Abstract
Recognition of drugs by immune cells is usually explained by the hapten model, which states that endogenous metabolites bind irreversibly to protein to stimulate immune cells. Synthetic metabolites interact directly with protein generating antigenic determinants for T-cells; however, experimental evidence relating intracellular metabolism in immune cells and the generation of physiologically relevant antigens to functional immune responses is lacking. The aim of this study was to develop an integrated approach using both animal and human experimental systems to characterize sulfamethoxazole (SMX) metabolism-derived antigenic protein adduct formation in immune cells and define the relationship between adduct formation, cell death, co-stimulatory signalling and stimulation of a T-cell response. Formation of SMX-derived adducts in antigen presenting cells was dose- and time-dependent, detectable at non-toxic concentrations and dependent on drug metabolizing enzyme activity. Adduct formation above a threshold induced necrotic cell death, dendritic cell co-stimulatory molecule expression and cytokine secretion. Antigen presenting cells cultured with SMX for 16h, the time needed for drug metabolism, stimulated T-cells from sensitized mice and lymphocytes and T-cell clones from allergic patients. Enzyme inhibition decreased SMX-derived protein adduct formation and the T-cell response. Dendritic cells cultured with SMX and adoptively transferred to recipient mice initiated an immune response; however, T-cells were stimulated with adducts derived from SMX metabolism in antigen presenting cells, not the parent drug. This study shows that antigen presenting cells metabolize SMX; subsequent protein binding generates a functional T-cell antigen. Adduct formation above a threshold stimulates cell death, which provides a maturation signal for dendritic cells.
Introduction
Hypersensitivity represents one of the most feared adverse events in the drug development process. The presence of drug-specific, cytotoxic T-cells in the peripheral circulation and target organs of hypersensitive patients, but not drug-exposed controls provide a robust case for their involvement in the pathogenesis of the reaction (1-4). To initiate an immune response, the drug antigen must be presented in the context of specific MHC molecules expressed on dendritic cells to specific T-cell receptors in a micro-environment rich in co-stimulatory signalling and cytokines, which are necessary for sustained T-cell expansion (5, 6).
Our understanding of the chemical basis of drug hypersensitivity reactions derives from the field of allergic contact dermatitis, where Landsteiner and Jacobs (7) defined low molecular weight chemical allergens as “incomplete antigens” since the compounds themselves were not directly antigenic (i.e., do not bind with high affinity to immunological receptors) and only gained immunogenic potential following conjugation with a protein carrier. The β-lactam antibiotics, which cause a high incidence of drug hypersensitivity reactions, also react spontaneously with protein (8, 9) and synthetically constructed penicillin protein adducts stimulate specific T-cells following processing (10). On this basis, the “hapten concept” (hapten referring to any substance that modifies protein to induce an immune response) is the hypothesis most commonly used to describe the interaction of drugs with immune cells. The picture is further complicated because most drugs are not directly protein-reactive. They may however gain protein reactivity through normal metabolic processes, generating a hapten with the potential to modify specific amino acid residues on protein. Using the antibacterial agent sulfamethoxazole (SMX)5 as a model drug allergen, independent research groups have shown that synthetic metabolites of SMX interact with MHC and T-cell receptors with sufficient affinity to stimulate blood and skin-derived T-cells from hypersensitive patients (4, 11-14). These data confirm that hapten-specific T-cells are present in hypersensitive patients and that T-cell responses are directed against drug metabolite conjugated protein. The current thinking is that hepatic metabolism first generates drug metabolites which form protein adducts that are then processed and presented by antigen presenting cells. It is not known whether the antigen presenting cells could also generate drug metabolites and subsequent haptenated antigens. Thus, critical experiments showing a causal relationship between compound distribution, drug metabolism and protein modification in antigen presenting cells and the development of T-cell responses have not been described. Detection of T-cell responses to protein adducts formed as a consequence of antigen presenting cell metabolism would signify that (1) hepatic metabolism is not necessarily involved in drug hypersensitivity and (2) the propensity to form chemically reactive metabolites by liver enzymes is not a sensitive method for predicting drugs which cause hypersensitivity.
Dendritic cells perform an important role in determining the equilibrium between immune tolerance and immune reactivity through the provision of receptor ligand interactions (co-stimulatory signalling) and cytokine secretion (15). Drugs including abacavir, amoxicillin and SMX have been shown to partially stimulate human dendritic cell co-stimulatory signalling (16-18), which could potentially drive pathogenic immune responses in hypersensitive patients. Dendritic cells express multiple drug metabolizing enzymes (16, 19-22) that can generate drug-derived protein adducts in increasing amounts when exposed to “danger” signals (22). Furthermore, SMX-mediated dendritic cell signalling has been shown to be dependent on intracellular metabolism and protein-adduct formation (16). One pathway for dendritic cell activation is through exposure to products derived from dying cells (eg ATP, Hsps, HMGB1, urate phosphatidyl serine). It is unclear whether dendritic cell activation in the context of drug hypersensitivity requires cytotoxicity and thus the relationship between drug-specific dendritic cell signalling and cytotoxicity remains controversial. In particular, the relationship between bystander cell death following exposure to haptenic drugs and dendritic cell activation has not been studied.
In this study we have utilized the drug SMX to explore the functional consequences of metabolite formation in immune cells (Figure 1). Data presented herein show that T-cells from animal models of SMX immunogenicity and SMX hypersensitive patients are stimulated by drug metabolite protein conjugates generated intracellularly as a consequence of metabolism in antigen presenting cells. We also show that bystander cell death associated with exposure to high levels of drug metabolite is required for the provision of maturation signals to dendritic cells. Our findings suggest that antigen presenting cells alone are sufficient to generate drug metabolites, haptenated antigens and to induce the drug specific T-cell response.
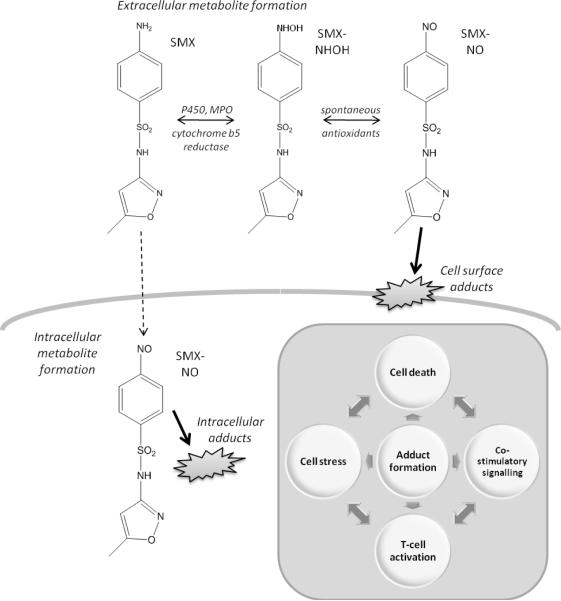
Figure 1.
Scheme depicting SMX metabolism and the possible involvement of protein adducts in cell stress, cell death, co-stimulatory signalling and the development of antigen-specific T-cell response.
Materials and Methods
Mice
Female Balb/c mice (6-10 weeks of age) were purchased from Charles River (Margate, Kent, UK) and maintained under specific pathogen-free conditions. All experiments were carried out under the provisions of the United Kingdom Animals (Scientific Procedures) Act, 1986. Drug-naive animals were used to obtain bone-marrow-derived dendritic cells, unless stated otherwise.
Immune cells
Bone marrow-derived mouse dendritic cells
Bone marrow-derived mouse dendritic cells were generated according to the procedure of Lutz (23), with slight modifications. Harvested cells were filtered, suspended in culture medium (RPMI-1640 supplemented with heat-inactivated and filtered FBS (10% v/v), penicillin (100 U / ml), streptomycin (100 μg / ml), L-glutamine (2 mM), HEPES (25 mM), 2-mercaptoethanol (50 μM) and recombinant murine GM-CSF (20 ng / ml)) and aliquoted in sterile Petri dishes (10ml, 2×105 cells / ml). Half of the medium was replaced with fresh complete medium on days 3, 6 and 8, and immature dendritic cells were ready for use on day 9.
Mouse splenocytes
Spleens were removed using aseptic technique and homogenized. Splenocytes were filtered prior to the removal of red blood cells by density centrifugation using Lymphoprep. Splenocytes were suspended in culture medium (RPMI-1640 supplemented with heat-inactivated and filtered FBS (10% v/v), penicillin (100 U / ml), streptomycin (100 μg / ml), L-glutamine (2 mM), HEPES (25 mM)) prior to each experiment.
