Abstract
Background:
Fibrillar amyloid deposition preferentially affects the frontal lobes, temporal pole/neocortex, and posterior cingulate by age 65 years in APOE ε4 carriers prior to the diagnosis of mild cognitive impairment (MCI) and Alzheimer disease (AD), but is it impairing frontally mediated neuropsychological performance?
Methods:
A total of 71 ε4 homozygotes (HMZ), 194 ε4 heterozygotes (HTZ), and 356 ε4 noncarriers (NC) who did not differ significantly in mean age (56.6 years), years of education (15.6), gender (70% women), or follow-up duration (6.3 years) had neuropsychological testing every 2 years including the Auditory Verbal Learning Test (AVLT) and frontal/executive tasks sensitive to psychomotor speed, working memory, problem solving, and activity. A subset also received the Iowa Gambling Task (IGT). Findings were then tested in a clinical sample of 27 patients with incident MCI and AD.
Results:
APOE ε4 carriers had greater acceleration of decline (quadratic effect) than NC on the AVLT (p = 0.04) but not on any frontal test. APOE ε4 HMZ had greater velocity of decline (linear effects) than NC on all mental arithmetic tests: paced auditory serial attention task (PASAT) 3 second (p = 0.01) and 2 second (p = 0.004) versions; and Wechsler Adult Intelligence Scale–Revised arithmetic (p = 0.048). IGT performance did not differ between 12 ε4 HMZ, 27 ε4 HTZ, and 44 NC. Among 27 patients with incident MCI and AD, the PASAT showed progressive decline preceding diagnosis in 50%.
Conclusions:
No frontal cognitive effects were as robust as memory decline. APOE ε4 HMZ declined more quickly than NC on mental arithmetic tests related to frontal lobe–mediated working memory ability.
In studies of preclinical1 and mild stage Alzheimer disease (AD), Pittsburgh compound B (PiB) uptake is most consistently maximal in frontal and posterior cingulate regions and minimal in medial temporal structures.2–6 Yet, in apparent contradiction to the amyloid cascade hypothesis,7 cortical atrophy is maximal, not in Aβ rich regions, but in medial temporal structures.5 Another recent challenge to Aβ's posited central role in AD pathogenesis, discussed by others,5 comes from the clinical underperformance of Aβ-modifying therapies including immunotherapy-mediated Aβ clearance that failed to halt the dementia progression.8
Paralleling the lack of frontal atrophy is an apparent lack of frontally mediated cognitive dysfunction in preclinical and mild AD,2–6 but this missing “frontal effect” might reflect insufficient study sensitivity due to small patient numbers and a limited range of frontal cognition measures. In patients with established dementia, cerebral Aβ deposition is widespread, but preclinically is more focally concentrated in the frontal lobe, temporal pole and neocortex, and posterior cingulate.1 If Aβ impairs neuronal function, then preclinical APOE ε4 carriers could show accelerated decline on tests sensitive to frontal lobe function as they do on memory tests.
We previously reported that memory, but not letter fluency (a frontally sensitive task), declines in APOE ε4 carriers during this preclinical period.9 Because a single frontal measure may not be sufficiently sensitive, we explored the differences between preclinical APOE ε4 carriers and noncarriers on a battery of frontal/executive measures to test the hypothesis that Aβ deposition adversely affects cognition.
METHODS
Study participants.
From January 1, 1994, through December 31, 2009, cognitively normal residents of Maricopa County age 21 years and older were recruited through local media ads and underwent APOE genotyping and longitudinal neuropsychological assessment every 2 years. Determination of APOE genotype was performed using Taqman single nucleotide polymorphism assays.10
All identified ε4 homozygotes (HMZ) were matched by age, gender, and education to one ε4 heterozygote (HTZ; all with the ε3/4 genotype) and 2 ε4 noncarriers, but many additional heterozygous persons and noncarriers were also eligible for enrollment. Each participant had screening tests that included a neurologic examination, the Folstein Mini-Mental State Examination (MMSE), Hamilton Depression Rating Scale (Ham-D), Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for DSM-III-R. We excluded anyone with potentially confounding medical, neurologic, or psychiatric problems. None met published criteria for MCI,11 AD,12 other forms of dementia, or major depressive disorder.13 Entry criteria included scores of at least 27 on the MMSE (with at least 1 of 3 on the recall subtest), 10 or less on the Ham-D, and perfect scores on the FAQ and IADL.
