:126–131 [DOI] [PubMed] [Google Scholar]
- 18. Eriksson SH, Nordborg C, Rydenhag B, Malmgren K. Parenchymal lesions in pharmacoresistant temporal lobe epilepsy dual and multiple pathology. Acta Neurol Scand 2005;112:151–156 [DOI] [PubMed] [Google Scholar]
- 19. Fauser F, Schulze-Bonhage A, Honegger J, et al. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain 2004;127:2406–2418 [DOI] [PubMed] [Google Scholar]
- 20. Riney CJ, Harding B, Harkness WJF, Scott RC, Cross JH. Hippocampal sclerosis in children with lesional epilepsy is influenced by age at seizure onset. Epilepsia 2006;47:159–166 [DOI] [PubMed] [Google Scholar]
- 21. Vernet O, Farmer J-P, Montes JL, Villemure J-G, Meagher-Villemure K. Dysgenetic mesial temporal sclerosis: an unrecognized entity. Child Nerv Syst 2000;16:719–723 [DOI] [PubMed] [Google Scholar]
- 22. Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain 2003;126:2551–2557 [DOI] [PubMed] [Google Scholar]
- 23. Briellmann RS, Jackson GD, Pell GS, Mitchell LA, Abbott DF. Structural abnormalities remote from the seizure focus: A study using T2 relaxometry at 3 T. Neurology 2004;62:2303–2308 [DOI] [PubMed] [Google Scholar]
- 24. Thom M, Sisodiya SM, Lin WR, et al. Bilateral isolated hippocampal malformation in temporal lobe epilepsy. Neurology 2002;58:1683–1686 [DOI] [PubMed] [Google Scholar]
- 25. Bernasconi N, Kinay D, Andermann F, Antel S, Bernasconi A. Analysis of shape and positioning of the hippocampal formation: an MRI study in patients with partial epilepsy and healthy controls. Brain 2005;128:2442–2452 [DOI] [PubMed] [Google Scholar]
- 26. Berg AT, Vickrey BG, Testa FM, et al. How long does it take epilepsy to become intractable? A prospective investigation. Ann Neurol 2006;60:73–79 [DOI] [PubMed] [Google Scholar]
- 27. Berg AT, Mathern GW, Bronen RA, et al. Frequency, prognosis, and surgical treatment of MRI structural abnormalities in childhood epilepsy. Brain 2009;132:2785–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 1992;42:1743–1750 [DOI] [PubMed] [Google Scholar]
- 29. Smith SM. Fast robust automated brain extraction. Human Brain Mapp 2002;17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berg AT, Levy SR, Testa FM, D'Souza R. Remission of epilepsy after 2 drug failures in children: a prospective study. Ann Neurol 2009;65:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685 [DOI] [PubMed] [Google Scholar]
- 32. Mohamed A, Wyllie E, Ruggieri P, et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology 2001;56:1643–1649 [DOI] [PubMed] [Google Scholar]
- 33. Briellmann RS, Syngeniotis A, Jackson GD. Comparison of hippocampal volumetry at 1.5 Tesla and at 3 Tesla. Epilepsia 2001;42:1021–1024 [DOI] [PubMed] [Google Scholar]
- 34. Pardoe HR, Pell GS, Abbott DF, Jackson GD. Hippocampal volume assessment in temporal lobe epilepsy: how good is automated segmentation? Epilepsia 2009;50:2586–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiol 2008;50:569–581 [DOI] [PubMed] [Google Scholar]
- 36. Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology 2005;65:1873–1887 [DOI] [PubMed] [Google Scholar]
- 37. Fernandez G, Effenberger O, Vinz B, et al. Hippocampal malformation as a cause of familial febrile convulsions and subsequent hippocampal sclerosis [see comments]. Neurology 1998;50:909–917 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.