Human blood lymphocytes
Lymphocytes were isolated from heparinised blood of 3 healthy volunteers and from 3 SMX hypersensitive patients via centrifugation through Lymphoprep. Approval for the study was obtained from Liverpool local research ethics committee; informed written consent was obtained from all donors. Clinical details of the hypersensitive patients have been described previously (14). Cells were suspended in cell culture medium (RPMI, 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 ug/mL streptomycin, 25 mM HEPES) prior to each experiment.
Human monocyte-derived dendritic cells
Monocyte-derived dendritic cells were generated using an established procedure (16). Adherent monocytes were then cultured in 1 ml of RPMI 1640 medium, supplemented with 10% FBS, HEPES buffer (25 mM), L-glutamine (2 mM), penicillin (100 U / ml), streptomycin (100 μg / ml), IL-4 (800 U / ml), and GM-CSF (800 U / ml). Half of the medium was replaced with fresh complete medium on days 1, 3, and 5, and immature dendritic cells were ready for use on day 6. Purity of dendritic cell populations was determined by FACS analysis with an anti-human CD11c antibody. CD11c expression was consistently >95%.
Epstein Barr Virus-transformed B-cell lines
EBV-transformed B-cell lines were generated from blood lymphocytes isolated from healthy volunteers (n=3) and SMX hypersensitive patients (n=3) using supernatant from the EBV-producing cell line B9-58 (24).
T cell clones
SMX (metabolite) stimulated lymphocytes from hypersensitive patients were cloned by serial dilution using previously described methodology (25). CD phenotype and monoclonality was determined by flow cytometry; a detailed account of the phenotypic data has been described previously (14). Clones were maintained in IL-2 containing medium (RPMI 1640 supplemented with 10% heat-inactivated human blood type AB serum, HEPES buffer (25 mM), L-glutamine (2 mM), transferrin (25 μg / ml), streptomycin (100 μg / ml) and penicillin (100 U / ml) and restimulated every 14 days with the mitogen phytohemagglutinin (5 μg/ml).
Reagents and chemicals
Human and murine GM-CSF and IL-4 were obtained from Peprotech (London, UK). Foetal bovine serum, mouse serum and human AB serum were purchased from Innovative Research (Michigan, USA). Lymphoprep was obtained from Axis-Shield PoC AA (Oslo, Norway. Multiplex kits for detection of secreted cytokines were purchased from Millipore (Herts, UK). [3H]thymidine was purchased from Moravek (Califonia, USA). The oxidative metabolites of SMX (SMX-hydroxylamine and nitroso SMX [SMX-NO]) were synthesized according to the method of Naisbitt et al (26). The following mouse antibodies were used for flow cytometric characterization of dendritic cell receptor expression: PE-labelled CD11c (Caltag, Bucks, UK), FITC-labelled CD40, FITC-labelled CD86, PE-labelled I-A/I-E (all BD Biosciences Pharmingen, Oxford, UK), FITC-labelled CD11b, PE-labelled Gr-1 (Miltenyi Biotec, Surrey, UK), FITC-labelled neutrophil, FITC-labelled CD19 and FITC-labelled CD204 (all AbD Serotec, Oxford, UK). All other reagents were purchased from Sigma-Aldrich (Gillingham, UK).
To generate an anti-SMX antibody for immunochemical detection of SMX protein adducts, SMX was conjugated to human serum albumin (for use as a positive control for the validation of the ELISA protocol), and to keyhole limpet hemocyanin for use as a soluble antigen for rabbit immunization, using a previously described procedure (27). Drug-protein conjugation was confirmed by spectrophotometry, and by reactivity with rabbit anti-SMX antibody, kindly provided by Dr. Michael Rieder (London Ontario, Canada).
The rabbit immunization against SMX-keyhole limpet hemocyanin was approved by an Institutional Animal Care and Use Committee, under a USDA license (# 35-B-0097 and 36-R-0108) and a PHS accreditation (# A4284-01) (Panigen Inc, Branchardville WI) (27). The reactivity of anti-sera against SMX-human serum albumin was confirmed by both ELISA and immunoblotting.
Drug exposure
Cells (1×106 / ml; unless indicated otherwise) were incubated with SMX (0.05-2mM) or SMX-NO (0.1-1000 μM; final DMSO concentration less than 0.5%) over various periods of time (5 min - 6 days). Certain experiments were conducted in the presence of the enzyme inhibitors methimazole (an inhibitor of peroxidises and flavin-monooxygenases) or 1-aminobenzotriazole (a non-selective suicide inhibitor), at a concentration that block CYP2C9 and peroxidase-catalyzed SMX metabolism (both 1 mM). On completion of the incubation period, cells were then washed three times with HBSS prior to processing for detection of SMX and SMX-metabolite derived adduct formation (confocal microscopy and ELISA), cell viability, depletion of intracellular glutathione, dendritic cell function and T-cell activation.
Detection of dendritic cell surface receptor expression and cytokine secretion
Bone marrow derived dendritic cells were incubated with SMX, SMX metabolites or LPS (1μg / ml) for 24 h. On completion of the incubation period, washed cells were analyzed by flow cytometry using antibodies described above on a Coulter Epics flow cytometer (Coulter Epics, XL software; Beckman Coulter). Data files were further processed using WinMDI software (version 2.9; Scripps Research Institute, CA, USA).
Levels of secreted dendritic cell cytokines (IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12, IL15, IFN-γ and TNF-α) were measured simultaneously using a Millipore multiplex assay kit (Millipore, Watford, UK) on a Bio-Plex Suspension Array System.
Detection of sulfamethoxazole metabolite protein adduct formation
Cells incubated with SMX or SMX metabolites were incubated with rabbit anti-SMX antisera (1 : 2000; overnight at 4°C), an alkaline phosphatase-conjugated anti-rabbit IgG (1 : 1000; 2 h, rt) and an alkaline phosphatase substrate (30min, rt) to quantify drug protein adducts. The optical density was then measured at 405 nm, using a microplate reader (MRX; Dynatech Laboratories Inc., Chantilly, VA, USA). Results are expressed as “ΔOD = sample OD − vehicle OD”. To verify that the antibody binding truly reflects expression of SMX-derived adducts, hapten inhibition experiments were performed using soluble SMX (2 mM) in the presence of the primary anti-SMX antibody. Detection of SMX-derived protein adducts was completely inhibited by soluble SMX (results not shown). To visualize SMX-derived protein adduct formation, fixed cells were incubated overnight with rabbit anti-SMX antibody (1:500), prior to incubation with FITC-labelled anti rabbit IgG for 2 hours. Slides were mounted in Vectashield H-1200 (Vector Laboratories Inc., Peterborough,UK) prior to analysis by confocal microscopy.
Analysis of cell viability
Viability of mouse dendritic cells and splenocytes incubated with SMX or SMX-NO was analyzed using the Annexin-V FITC / propidium iodide double staining method originally described by Vermes et al (28) to detect apoptotic and necrotic cell death. The combination of these two characteristics permits simultaneous detection of viable (Annexin − / propidium iodide −), apoptotic (Annexin + / propidium iodide −) and necrotic cells (Annexin + / propidium iodide +). Cells incubated with formaldehyde or DMSO (10 %) served as a positive control.
Analysis of intracellular glutathione content
Glutathione content in SMX and SMX-metabolite treated bone marrow-derived mouse dendritic cells was measured using a microtitre plate assay according to the method of Vandeutte (29).
Co-culture of dendritic cells with nitroso sulfamethoxazole-treated splenocytes
Splenocytes were incubated with SMX-NO (10-500 μM) for 16 h. After repeated washing to remove non-covalently bound drug, splenocytes (3 × 106 / ml) were cultured with mouse bone marrow-derived dendritic cells (1 × 106 / ml) in 24 well cell culture plates. After 16 h, dendritic cells were assayed for cell viability, expression of cell surface receptors and cytokine secretion using methods described above.
Immunization protocol for the analysis of sulfamethoxazole-metabolite-specific T-cell responses in the mouse
Female Balb-c strain mice were administered SMX-NO (5 mg / kg; n = 4) in DMSO (100 μL) via i.p. injection four times weekly for 2 weeks according to a previously described protocol (30). On completion of this dosing regimen, animals were killed and the spleen was removed for analysis of splenocyte proliferation.
Spleens were homogenized in HBSS (10 mL) and filtered to liberate a single cell suspension. Red cells were removed by centrifugation with Lymphoprep. Splenocytes were washed, suspended in culture medium at a concentration of 1.5 × 106 cells / mL and placed in a round-bottom 96-well plate (1.5 × 105 / well) with SMX-NO (10–100 μM) or SMX (100–1000 μM) for 72 h (37 °C, 5% CO2). After 4 days, proliferation was measured by the addition of [3H]thymidine for 16 h. Cells were harvested and incorporated radioactivity measured as counts per minute on a β-counter (PerkinElmer Life Sciences, Cambridge, UK). Data are presented as stimulation indices (cpm in drug-treated cultures / cpm in cultures containing solvent alone).