Clinical sample.
Those who subsequently met published criteria for MCI, AD, or any form of dementia during follow-up, and were thus excluded, were used to test any findings from the main analysis. The longitudinal neuropsychological data from these 27 individuals were examined to determine whether any identified frontal measures showed actual decline from the normal to MCI and dementia states. Test decline was considered present only if both of 2 raters (R.J.C. and D.E.C.L.) agreed in independent ratings.
Standard protocol approvals, registrations, and patient consents.
All individuals gave their written, informed consent to participate in the study and have the results of the APOE test withheld from them, which was approved by the Mayo Clinic Institutional Review Board.
Neuropsychological testing.
The following tests were the endpoints of this study14:
Memory: The long-term memory score (LTM) of the Auditory Verbal Learning Test (AVLT) is a sensitive marker of memory decline in preclinical APOE ε4 carriers.9
- Longitudinal frontal lobe/executive measures.
- Psychomotor speed: Controlled Oral Word Association Test: a timed letter fluency task; Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol Substitution (DSS): patients transcribe, as many as possible within 90 seconds, a simple symbol for a number.
- Working memory: Paced Auditory Serial Attention Task (PASAT) 2- and 3-second versions: a mental arithmetic task in which patients consecutively add pairs of numbers such that each number is added to the one that immediately preceded it. Problems are given at rates of one every 3 or 2 seconds, 60 trials each; WAIS-R Mental Arithmetic (WAIS-arith): another timed mental arithmetic test but each problem is wholly separate; WAIS-R Digit Span (WAIS-DiSp): forward and backward spans are used to generate a total single score. All 3 WAIS-R scores were age-scaled.
- Problem solving: Wisconsin Card Sorting Test (WCST): patients sort a deck of cards depicting designs that vary by shape, color, and the number of designs with the task of inferring the sorting rule receiving feedback limited to whether their choice is correct or not. Three scores included the number of categories completed up to 6, total number of errors, and perseverative errors made.
- Activity: Personality Assessment Inventory Mania Activity Subscale. Participants answer questions related to their desire for outings and nonsedentary events to capture possible changes in drive/motivation. T scores are reported.
Iowa Gambling Task (IGT): This was not part of our longitudinal battery and was recently added for coverage of orbitofrontal cortices.15 The IGT is more sensitive to impairment in mild behavioral variant frontotemporal dementia (bvFTD) than traditional measures.16 Participants select cards from a series of 4 decks including 2 that are high reward/high penalty (that result in net loss and are less favorable) and 2 that are low reward/low penalty (that result in net gain and are more favorable) over 100 trials to gain as much money as possible. The total net score is the number of selections from the more favorable decks minus selections from the less favorable decks. Cross-sectional data only will be presented in a subset of our cohort, all of who are more than 50 years old. Age- and education-based t scores, net total raw scores, and total money gained was compared between the APOE genotype groups.
Longitudinal modeling.
For additional data on longitudinal modeling, see appendix e-1 on the Neurology® Web site at www.neurology.org. To isolate the effect of longitudinal cognitive change for our neuropsychological measures, we used a statistical method to gauge change in performance over time.17 The acceleration of the rate of decline for each of the predetermined measures for carriers (collectively and also separately for each of the subgroups of HMZ and HTZ) was compared to those of the noncarriers using a mixed model approach for modeling cross-sectional and longitudinal data.17,18 A quadratic model was selected to allow for comparison of the acceleration in the rate of decline between groups, as well as linear effects (velocity of decline). Modeling was carried out using SAS Proc Mixed (SAS version 9). In subsequent analyses, the model was modified to replace the carrier variable with a continuous variable equal to 0 for NC, 1 for HTZ, and 2 for HMZ used only to test for linear trend associated with gene dose. These analyses were pre-specified. Baseline characteristics and those recorded during follow-up were compared among groups by using the 2-sample t test/analysis of variance (analysis of variance) F test or Pearson χ2 test.