To explore the ability of protein conjugates, generated through direct conjugation of SMX-NO or through metabolism of SMX, to stimulate a proliferative response, spleen cells and dendritic cells from naive mice were pulsed with SMX or SMX-NO for 1 – 16 h, washed repeatedly to remove unbound drug(metabolite), irradiated to prevent proliferation and incubated with splenocytes from SMX-NO sensitized mice. After 4 days, proliferation was measured by the addition of [3H]thymidine for 16 h.
Adoptive transfer of drug-treated dendritic cells and analysis of sulfamethoxazole-metabolite-specific T-cell responses in the mouse
Bone marrow-derived dendritic cells, cultured in medium supplemented with mouse serum to prevent T-cell responses against FBS-derived antigens were incubated for 16 h in culture medium with SMX (2 mM) or SMX-NO (100 μM). For immunization, dendritic cells were harvested, washed extensively, and injected via i.v. injection in the lateral tail vein of Balb/c strain mice. Each mouse received one injection of 0.5 × 106 viable dendritic cells in HBSS (200 μl). Control mice received unmodified dendritic cells or dendritic cells treated with DMSO. After 21 days, mice were sacrificed and splenocytes isolated as described above. Splenocytes (1.5 × 106 / ml) were stimulated in 96-well U-bottom culture plates with SMX (0.25 – 2 mM), SMX-NO (10 – 50 μM) or modified spleen cells. Proliferation was quantified by the addition of [3H]thymidine.
Analysis of the drug metabolite-specific proliferative response of lymphocytes and T-cell clones from hypersensitive patients
Proliferation of lymphocytes (1.5×105 / well) from hypersensitive patients and SMX-exposed non-hypersensitive volunteers with SMX (250 – 2000 μM) and SMX-NO (20 – 80 μM) was quantified by measurement of [3H]thymidine incorporation after 6 days, as described previously (31). Proliferative responses were calculated as stimulation indices (cpm in drug-treated cultures / cpm in cultures with solvent alone).
CD4+ T-cell clones (5 × 104 / well) were aliquoted in 96 well U bottomed cell culture plates and incubated with irradiated autologous EBV transformed B-cells (1×104; 60Gy) with or without SMX or SMX-NO. After a 48 h, [3H]thymidine was added for the analysis of proliferation.
To evaluate the role of SMX metabolism by human antigen presenting cells in drug-specific T-cell proliferation, lymphocytes (used as additional antigen presenting cells in the lymphocyte proliferation assay) or EBV transformed B-cells (used as antigen presenting cells with T-cell clones) were incubated with SMX or SMX-NO for 16 h, prior to repeated washing to remove unbound drug, irradiated to prevent proliferation and co-incubated with autologous lymphocytes or antigen-specific T-cell clones. Proliferation was measured using [3H]thymidine incorporation as described above.
Levels of secreted cytokines (IL-1β, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, IL-17, IFN-γ, TNF-α, MCP-1 and GMCSF) were measured from the supernatant of SMX and SMX-NO stimulated T-cell clones using a Millipore multiplex assay kit as described above.
Enzyme inhibition experiments
To confirm that SMX-derived protein adduct formation and T-cell proliferation is dependent on the enzymatic oxidation of SMX, certain experiments were conducted in the presence of various enzyme inhibitors. APC were pre-incubated for 1 h with 1-aminobenzotriazole ((a non-selective suicide inhibitor; 1 mM) (32) or methimazole (an inhibitor of peroxidises and flavin-monooxygenase; 1 mM) (33).
Statistical analysis
Values to be compared were analyzed for normality using Shapiro-Wilks test. Normally distributed data were analyzed using a Student T test. Non-normally distributed data were compared with the Mann-Whitney test using the SPSS 16.0 software (SPSS Inc., Chicago, USA). In all cases, p < 0.05 was considered statistically significant.
Results
Sulfamethoxazole, intracellular sulfamethoxazole metabolites and nitroso sulfamethoxazole do not activate mouse dendritic cells
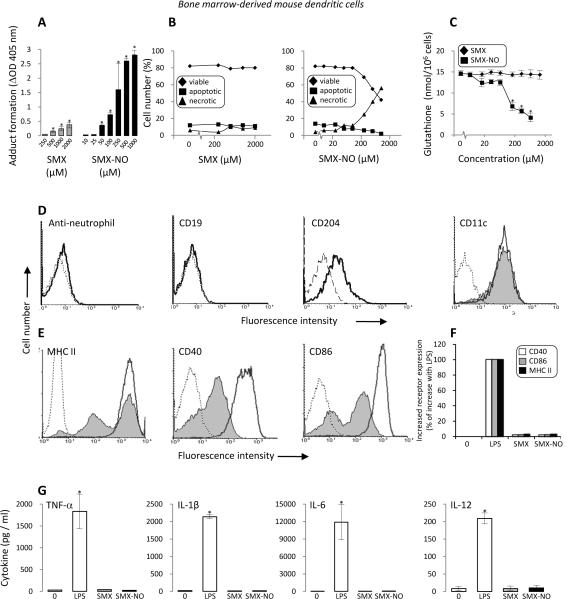
To study whether SMX or the metabolite SMX-NO activate bone marrow-derived dendritic cells directly, both compounds were cultured with dendritic cells, prior to analyzing cell viability, depletion of intracellular glutathione, adduct formation, co-stimulatory receptor expression and cytokine secretion. Dendritic cell death and glutathione depletion were detected at SMX-NO concentrations of 250 μM and above, whereas there was no significant effect when dendritic cells were incubated with SMX (Figures 2B and C). Binding of SMX-NO to dendritic cells was concentration-dependent, with adducts detected at concentrations of 50 μM and above. SMX treatment of dendritic cells was associated with intracellular adduct formation at a concentration of 500 μM. Thus, direct binding of SMX-NO to dendritic cells and binding following SMX metabolism was detectable on viable cells.
Figure 2. SMX metabolism and protein adduct formation in mouse bone marrow-derived dendritic cells is not associated with co-stimulatory signalling and cytokine secretion.
Dendritic cells were incubated with SMX (250 – 2000 μM) or SMX-NO (10 – 1000 μM) for 16 h. (A) Concentration-dependent protein adduct formation in SMX and SMX-NO treated cells was quantified by ELISA using a specific anti-SMX antibody. (B) Necrotic and apoptotic cell death was quantified by flow cytometry using AnnexinV/PI staining. (C) Intracellular glutathione levels were quantified using the method of Vandeputte et al.(29) (D) Cells were stained with CD11c, CD204, CD19 and an anti-neutrophil antibody and subjected to flow cytometric analysis to confirm the dendritic cell phenotype (dotted line [background fluorescence], solid line [antibody stained, LPS treated] and shaded [antibody stained, untreated]). (E) Up-regulation of CD40, CD86 and MHC class II expression in response to LPS (dotted line [background fluorescence], solid line [antibody stained, LPS treated] and shaded [antibody stained, untreated)]. (F) CD40, CD86 and MHC class II expression on dendritic cells cultured with SMX or SMX-NO. Up-regulation of cell surface receptor expression is presented as the percentage of the increase with LPS over control. (G) LPS, SMX and SMX-NO mediated dendritic cell cytokine secretion measured using multiplex analysis. Data are presented as mean±SD of at least 3 separate experiments. *significantly different from untreated cells; P < 0.05. Flow cytometry traces are representative of 3 experiments.
Dendritic cells stimulated with LPS displayed an increase in cell surface markers (CD40, CD86 and MHC class II) and cytokine secretion (TNF-α, IL-1β, IL-6 and IL-12). However, SMX and SMX-NO exposure, at toxic and non-toxic concentrations, failed to stimulate dendritic cell activation (Figures 2D, E, F and G).