RESULTS
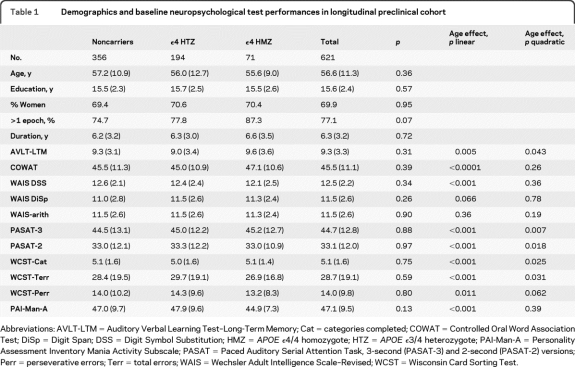
A total of 356 ε4 noncarriers, 194 e3/4 HTZ, and 71 ε4 HMZ were included and did not differ at entry by age (mean 56.6 ± 11.3 years, p = 0.36), years of education (15.6 ± 2.4, p = 0.57), gender (69.9% women, p = 0.95), follow-up duration (6.3 ± 3.2 years, p = 0.72), or entry scores on any of the outcome measures. The main effects of age were significant for every measure (either linear or quadratic effects or both) save for 2 of the age-scaled WAIS-R measures (table 1).
Table 1.
Demographics and baseline neuropsychological test performances in longitudinal preclinical cohort
Abbreviations: AVLT-LTM = Auditory Verbal Learning Test–Long-Term Memory; Cat = categories completed; COWAT = Controlled Oral Word Association Test; DiSp = Digit Span; DSS = Digit Symbol Substitution; HMZ = APOE ϵ4/4 homozygote; HTZ = APOE ϵ3/4 heterozygote; PAI-Man-A = Personality Assessment Inventory Mania Activity Subscale; PASAT = Paced Auditory Serial Attention Task, 3-second (PASAT-3) and 2-second (PASAT-2) versions; Perr = perseverative errors; Terr = total errors; WAIS = Wechsler Adult Intelligence Scale–Revised; WCST = Wisconsin Card Sorting Test.
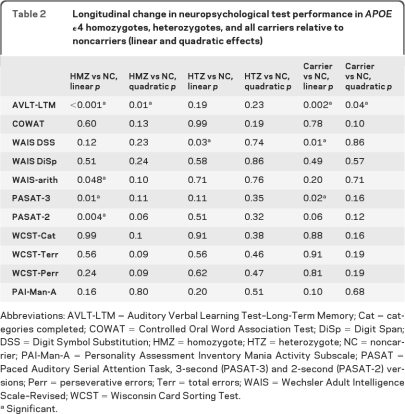
Longitudinal change results are summarized in table 2. As previously reported, only AVLT-LTM showed greater acceleration of decline (quadratic effect) in APOE ε4 carriers relative to noncarriers (p = 0.04). WAIS-DSS (p = 0.01) and PASAT-3 (p = 0.02) showed greater velocity of decline (linear effect) of ε4 carriers relative to noncarriers. When carriers were subdivided into HMZ and HTZ and compared to noncarriers, significant quadratic effects were found only for the AVLT-LTM and only in HMZ (p = 0.01). WAIS-DSS was the only measure to show a significantly steeper decline in HTZ than noncarriers (linear trend, p = 0.03). HMZ declined more than noncarriers on all mental arithmetic tests (linear trends, PASAT-3, p = 0.01; PASAT-2, p = 0.004; WAIS-arith, p = 0.048) in addition to the AVLT-LTM (p < 0.001). When frontal/executive measure comparisons were adjusted for AVLT-LTM, the results did not change. APOE ε4 gene dose effect was significant for AVLT-LTM (p = 0.01, linear trend) and borderline for the PASAT-2 second (p = 0.06, linear trend).
Table 2.
Longitudinal change in neuropsychological test performance in APOE ϵ4 homozygotes, heterozygotes, and all carriers relative to noncarriers (linear and quadratic effects)
Abbreviations: AVLT-LTM = Auditory Verbal Learning Test–Long-Term Memory; Cat = categories completed; COWAT = Controlled Oral Word Association Test; DiSp = Digit Span; DSS = Digit Symbol Substitution; HMZ = homozygote; HTZ = heterozygote; NC = noncarrier; PAI-Man-A = Personality Assessment Inventory Mania Activity Subscale; PASAT = Paced Auditory Serial Attention Task, 3-second (PASAT-3) and 2-second (PASAT-2) versions; Perr = perseverative errors; Terr = total errors; WAIS = Wechsler Adult Intelligence Scale–Revised; WCST = Wisconsin Card Sorting Test.
Significant.
The IGT results are summarized in table e-1. A total of 44 ε4 noncarriers, 27 e3/4 HTZ, and 12 ε4 HMZ over age 50 years completed the IGT. The groups did not differ by age, educational background, or performance on any neuropsychological measure. IGT t score (p = 0.046) and total net score (p = 0.021) declined with increasing age for the group overall, but there were no significant interactions with APOE genotype or carrier status.
A total of 13 ε4 HMZ, 9 ε4 HTZ, and 5 ε4 noncarriers (11 women and 16 men) who were cognitively normal at entry converted to MCI (n = 25) or AD (n = 2) after a mean follow-up interval of 86.6 ± 44.0 months (3.8 ± 1.6 test epochs) and so were excluded from the above analyses. The frequency of test decline is summarized in table e-2. Memory was clinically impaired in all cases and in 75%–78% of cases AVLT LTM and complex figure test recall scores showed clear evidence of progressive decline over successive epochs up to the clinical diagnosis. PASAT declined in 50% of cases, all ε4 carriers (p = 0.042). WCST declined with similar frequency but with no difference between ε4 carriers and noncarriers.
DISCUSSION
APOE ε4 influences the age at onset of AD in a gene dose-dependent fashion.19,20 By age 65 years, healthy-appearing APOE ε4 carriers have higher amounts of cerebral amyloid than noncarriers evident both on autopsy21 and amyloid-ligand (PiB) PET,1 reduced CSF Aβ levels,22 and declining memory performance on neuropsychological tests.9 Collectively, these changes define and characterize the preclinical stage of AD.
The amyloid cascade hypothesis based upon autosomal dominant mutations that cause early onset familial AD is the prevailing model for AD pathogenesis.7 It is supported by numerous studies that collectively reveal a variety of Aβ-mediated adverse effects on neuronal physiology23–25 implying that areas of Aβ deposition in the brain should manifest impaired function.26 In transgenic mice, Aβ's role in neurodegeneration and memory loss is upstream from and dependent upon tau.27–29 In patients, Aβ deposition precedes hippocampal atrophy that in turn correlates most directly with memory loss.2
We again found an acceleration of memory decline in preclinical APOE ε4 carriers beginning around age 55–60 years, but no similar acceleration of decline on any frontal/executive measure despite the greater amount of fibrillar amyloid in frontal than medial temporal regions.1–6 The frontal/executive measures we used were age-sensitive, surveyed a wide range of relevant skills, and comprised a unique dataset in the extensive literature of cognitive aging. Although none demonstrated significant quadratic effects, several showed significant linear effects (uncorrected for multiple comparisons), particularly mental arithmetic tasks in ε4 HMZ, the group with the heaviest frontal amyloid burden.1 This could reflect the direct effect of Aβ itself on neuronal function or another pathophysiologic factor that causes both Aβ deposition and neuronal dysfunction.