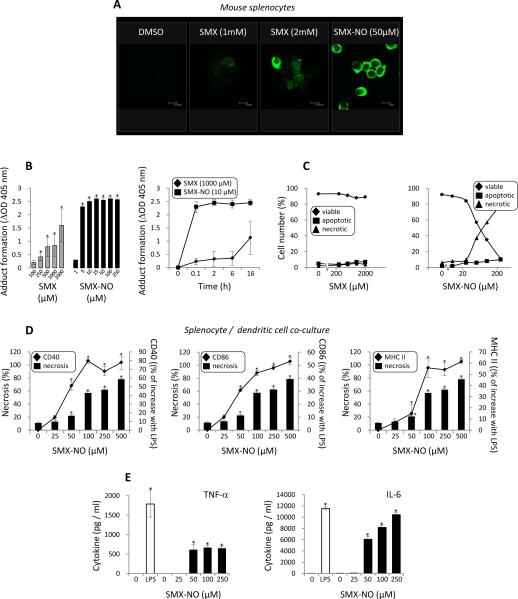
Activation of dendritic cells by necrotic cells modified with nitroso sulfamethoxazole
To explore the relationship between SMX metabolite-mediated cell death and dendritic cell activation, SMX and SMX-NO treated splenocytes were cultured with dendritic cells prior to analysis of dendritic cell activation. SMX-NO bound rapidly to splenocytes (within 1 h) and necrotic cell death was detectable at concentrations of 50 μM and above. Adduct formation in splenocytes cultured with SMX was both time and concentration-dependent and detectable by both ELISA and confocal microscopy. However, adduct formation with SMX was not associated with an increase in either apoptotic or necrotic cell death (Figures 3A, B and C), indicating that the number of modified proteins did not exceed a threshold that must be surmounted prior to the development of proportionate increases in cell death (34).
Figure 3. SMX metabolite-mediated necrotic cell death provides a potent signal for dendritic cell co-stimulatory signalling and cytokine secretion.
SMX and SMX-NO treated mouse splenocyte were incubated in a co-culture system with dendritic cells prior to the analysis of co-stimulatory signalling and cytokine secretion. (A) Confocal microscopy imaging showing the formation of protein adducts when splenocytes were exposed to SMX and the protein reactive metabolite SMX-NO. Protein adducts were not detected in cells exposed to DMSO alone. (B) Concentration- and time-dependent protein adduct formation in SMX and SMX-NO treated splenocytes quantified by ELISA using a specific anti-SMX antibody. (C) Necrotic and apoptotic splenocyte death quantified by flow cytometry using AnnexinV/PI staining. (D) CD40, CD86 and MHC class II on dendritic cells cultured with SMX or SMX-NO treated splenocytes. Up-regulation of cell surface receptor expression is presented as the percentage of the increase with LPS over control. Graphical representations compare SMX-NO mediated necrotic cell death with up-regulation of co-stimulatory receptor expression. (G) Dendritic cell cytokine secretion measured using multiplex analysis following co-culture with SMX-NO modified splenocytes. Data are presented as mean±SD of at least 3 separate experiments. *significantly different from untreated cells; P < 0.05.
The addition of untreated mouse splenocytes to dendritic cells did not increase dendritic cell co-stimulatory receptor expression or cytokine secretion. In contrast, SMX-NO treated splenocytes activated dendritic cells in a concentration-dependent manner (co-stimulatory receptors, MHC class II and cytokine release; Figures 3D and E). The number of activated dendritic cells was associated with increasing quantities of SMX-NO modified necrotic splenocytes. Dendritic cell activation was not detectable with non-toxic concentrations of SMX-NO.
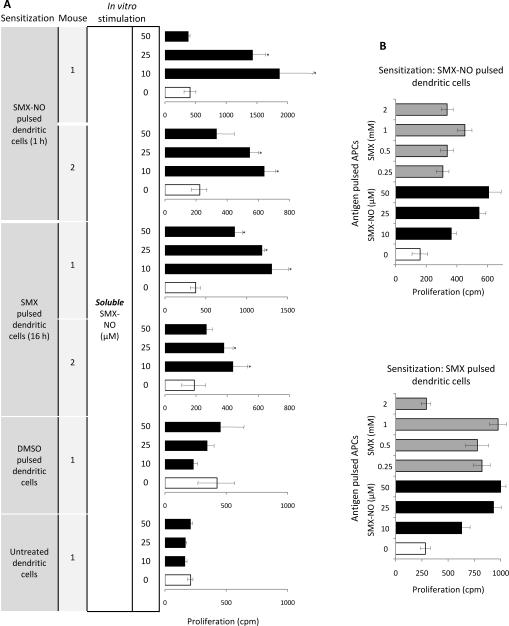
Sulfamethoxazole metabolism in antigen presenting cells generates a functional antigen for T-cells
Splenocytes from mice administered SMX-NO displayed a concentration-dependent proliferative response on in vitro stimulation with soluble synthetic SMX-NO (10–50 μM; Figure 4B). Splenocytes from sensitized mice were additionally stimulated with SMX-NO modified (10–100 μM; 1 h), irradiated cells from naive syngeneic mice (Figure 4C). Thus, SMX-NO bound to the surface of antigen presenting cells stimulates T-cells.
Figure 4. SMX-derived protein adduct formation in antigen presenting cells represents a functional antigen for SMX-NO sensitized mice.
(A) Inhibition of protein adduct formation in SMX and SMX-NO treated splenocytes quantified by ELISA using a specific anti-SMX antibody. The inhibitors 1-aminobenzotriazole (ABT) and methimazole (Meth) were incubated with cells for 1 h before the addition of SMX or SMX-NO. (B, C) Dose-dependent proliferative response of splenocytes from SMX-NO sensitized mice cultured with (B) soluble SMX-NO or (C) syngeneic naive irradiated splenocytes pulsed with SMX-NO (1 h) or SMX (16 h). After 72 h, proliferation was measured by incorporation of [3H]thymidine over an additional 16 h. (D, E) Time-dependent proliferative response of splenocytes from SMX-NO sensitized mice cultured with (D) syngeneic naive irradiated splenocytes pulsed with SMX-NO or SMX (0.1 - 16 h) and (E) bone-marrow derived dendritic cells pulsed with SMX-NO or SMX (1 and 16 h). In these pulsing experiments, sensitized splenocytes were not exposed to soluble drug. After 72 h, proliferation was measured by incorporation of [3H]thymidine over an additional 16 h. (F) 1-Aminobenzotriazole and methimazole inhibited the concentration-dependent proliferation of sensitized splenocytes cultured with naive irradiated splenocytes pulsed with SMX (250 – 1000 μM; 16 h). Enzyme inhibition did not block the proliferative response directed against SMX-NO modified splenocytes. Proliferation data represented as mean ± SD from three sensitized mice, with incubations carried out in triplicate. Statistical analysis compares the proliferative response of sensitized splenocytes after the addition of drug antigen and solvent alone (*P < 0.05).
Splenocytes from SMX-NO sensitized mice were stimulated to proliferate when incubated with irradiated splenocytes and dendritic cells pulsed with SMX (0.1 – 2 mM) for 16 h (Figure 4C). To investigate the relationship between the dynamics of SMX metabolism and the generation of a functional antigen for spleen cells, antigen presenting cells were pulsed with SMX or SMX-NO for 0.1 – 16 h in the presence or absence of the enzyme inhibitors methimazole (an inhibitor of peroxidises and flavin-monooxygenases) and 1-aminobenzotriazole (a non-selective suicide inhibitor), at a concentration that block CYP2C9 and peroxidase-catalyzed SMX metabolism (both 1 mM). The proliferative response of splenocytes against SMX-pulsed antigen presenting cells (naive splenocytes and dendritic cells) was time-dependent; significant proliferation was detectable at 6 – 16 h (Figures 4D and E), the time required for SMX metabolism and protein binding (Figure 3B). In contrast, a 6 min pulse with SMX-NO was sufficient for adduct formation and T-cell proliferation.
Importantly, methimazole and 1-aminobenzotriazole pre-treatment of splenocytes blocked binding of SMX-derived metabolites (Figure 4A) and eliminated the concentration-dependent T-cell response against antigen presenting cells pulsed with SMX for 16 h (Figure 4F). Adduct formation and T-cell responses to the directly reactive SMX-NO were unchanged by enzyme inhibition (Figures 4A and F).
These data show for the first time that drug metabolites formed locally in antigen presenting cells bind irreversibly to intracellular protein and the resultant protein adducts stimulate T-cells.
Dendritic cells generate sulfamethoxazole-derived antigen and can induce an in vivo immune response against nitroso sulfamethoxazole
To test whether protein adducts derived from SMX metabolism in antigen presenting cells can sensitize mice, dendritic cells incubated with SMX (and SMX-NO) for 16 h were administered to naive recipient mice via a single i.v. injection. SMX treatment of dendritic cells was associated with adduct formation (Figure 2A) and provoked an immune response (Figure 5). Splenocytes isolated 21 days after the adoptive transfer were stimulated to proliferate ex vivo. The proliferative response was directed against SMX-NO (Figure 5A) and SMX-derived metabolites (Figure 5B), but not the parent drug. The absence of soluble SMX after washing and prior to dendritic cell transfer was confirmed using 14C-labelled SMX (results not shown).
Figure 5. Stimulation of drug(metabolite)-specific T-cells following adoptive transfer of SMX and SMX-NO treated dendritic cells.
SMX- and SMX-NO-derived dendritic cell protein adducts stimulated a cellular immune response in naive recipient mice. However, the response of splenocytes from mice immunized with SMX-treated dendritic cells was directed against (A) SMX-NO and (B) SMX-derived metabolites, not the parent drug. Bone marrow-derived dendritic cells were incubated for 16 h in culture medium with SMX or SMX-NO. For immunization, dendritic cells, were washed extensively, and administered via i.v. injection in the lateral tail vein. Control mice received unmodified dendritic cells or dendritic cells treated with DMSO. After 21 days, mice were sacrificed. Splenocytes were stimulated in vitro with (A) soluble antigen and (B) antigen-pulsed splenocytes. Proliferation was quantified by the addition of [3H]thymidine. Data presented as mean ± SD, with incubations carried out in triplicate. Statistical analysis compares the proliferative response of sensitized splenocytes after the addition of drug and solvent alone (*P < 0.05).
Human antigen presenting cells form intracellular SMX-derived protein adducts that stimulate T-cells from drug hypersensitive patients
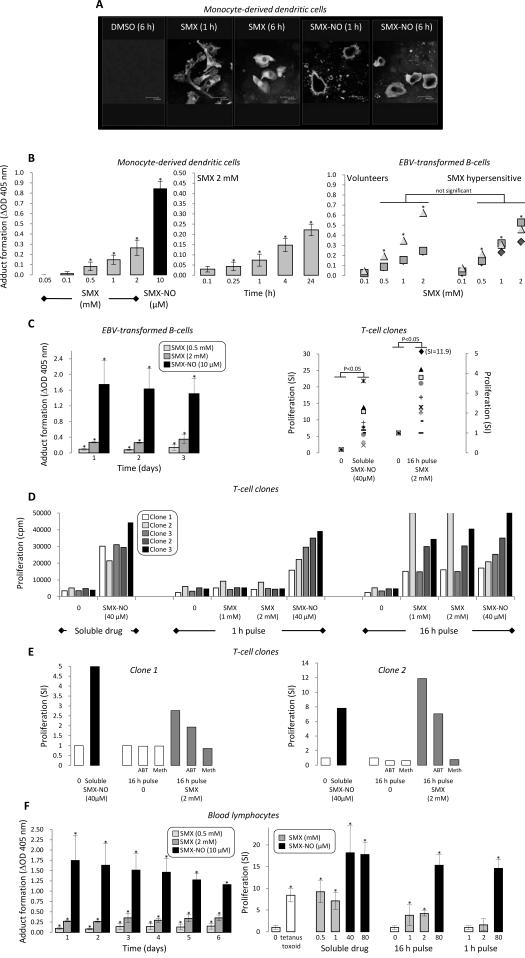
Using ELISA, SMX-derived protein adducts were detected in human lymphocytes, monocyte-derived dendritic cells and EBV-transformed B-cell lines. Confocal microscopy showed that, while SMX-NO first binds to the membrane of human monocyte-derived dendritic cells and is then incorporated intracellularly, SMX forms intracellular protein adducts (Figure 6A). Adduct formation with SMX was concentration- and time-dependent (Figure 6B). Protein adducts were detected instantaneously with SMX-NO (results not shown).
Figure 6. SMX-derived protein adduct formation in antigen presenting cells represents a functional antigen for lymphocytes and T-cell clones from hypersensitive human patients.
(A) Confocal microscopy imaging showing formation of cell membrane adducts when monocyte-derived dendritic cells are incubated with SMX-NO and the parent drug. SMX-NO derived adducts become internalized and are detected inside cells after 6 h. In contrast, intracellular cytoplasmic adducts are detected with dendritic cells exposed to the parent drug. Protein adducts were not detected with dendritic cells exposed to DMSO. (B) Concentration- and time-dependent protein adduct formation in SMX and SMX-NO treated dendritic cells and EBV-transformed B-cells from hypersensitive patients and healthy volunteers, quantified by ELISA using a specific anti-SMX antibody. (C) The quantity of SMX- and SMX-NO-derived protein adducts in EBV-transformed B-cells from hypersensitive patients remains constant for 3 days, the duration of a proliferation assay with T-cell clones. EBV-transformed B-cells pulsed with SMX for 16 h stimulated 9 out of 11 SMX-NO-responsive T cell clones. (D) Stimulation of a second independent panel of T-cell clones with EBV-transformed B-cells pulsed with SMX-NO for 16 h. (E) Enzyme inhibition with 1-aminobenzotriazole (ABT) and methimazole (meth) diminished proliferative response of drug-specific T cell clones to SMX-pulsed EBV-transformed B-cells. (F) SMX-pulsed lymphocytes generate a functional antigen that stimulates the proliferation of lymphocytes from hypersensitive patients. The quantity of SMX- and SMX-NO-derived protein adducts in lymphocytes from hypersensitive patients remains constant for 6 days, the duration of the lymphocyte transformation test. Lymphocytes from hypersensitive patients proliferated in presence of soluble SMX and SMX-NO and irradiated lymphocytes pulsed overnight with SMX-NO and the parent drug. In contrast, lymphocytes pulsed with SMX 1 h and used as a source of antigen did not stimulate a proliferative response.
EBV-transformed B-cells from SMX hypersensitive patients and volunteers displayed no discernable difference between the quantity of protein adducts formed when cultured with SMX for 16 h (Figure 6 B). Adducts were subsequently detectable for 3 days (Figure 6C), the duration of a proliferation assay. EBV-transformed B-cells pulsed with SMX (1 and 2 mM) overnight stimulated 9 out of 11 SMX-NO specific T cell clones, with a stimulation index ranging from 2.0 to 11.9 (Figure 6C).
These data provide the first evidence that T-cells from allergic patients are stimulated with SMX-derived protein conjugates generated as a consequence of intracellular metabolism by antigen presenting cells. To validate these results, a second panel of clones were generated; ten antigen-specific T-cell clones were stimulated to proliferate with antigen presenting cell pulsed with SMX for 16 h, but not 1 h (Figure 6D).
Adduct formation and the T-cell response directed against SMX-derived protein adducts was decreased by pre-incubating EBV-transformed B-cells with 1-aminobenzotriazole or methimazole (adduct formation: SMX 2 mM, OD 0.84±0.24; + 1-aminobenzotriazole, OD 0.29±0.14% [% inhibition 65.0 %, p < 0.05]; + methimazole, OD 0.24±0.13 [% inhibition 71.4 %, p < 0.05]: SMX-NO 50 μM, OD 2.65±0.27; + 1-aminobenzotriazole, OD 2.64±0.21% [% inhibition 0.4 %, ns]; + methimazole, OD 2.60±0.25 [% inhibition 1.9 %, ns]) (Figure 6E). The low numbers of clones that respond exclusively to the parent drug SMX were not stimulated with SMX-pulsed EBV-transformed B-cells.
Adduct formation in blood lymphocytes incubated with SMX was also time dependent for 16 h, while subsequently, the quantity of adducts formed remained relatively stable for 6 days, the duration of the lymphocyte transformation test. Lymphocytes from hypersensitive patients were found to proliferate in the presence of soluble SMX and SMX-NO and SMX pulsed (1 and 2 mM; 16 h) and irradiated autologous lymphocytes (Figure 6F). In contrast, antigen presenting cells pulsed with SMX for 1 h did not stimulate a proliferative response.
Lymphocytes from non-hypersensitive patients (n=3) were not stimulated with SMX, synthetic SMX-NO or SMX-derived protein conjugates (see supplemental data).6
Cytokine secretion from SMX and SMX metabolite stimulated T-cell clones
When culture supernatants from incubations containing T-cells, antigen presenting cells and SMX or SMX-NO were analysed for cytokine expression, high levels of IL-5, IL-13, IFNγ and TNF-α were detected (Table I) Antigen stimulation of certain clones was also associated with secretion of lower levels of IL-4, IL-6, IL-8 and IL-17.
Table I.