In a small but separate group of individuals with incident MCI and AD who were excluded from the study, the PASAT declined in 50% of cases (as did the WCST), which was far more than other frontal measures. We did not find PASAT decline in the 5 APOE ε4 noncarriers, but this probably reflects the small size of our clinical sample. Although it has been reported that executive measures are more likely to decline in ε4 noncarriers with very mild AD,30 differences in test batteries, cohort characteristics, and study design may account for the seemingly divergent findings obtained in our study. However, it should be noted that both studies concur on the absence of robust frontal dysfunction in APOE ε4 carriers.
Cognitive studies of normal aging have consistently demonstrated declining frontally mediated skills including working memory and psychomotor speed.31–33 Our findings are consistent with this age effect, but less so with a further APOE genotype interaction. Our IGT administration was a first attempt to encompass an aspect of frontally mediated cognition that is not generally surveyed in cognitive aging studies or well-captured by traditional psychometric tests. Others have shown that the IGT in cross-sectional studies is sensitive to early stage bvFTD.16 Our cohort of ε4 carriers was similar in size to these bvFTD cohorts, yet they did not perform less well than noncarriers despite their greater frontal Aβ burden.
Preclinical AD is a recently characterized entity34 that provides the opportunity to directly test brain–behavior relationships related to the relatively focal patches of specific pathology including frontal lobe Aβ and medial temporal neurofibrillary tangles (NFT). While the tests in our battery should not be inferred to reflect precise anatomically defined frontal subsectors, they are nonetheless sensitive to frontal/executive function more generally. The Alzheimer's Disease Neuroimaging Initiative (ADNI),2 Melbourne Healthy Aging Study,4 and others5,6 have examined the relationship between neuropsychological test performance and cerebral Aβ. All found memory to be the major area of decline; none found significant frontal effects. In the ADNI cohort, memory decline correlated most directly not with PiB uptake, but with hippocampal atrophy, although the atrophy itself correlated with PiB uptake.2 There was no clinical or cognitive decline associated with progression of PiB retention in an ADNI–Mayo Clinic preclinical cohort, although the cognitive battery used was abbreviated to the MMSE and Clinical Dementia Rating.35 Areas of maximum PiB uptake correlated poorly with cortical atrophy, leading to speculation about selective vulnerability of the hippocampus to Aβ-mediated toxicity.5 Neuropathologic studies have shown that Aβ deposition begins in neocortical regions and not the hippocampus. Conversely, NFT formation begins in the entorhinal cortex, is nearly universal over age 65 years, and is part of normal cognitive aging (including preclinical stage AD).36,37 Medial temporal NFT pathology provides a more proximate explanation for preclinical stage hippocampal atrophy and memory loss38 although acceleration of NFT formation in the presence of Aβ is possible.
Through longitudinal modeling of a comprehensive neuropsychological battery in a large cohort, we found faster decline on mental arithmetic tests in APOE ε4 homozygotes. Yet none showed an acceleration of decline as did memory, and a large number of tests in other domains failed to discriminate APOE ε4 carriers and noncarriers at all. There are several possible explanations for this. First, Aβ may not be as neurotoxic in humans as animal models have suggested. We cannot distinguish between the direct toxicity of amyloid itself or another factor that caused both cognitive decline and amyloid deposition, the latter even potentially formed in reaction to the insulting event as a protective mechanism (thus attenuating frontal dysfunction). Second, assuming Aβ is neurotoxic, its density may have been insufficient during the preclinical period to cause enough dysfunction for these tests to detect. Consistent with (but not proving) this possibility, we found clear examples of progressive decline on the WCST in individual cases at the later stage of incident MCI and AD. Third, poor baseline test performance could reduce our power to detect subsequent decline, particularly on the more difficult tests in our battery. However, even in a post hoc analysis when poor baseline performers of the WCST were excluded, we still did not find evidence of greater decline in ε4 carriers. Fourth, other tests and frontal lobe–mediated functions were not fully assessed, such as social cognition, response inhibition, interference/conflict monitoring and control, and source memory. Finally, soluble amyloid monomers and oligomers not imaged with PiB-PET may cause greater neuronal dysfunction than fibrillar amyloid.23,24 This might explain the greater impairment of memory in the absence of medial temporal PiB uptake, but is speculative.