Cytokine secretion from SMX and SMX metabolite stimulated T-cell clones from hypersensitive patients.
| Cytokine (pg/ml)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| id | CD | IL4 | IL5 | IL6 | IL8 | IL13 | IL17 | IFNγ | TNFα | GMCSF |
| SMX-stimulated clones | ||||||||||
| 1 | 8 | -b | - | - | - | 426 | 32 | 544 | 358 | 271 |
| 2 | 4 | 137c | 28190 | - | 25 | 16157 | 62 | 33 | 149 | 273 |
| 3 | 8 | 136 | 373 | 13 | 59 | 18204 | 71 | 1202 | 1967 | 991 |
| 4 | 4 | - | - | 56 | 1408 | 7375 | 57 | 8004 | 3346 | 1676 |
| 5 | 8 | - | 14 | - | - | 726 | 36 | 108 | 34 | 108 |
| SMX-metabolite-stimulated clones | ||||||||||
| 1 | 8 | 55 | 1791 | 323 | 56 | >20000 | 77 | 817 | 2058 | 827 |
| 2 | 4 | - | - | - | - | 1012 | 37 | 441 | 953 | 441 |
| 3 | 4 | - | - | 11 | 56 | 978 | 35 | 326 | 855 | 326 |
| 4 | 4 | 16 | 12 | 186 | 295 | 7742 | 56 | 947 | 2499 | 947 |
No detectable drug-specific secretion of IL-1β, IL-7, IL-10 & MCP-1.
-; not detectable.
Data represents mean of duplicate cultures with cytokine levels (less than 10pg/ml IL4, IL5, IL6, IL-8, TNFα; less than 20pg/ml IFNγ; less than 50pg/ml IL13, IL17, GMCSF) in drug-free wells subtracted.
Discussion
In this study, we have embarked upon a series of experiments to address the hypothesis “antigen presenting cells metabolize drugs in sufficient quantities to form protein adducts and haptenated antigens that stimulate both dendritic cells and T-cells”. The immune response may arise directly through drug-protein adduction of viable cells or indirectly through inducing necrotic bystander cell death and subsequent release of endogenous antigen and possibly “danger signals” (35-39).
The oxidative metabolite of SMX, SMX-NO stimulates skin and blood derived T-cells from all SMX-hypersensitive patients to proliferate, secrete cytokines and kill target cells (4, 11, 13, 14). These data indicate that T-cells play a causal role in the development of clinical signs. However, the way in which drugs stimulate T-cells remains an area of intense debate and is complicated by the fact that until now, it has been impossible to generate and characterize physiologically relevant intracellular drug protein adducts in biological test systems. In in vitro assays, synthetic drug metabolites (e.g., SMX-NO) generate cell surface adducts that are unlikely to be formed in patients where metabolism occurs inside the cell. In this study, immunochemical methods to quantify SMX metabolite protein binding and enzyme inhibitors were used to relate drug metabolism in antigen presenting cells to T-cell activation. We show for the first time that antigen presenting cell drug metabolism and irreversible binding of the derived metabolites to intracellular protein results in the capacity to stimulate T-cell responses. These data are the first to show a pro-hapten mechanism of T-cell activation that is active in hypersensitive patients and animal models of immunogenicity.
The detection of T-cell responses against protein adducts formed as a consequence of antigen presenting cell drug metabolism have important ramifications for future drug development. The pharmaceutical industry test molecules for their propensity to form chemically reactive metabolites in the liver and attempt to relate this to risk/hazard identification. Our data suggest that this approach may not be relevant for predicting/assessing the risk for drug hypersensitivity.
The distribution of SMX-NO in in vitro culture is complex. Auto-oxidation generates nitro SMX, self-conjugation generates azo and azoxy dimers and reduction through enzymatic reactions or glutathione dependent processes liberates a hydroxylamine and the parent drug (Figure 1) (26, 34, 40-43). Although these reactions result in rapid (within 5 min) and complete degradation of SMX-NO; multiple meta-stable protein adducts that become internalized via a caveole-dependent mechanism (16, 44, 45), are readily detectable. In contrast, SMX is relatively stable; with most of the parent drug being recovered unchanged after 6 days in culture. Nevertheless, human monocyte-derived dendritic cells (16) and dendritic cell-like cell lines (46) have been shown to metabolize SMX to an unstable intermediate that forms intracellular protein adducts. Data presented herein show the presence of T-cell stimulatory intracellular protein adducts in viable mouse and human antigen presenting cells (splenocytes, lymphocytes, EBV-transformed B-cell lines and dendritic cells) incubated with the parent drug SMX (Figures 2 and 3). Importantly, metabolism and adduct formation was time- and dose-dependent and not detectable immediately after sulfamethoxazole exposure. Consistent with these findings, T-cell responses were only obtained with antigen presenting cells pulsed with SMX for 6 - 16 h.
Drug-protein adducts formed in human antigen presenting cells exposed to the parent drug SMX were detectable over several days (Figure 6). These data have implications regarding the use of drugs in biological tests to diagnose drug hypersensitivity. To fully define the sensitivity and specificity of the test, future validation studies must incorporate steps to relate the nature of the antigenic determinant(s) formed in situ to the biological readout. The development of sensitive mass spectrometers to characterize drug metabolites and protein adducts makes this approach feasible.
We have previously reported that human monocyte-derived dendritic cells expressed consistently high levels of myeloperoxidase, but only low levels of CYPs, leading us to propose that a peroxidase is responsible for SMX metabolism in dendritic cells (16). More recently, we have shown that pathogenic conditions, which potentially activate peroxidise enzymes, enhance the formation of SMX-derived protein adducts in immune cells (22). To substantiate a causal relationship between peroxidise-mediated SMX metabolism and T-cell immunogenicity, enzyme inhibitor studies were performed with 1-aminobenzotriazole, a non-selective suicide inhibitor, and methimazole, an inhibitor of peroxidises and flavin-monooxygenases. Both inhibitors blocked SMX-derived adduct formation and the T-cell response in mouse and human systems.
Detection of T-cells with reactivity to functional intracellular antigens arising as a consequence of metabolism and extracellular antigens generated through direct conjugation of synthetic SMX-NO to cell surface proteins, implies that the T-cell response is directed against multiple peptide sequences. The one common component is the necessity for the drug metabolite to irreversibly modify specific cysteine residues on protein (45). SMX-derived adduct formation by splenocytyes was 3 fold higher when compared with dendritic cells (Figures 2A and 3B). Despite the lower quantity of adduct formed, dendritic cells stimulated a 10 fold stronger T-cell response (Figures 4C and E), which likely relates to their superior capacity to present endogenous antigens.
Previous studies utilizing lymphocytes from SMX hypersensitive patients and animal models of SMX immunogenicity demonstrated that antigen-stimulated T-cells secrete high levels of the Th2 cytokine IL-5 and the Th1 cytokine IFN-γ (41, 47, 48). Data presented in the current study using multiplex methods and cloned T-cells confirm these initial reports. Antigen stimulated T-cell clones also secreted of IL-4, IL-6, IL-8, IL-13, IL-17, and TNF-α. The profile of cytokine secretion did not differ when SMX, synthetic SMX-NO or SMX-derived metabolites were used.
The role of metabolism in the development of a primary immune response is ill-defined as an experimental approach to relate metabolism to immune activation is lacking. Antigen-specific T-cells from hypersensitive patients cannot be used to define antigenic determinants that prime T-cells since it is impossible to differentiate between cross-reactive drug antigens. Thus, to evaluate whether protein adducts formed intracellularly in antigen presenting cells drive T-cell responses, a mouse adoptive transfer system was developed. SMX and SMX-NO were cultured with bone-marrow derived dendritic cells and administered to syngeneic mice following quantification of adduct formation. Splenocytes isolated from mice that received SMX-treated dendritic cells were stimulated in vitro with SMX-NO and SMX-derived intracellular metabolites, but not the parent drug (Figure 5). These data provide unequivocal evidence that protein adducts generated following SMX metabolism by dendritic cells represent functional antigens that drive drug-specific immunity.
The antigen presenting capacity of dendritic cells is enhanced in response to toll-like receptor signalling (e.g., LPS, CpG oligodeoxynucleotides) (49, 50) and molecules released from necrotic (e.g., uric acid high-mobility group box 1 protein) (36, 51) or oxidatively stressed cells (52). Dendritic cells respond to these signals by secreting polarizing cytokines, and increased expression of co-stimulatory receptors and MHC class II. Previously, we have shown that exposure of human monocyte-derived dendritic cells to non-toxic concentrations of SMX selectively increased CD40 expression. Furthermore, CD40 expression was 5-fold higher on drug-treated cells from a hypersensitive patient, compared with volunteers (16). Our experiments using mouse bone-marrow-derived dendritic cells did not reproduce these findings, indicating that drug-specific CD40 signalling might be restricted to human cells. Drug-protein adducts were detected on viable human and mouse dendritic cells incubated with SMX-NO (50 – 100 μM; Figure 2A). In mouse cells, increasing the concentration of SMX-NO (250 – 1000 μM) was associated with depletion of intracellular glutathione and increased necrotic cell death (Figure 2). SMX-derived adducts were detected in dendritic cells; however, glutathione depletion and necrotic cell death was not seen with SMX. Although no increase in co-stimulatory receptor expression, MHC class II or cytokine secretion were detected following the direct application of SMX or SMX-NO, in a co-culture system consisting of SMX/SMX-NO treated splenocytes as bystander cells and dendritic cells, there was a strong association between SMX metabolite-mediated necrotic cell death and dendritic cell activation. Splenocytes incubated with SMX-NO (at concentrations of 50 μM and above) stimulated an increase in dendritic cell CD40, CD86 and MHC class II expression and cytokine secretion (Figure 3D and E). Previously, we have reported that SMX metabolite-derived adduct formation on splenocytes must surpass a critical threshold to induce necrosis (34). The cells that became intensely haptenated were the same as those that underwent necrotic cell death. Collectively, these data imply that high drug concentrations, which are observed clinically with many drugs associated with a high incidence of hypersensitivity reactions, may be needed to drive T-cell responses to drugs.
The quantity of adduct formation in SMX-treated splenocytes presumably did not reach the threshold to induce necrosis and therefore dendritic cell activation. It is however possible that this may occur in patients where environmental factors (bacterial endotoxins, flu viral proteins and cytokines) alter the oxidation state of cysteine residues on protein and increase SMX-derived protein adduct formation (22). These observations may explain the increased in vitro drug-specific cell death observed in hypersensitive patients (53-56), as patients administered SMX are carriers of such factors.
Previous studies exploring the importance of drug metabolism in the pathogenesis of hypersensitivity reactions have focussed on genetic polymorphisms and/or metabolism in liver (57, 58) and target organs such as the skin (59, 60), and produced largely negative results. Using cells from animal models and hypersensitive human patients, the present study shows that metabolism in antigen presenting cells generates functional antigen/s for T-cells. Given that environmental factors enhance metabolism-derived adduct formation, it is possible that at the time of drug exposure the levels of adduct formed in various cell types exceeds the threshold needed to induce cell death and the activation of dendritic cells, providing multiple co-stimulatory signals to initiate and drive the pathogenic immune response resulting in hypersensitivity.
Supplementary Material
Acknowledgements
The authors would like to thank the blood donors for their generous blood donations.
Footnotes
This work was funded by a grant from the Wellcome Trust (078598/Z/05/Z) as part of the Centre for Drug Safety Science supported by the Medical Research Council (G0700654).
- SMX
- sulfamethoxazole
- SMX-NO
- nitroso sulfamethoxazole
The online version of this article contains supplemental material.
References
- 1.Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. 2006;117:455–462. doi: 10.1016/j.jaci.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, Burkhart C, Yawalkar N, Pichler WJ. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107:1433–1441. doi: 10.1172/JCI12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Sanderson JP, Farrell J, Drummond NS, Hanson A, Bowkett E, Berry N, Stachulski AV, Clarke SE, Pichler WJ, Pirmohamed M, Park BK, Naisbitt DJ. Activation of T cells by carbamazepine and carbamazepine metabolites. J Allergy Clin Immunol. 2006;118:233–241. doi: 10.1016/j.jaci.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Nassif A, Bensussan A, Boumsell L, Deniaud A, Moslehi H, Wolkenstein P, Bagot M, Roujeau JC. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol. 2004;114:1209–1215. doi: 10.1016/j.jaci.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Gerner MY, Mescher MF. Antigen processing and MHC-II presentation by dermal and tumor-infiltrating dendritic cells. J Immunol. 2009;182:2726–2737. doi: 10.4049/jimmunol.0803479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 7.Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J Exp Med. 1935;61:643–656. doi: 10.1084/jem.61.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins RE, Meng X, Elliott VL, Kitteringham NR, Pirmohamed M, Park BK. Characterisation of flucloxacillin and 5-hydroxymethyl flucloxacillin haptenated HSA in vitro and in vivo. Proteomics Clinical Applications. 2009;3:720–729. doi: 10.1002/prca.200800222. [DOI] [PubMed] [Google Scholar]
- 9.Padovan E, Mauri-Hellweg D, Pichler WJ, Weltzien HU. T cell recognition of penicillin G: structural features determining antigenic specificity. Eur J Immunol. 1996;26:42–48. doi: 10.1002/eji.1830260107. [DOI] [PubMed] [Google Scholar]
- 10.Brander C, Mauri-Hellweg D, Bettens F, Rolli H, Goldman M, Pichler WJ. Heterogeneous T cell responses to beta-lactam-modified self-structures are observed in penicillin-allergic individuals. J Immunol. 1995;155:2670–2678. [PubMed] [Google Scholar]
- 11.Farrell J, Naisbitt DJ, Drummond NS, Depta JP, Vilar FJ, Pirmohamed M, Park BK. Characterization of sulfamethoxazole and sulfamethoxazole metabolite-specific T-cell responses in animals and humans. J Pharmacol Exp Ther. 2003;306:229–237. doi: 10.1124/jpet.103.050112. [DOI] [PubMed] [Google Scholar]
- 12.Burkhart C, von Greyerz S, Depta JP, Naisbitt DJ, Britschgi M, Park KB, Pichler WJ. Influence of reduced glutathione on the proliferative response of sulfamethoxazole-specific and sulfamethoxazole-metabolite-specific human CD4+ T-cells. Br J Pharmacol. 2001;132:623–630. doi: 10.1038/sj.bjp.0703845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J Immunol. 2000;164:6647–6654. doi: 10.4049/jimmunol.164.12.6647. [DOI] [PubMed] [Google Scholar]
- 14.Castrejon JL, Berry N, El-Ghaiesh S, Gerber BO, Pichler WJ, Park BK, Naisbitt DJ. Stimulation of T-cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125:184–192. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Novak N, Bieber T. 2. Dendritic cells as regulators of immunity and tolerance. J Allergy Clin Immunol. 2008;121:S370–374. doi: 10.1016/j.jaci.2007.06.001. quiz S413. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson JP, Naisbitt DJ, Farrell J, Ashby CA, Tucker MJ, Rieder MJ, Pirmohamed M, Clarke SE, Park BK. Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007;178:5533–5542. doi: 10.4049/jimmunol.178.9.5533. [DOI] [PubMed] [Google Scholar]
- 17.Martin AM, Almeida CA, Cameron P, Purcell AW, Nolan D, James I, McCluskey J, Phillips E, Landay A, Mallal S. Immune responses to abacavir in antigen-presenting cells from hypersensitive patients. AIDS. 2007;21:1233–1244. doi: 10.1097/QAD.0b013e3280119579. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Pena R, Lopez S, Mayorga C, Antunez C, Fernandez TD, Torres MJ, Blanca M. Potential involvement of dendritic cells in delayed-type hypersensitivity reactions to beta-lactams. J Allergy Clin Immunol. 2006;118:949–956. doi: 10.1016/j.jaci.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Sieben S, Baron JM, Blomeke B, Merk HF. Multiple cytochrome P450-isoenzymes mRNA are expressed in dendritic cells. Int Arch Allergy Immunol. 1999;118:358–361. doi: 10.1159/000024135. [DOI] [PubMed] [Google Scholar]
- 20.Tafazoli S, Spehar DD, O′Brien PJ. Oxidative stress mediated idiosyncratic drug toxicity. Drug Metab Rev. 2005;37:311–325. doi: 10.1081/dmr-55227. [DOI] [PubMed] [Google Scholar]
- 21.Siest G, Jeannesson E, Marteau JB, Samara A, Marie B, Pfister M, Visvikis-Siest S. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab Dispos. 2008;36:182–189. doi: 10.1124/dmd.107.017228. [DOI] [PubMed] [Google Scholar]
- 22.Lavergne SN, Wang H, Callan HE, Park BK, Naisbitt DJ. “Danger” conditions increase sulfamethoxazole-protein adduct formation in human antigen-presenting cells. J Pharmacol Exp Ther. 2009;331:372–381. doi: 10.1124/jpet.109.155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Lacelle C, Wang E. Establishing lymphoblastoid cell lines from frozen blood of extremely old individuals. Mech Ageing Dev. 2002;123:1415–1418. doi: 10.1016/s0047-6374(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Farrell J, Pirmohamed M, Park BK, Naisbitt DJ. Generation and characterization of antigen-specific CD4(+), CD8(+), and CD4(+)CD8(+) T-cell clones from patients with carbamazepine hypersensitivity. J Allergy Clin Immunol. 2007;119:973–981. doi: 10.1016/j.jaci.2006.12.617. [DOI] [PubMed] [Google Scholar]