Preclinical acceleration of memory decline in APOE ε4 carriers was not accompanied by acceleration of any frontal measure decline. APOE ε4 HMZ had greater velocity of decline on mental arithmetic tests, but our data cannot distinguish between the potentially direct neurotoxic effects of Aβ itself, or another factor that caused both neuronal dysfunction and amyloid deposition. This finding should be regarded as exploratory and warrants confirmation in an independent cohort. While autosomal dominant familial AD clearly establishes a role for amyloid in AD pathogenesis, and multiple lines of evidence have demonstrated the potentially deleterious effects of amyloid on neuronal function and survival, our relative paucity of findings, taken together with prior studies showing poor correlation between amyloid and atrophy, and the therapeutic failures to date of amyloid-modifying therapies, suggest that it may be time to reconsider amyloid's currently envisioned role in pathophysiologic models of AD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Sandra Yee-Benedetto, Bruce Henslin, Jessie Jacobsen, Jeanne Young, Allyson Jensen, Jennifer Pichon, and Marci Zomok for technical assistance.
- AD
- Alzheimer disease
- ADNI
- Alzheimer's Disease Neuroimaging Initiative
- AVLT
- Auditory Verbal Learning Test
- bvFTD
- behavioral variant frontotemporal dementia
- DSM-III-R
- Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- DSS
- Digit Symbol Substitution
- FAQ
- Functional Activities Questionnaire
- Ham-D
- Hamilton Depression Rating Scale
- HMZ
- homozygote
- HTZ
- heterozygote
- IADL
- Instrumental Activities of Daily Living
- IGT
- Iowa Gambling Task
- LTM
- long-term memory
- MCI
- mild cognitive impairment
- MMSE
- Mini-Mental State Examination
- NC
- noncarrier
- NFT
- neurofibrillary tangle
- PASAT
- paced auditory serial attention task
- PiB
- Pittsburgh compound B
- WAIS-arith
- WAIS-R mental arithmetic
- WAIS-DiSp
- WAIS-R Digit Span
- WAIS-R
- Wechsler Adult Intelligence Scale–Revised
- WCST
- Wisconsin Card Sorting Test
Supplemental data at www.neurology.org
DISCLOSURE
Dr. Caselli serves as Medical Editor for Clinical Neurology News and receives research support from the NIH/NIA and the Arizona Alzheimer's Research Consortium. Dr. Dueck, Dr. Locke, and C.R. Hoffman-Snyder report no disclosures. Dr. Woodruff serves on a scientific advisory board for the Desert Southwest Chapter of the Alzheimer's Association and receives research support from the NIH/NIA. Dr. Rapcsak receives research support from the NIH (NIA/NIDCD). Dr. Reiman serves on scientific advisory boards for Accera, Inc., Bayer Schering Pharma, Elan Corporation, Eli Lilly and Company, AstraZeneca, GlaxoSmithKline, Siemens, Takeda Pharmaceutical Company Limited, and Eisai Inc.; serves as Deputy Editor for the Journal of Clinical Psychiatry; holds a patent re: Methods for tracking the progression of Alzheimer's disease identifying treatment using transgenic mice; serves as a consultant for Amnestix/Sygnis; receives research support from Kronos Life Sciences, GlaxoSmithKline, AstraZeneca, Avid Radiopharmaceuticals, Inc., the NIH (NIA, NIMH), and the state of Arizona; and serves as Executive Director of the Banner Alzheimer's Institute and Director of the Arizona Alzheimer's Consortium, 501C3 organizations which have emphasized the use of brain imaging and other biomarker measurements in the evaluation of putative pre-symptomatic treatments for Alzheimer's disease.
REFERENCES
- 1. Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 2009;106:6820–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated B-amyloid deposition in elderly subjects. Brain 2008;132:1310–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci 2007;27:6174–6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villemagne VL, Pike KE, Darby D, et al. AB deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia 2008;46:1688–1697 [DOI] [PubMed] [Google Scholar]
- 5. Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinno JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology 2009;72:1504–1511 [DOI] [PubMed] [Google Scholar]
- 6. Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology 2010;74:807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 1992;256:184–185 [DOI] [PubMed] [Google Scholar]
- 8. Nicoll JA, Wilkinson D, Holmes C, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 2003;9:448–452 [DOI] [PubMed] [Google Scholar]
- 9. Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 2009;361:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crook R, Hardy J, Duff K. Single-day apolipoprotein E genotyping. J Neurosci Methods 1994;53:125–127 [DOI] [PubMed] [Google Scholar]
- 11. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133–1142 [DOI] [PubMed] [Google Scholar]
- 12. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurol 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 14. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, 4th edition. New York: Oxford University Press; 2004 [Google Scholar]
- 15. Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science 1997;275:1293–1295 [DOI] [PubMed] [Google Scholar]
- 16. Torralva T, Roca M, Gleichgerracht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain 2009;132:1299–1309 [DOI] [PubMed] [Google Scholar]
- 17. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis (Section 15.4). Hoboken: John Wiley & Sons, Inc.; 2004 [Google Scholar]
- 18. Ware JH, Dockery DW, Louis TA, Xu X, Ferris BG, Speizer FE. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiol 1990;132:685–700 [DOI] [PubMed] [Google Scholar]
- 19. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of AD in late onset families. Science 1993;261:921–923 [DOI] [PubMed] [Google Scholar]
- 20. Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele ϵ4 with late onset familial and sporadic Alzheimer's disease. Neurology 1993;43:1467–1472 [DOI] [PubMed] [Google Scholar]
- 21. Kok E, Haikonen S, Luoto T, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age, Ann Neurol 2009;65:650–657 [DOI] [PubMed] [Google Scholar]
- 22. Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 2007;27:2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 2008;14:837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu HY, Hudry E, Hashimoto T, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci 2010;30:2636–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid B-peptide in the brain and cognitive decline. JAMA 2000;283:1571–1577 [DOI] [PubMed] [Google Scholar]
- 27. Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to B-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA 2002;99:6364–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid B-induced deficits in Alzheimer's disease mouse model. Science 2007;316:750–754 [DOI] [PubMed] [Google Scholar]
- 29. Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-B toxicity in Alzheimer's disease mouse models. Cell 2010;142:387–397 [DOI] [PubMed] [Google Scholar]
- 30. Wolk DA, Dickerson BC. ADNI. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci USA 2010;107:10256–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull 1996;120:272–292 [DOI] [PubMed] [Google Scholar]
- 32. Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neurosci Behav Rev 2002;26:849–857 [DOI] [PubMed] [Google Scholar]
- 33. Treitz FH, Heyder K, Daum I. Differential course of executive control changes during normal aging. Aging Neuropsychol Cogn 2007;14:370–393 [DOI] [PubMed] [Google Scholar]
- 34. Preclinical Alzheimer's Disease Workgroup Criteria for Preclinical Alzheimer's Disease. National Institute on Aging and the Alzheimer's Disease and Related Disorders Association; 2010 [Google Scholar]
- 35. Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment, and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009;132:1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bouras C, Hof PR, Giannakopoulos P, Michel J-P, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex 1994;4:138–150 [DOI] [PubMed] [Google Scholar]
- 37. Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997;18:351–357 [DOI] [PubMed] [Google Scholar]
- 38. Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science 1984;225:1168–1170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.