- 26.Naisbitt DJ, O’Neill PM, Pirmohamed M, Park BK. Synthesis and reactions of nitroso sulfamethoxazole with biological nucleophiles - implications for immune-mediated toxicity. Bioorg Med Chem Let. 1996;6:1511–1516. [Google Scholar]
- 27.Lavergne SN, Kurian JR, Bajad SU, Maki JE, Yoder AR, Guzinski MV, Graziano FM, Trepanier LA. Roles of endogenous ascorbate and glutathione in the cellular reduction and cytotoxicity of sulfamethoxazole-nitroso. Toxicology. 2006;222:25–36. doi: 10.1016/j.tox.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 29.Vandeputte C, Guizon I, Genestie-Denis I, Vannier B, Lorenzon G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol Toxicol. 1994;10:415–421. doi: 10.1007/BF00755791. [DOI] [PubMed] [Google Scholar]
- 30.Naisbitt DJ, Gordon SF, Pirmohamed M, Burkhart C, Cribb AE, Pichler WJ, Park BK. Antigenicity and immunogenicity of sulphamethoxazole: demonstration of metabolism-dependent haptenation and T-cell proliferation in vivo. Br J Pharmacol. 2001;133:295–305. doi: 10.1038/sj.bjp.0704074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy. 1997;27:175–181. [PubMed] [Google Scholar]
- 32.Emoto C, Murase S, Sawada Y, Iwasaki K. In vitro inhibitory effect of 1-aminobenzotriazole on drug oxidations in human liver microsomes: a comparison with SKF-525A. Drug Metab Pharmacokinet. 2005;20:351–357. doi: 10.2133/dmpk.20.351. [DOI] [PubMed] [Google Scholar]
- 33.Wagner BA, Buettner GR, Oberley LW, Darby CJ, Burns CP. Myeloperoxidase is involved in H2O2-induced apoptosis of HL-60 human leukemia cells. J Biol Chem. 2000;275:22461–22469. doi: 10.1074/jbc.M001434200. [DOI] [PubMed] [Google Scholar]
- 34.Naisbitt DJ, Farrell J, Gordon SF, Maggs JL, Burkhart C, Pichler WJ, Pirmohamed M, Park BK. Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol Pharmacol. 2002;62:628–637. doi: 10.1124/mol.62.3.628. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J Immunol. 2006;176:3905–3908. doi: 10.4049/jimmunol.176.7.3905. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 37.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 38.Park BK, Pirmohamed M, Kitteringham NR. Role of Drug Disposition in Drug Hypersensitivity: A Chemical,Molecular and Clinical Perspective. Chem Res Toxicol. 1998;9:969–988. doi: 10.1021/tx980058f. [DOI] [PubMed] [Google Scholar]
- 39.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 40.Kurian JR, Bajad SU, Miller JL, Chin NA, Trepanier LA. NADH cytochrome b5 reductase and cytochrome b5 catalyze the microsomal reduction of xenobiotic hydroxylamines and amidoximes in humans. J Pharmacol Exp Ther. 2004;311:1171–1178. doi: 10.1124/jpet.104.072389. [DOI] [PubMed] [Google Scholar]
- 41.Castrejon JL, Lavergne SN, El-Sheikh A, Farrell J, Maggs JL, Sabbani S, O’Neill PM, Park BK, Naisbitt DJ. Metabolic and Chemical Origins of Cross-Reactive Immunological Reactions to Arylamine Benzenesulfonamides: T-Cell Responses to Hydroxylamine and Nitroso Derivatives. Chem Res Toxicol. 2009;23:184–192. doi: 10.1021/tx900329b. [DOI] [PubMed] [Google Scholar]
- 42.Cribb AE, Miller M, Leeder JS, Hill J, Spielberg SP. Reactions of the nitroso and hydroxylamine metabolites of sulfamethoxazole with reduced glutathione. Implications for idiosyncratic toxicity. Drug Metab Dispos. 1991;19:900–906. [PubMed] [Google Scholar]
- 43.Sacco JC, Trepanier LA. Cytochrome b5 and NADH cytochrome b5 reductase: genotype-phenotype correlations for hydroxylamine reduction. Pharmacogenet Genomics. 20:26–37. doi: 10.1097/FPC.0b013e3283343296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manchanda T, Hess D, Dale L, Ferguson SG, Rieder MJ. Haptenation of sulfonamide reactive metabolites to cellular proteins. Mol Pharmacol. 2002;62:1011–1026. doi: 10.1124/mol.62.5.1011. [DOI] [PubMed] [Google Scholar]
- 45.Callan HE, Jenkins RE, Maggs JL, Lavergne SN, Clarke SE, Naisbitt DJ, Park BK. Multiple Adduction Reactions of Nitroso Sulfamethoxazole with Cysteinyl Residues of Peptides and Proteins: Implications for Hapten Formation. Chemical Research in Toxicology. 2009;22:937–948. doi: 10.1021/tx900034r. [DOI] [PubMed] [Google Scholar]
- 46.Roychowdhury S, Vyas PM, Svensson CK. Formation and uptake of arylhydroxylamine-haptenated proteins in human dendritic cells. Drug Metab Dispos. 2007;35:676–681. doi: 10.1124/dmd.106.013680. [DOI] [PubMed] [Google Scholar]
- 47.Pichler WJ, Zanni M, von Greyerz S, Schnyder B, Mauri-Hellweg D, Wendland T. High IL-5 production by human drug-specific T cell clones. Int Arch Allergy Immunol. 1997;113:177–180. doi: 10.1159/000237539. [DOI] [PubMed] [Google Scholar]
- 48.Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Hunziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J Immunol. 1995;155:462–472. [PubMed] [Google Scholar]
- 49.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiba H, Satoh M, Iwatsuki K, Kaiserlian D, Nicolas JF, Kaneko F. CpG immunostimulatory sequences enhance contact hypersensitivity responses in mice. J Invest Dermatol. 2004;123:488–493. doi: 10.1111/j.0022-202X.2004.23318.x. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 52.Becker D, Valk E, Zahn S, Brand P, Knop J. Coupling of contact sensitizers to thiol groups is a key event for the activation of monocytes and monocyte-derived dendritic cells. J Invest Dermatol. 2003;120:233–238. doi: 10.1046/j.1523-1747.2003.12026.x. [DOI] [PubMed] [Google Scholar]
- 53.Shear NH, Spielberg SP, Grant DM, Tang BK, Kalow W. Differences in metabolism of sulfonamides predisposing to idiosyncratic toxicity. Ann Intern Med. 1986;105:179–184. doi: 10.7326/0003-4819-105-2-179. [DOI] [PubMed] [Google Scholar]
- 54.Spielberg SP, Gordon GB, Blake DA, Goldstein DA, Herlong HF. Predisposition to phenytoin hepatotoxicity assessed in vitro. N Engl J Med. 1981;305:722–727. doi: 10.1056/NEJM198109243051302. [DOI] [PubMed] [Google Scholar]
- 55.Rieder MJ, Uetrecht J, Shear NH, Cannon M, Miller M, Spielberg SP. Diagnosis of sulfonamide hypersensitivity reactions by in-vitro ‘rechallenge” with hydroxylamine metabolites. Ann Intern Med. 1989;110:286–289. doi: 10.7326/0003-4819-110-4-286. [DOI] [PubMed] [Google Scholar]
- 56.Pirmohamed M, Graham A, Roberts P, Smith D, Chadwick D, Breckenridge AM, Park BK. Carbamazepine hypersensitivity: assessment of clinical and in vitro chemical cross-reactivity with phenytoin and oxcarbazepine. Br J Clin Pharmacol. 1991;32:741–749. [PMC free article] [PubMed] [Google Scholar]
- 57.Cribb AE, Spielberg SP, Griffin GP. N4-hydroxylation of sulfamethoxazole by cytochrome P450 of the cytochrome P4502C subfamily and reduction of sulfamethoxazole hydroxylamine in human and rat hepatic microsomes. Drug Metab Dispos. 1995;23:406–414. [PubMed] [Google Scholar]
- 58.Trepanier LA, Miller JL. NADH-dependent reduction of sulphamethoxazole hydroxylamine in dog and human liver microsomes. Xenobiotica. 2000;30:1111–1121. doi: 10.1080/00498250010013908. [DOI] [PubMed] [Google Scholar]
- 59.Roychowdhury S, Vyas PM, Reilly TP, Gaspari AA, Svensson CK. Characterization of the formation and localization of sulfamethoxazole and dapsone-associated drug-protein adducts in human epidermal keratinocytes. J Pharmacol Exp Ther. 2005;314:43–52. doi: 10.1124/jpet.105.086009. [DOI] [PubMed] [Google Scholar]
- 60.Bhaiya P, Roychowdhury S, Vyas PM, Doll MA, Hein DW, Svensson CK. Bioactivation, protein haptenation, and toxicity of sulfamethoxazole and dapsone in normal human dermal fibroblasts. Toxicol Appl Pharmacol. 2006;215:158–167. doi: 10.1016/j.taap.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.