Abstract
Purpose
Heterozygous mutations around codon 838 of the guanylate cyclase 2D (GUCY2D) gene have recently been associated with more than a third of autosomal dominant macular dystrophy patients. The aim of our study was to evaluate the prevalence of these mutations in Spanish families with autosomal dominant cone, cone-rod, and macular dystrophies.
Methods
Mutation analysis was performed by PCR amplification of exon 13 of GUCY2D and subsequent restriction analysis. To confirm the results, automatic sequencing analysis was also performed.
Results
Among the 22 unrelated Spanish families included in the study, we found two associated disease mutations at codon 838 of the GUCY2D gene, one of which had not been previously described (p.R838P). This novel mutation exhibited phenotypic variability.
Conclusions
The prevalence of mutations around codon 838 of GUCY2D in our group of families (9.09%) is lower than that previously reported in other populations. However, the discovery of a novel mutation at codon 838 further suggests that this locus is a mutation hotspot within the GUCY2D gene, and confirms the importance of analyzing this codon to characterize molecularly these autosomal dominant retinal disorders.
Introduction
Cone-rod dystrophy (CORD) and cone dystrophy (COD) are a subgroup of inherited retinal dystrophies characterized by progressive loss of photoreceptor function. In CORD, there is a progressive loss of cone photoreceptor function followed by gradual loss of rod photoreceptor function and retinal degeneration. In COD however, cone function decreases progressively from its onset, though rod function is well preserved until the late stages of the disease. The predominant symptoms of these disorders are decreased visual acuity, bright light aversion, and decreased sensitivity in the central visual field, sometimes followed by progressive loss of peripheral vision and night blindness [1-6].
CORD and COD are genetically heterogeneous, with described dominant, recessive and X-linked inheritance patterns. To date, ten loci for autosomal dominant CORD and COD have been identified: cone-rod dystrophy 4 (CORD4) [7], retinal cone dystrophy 1 (RCD1) [8], arylhydrocarbon-interacting receptor protein-like 1 (AIPL1) [9], cone-rod homeobox-containing gene (CRX) [10], guanylate cyclase activator 1A (GUCA1A) [11], guanylate cyclase 2D (GUCY2D) [12], phosphatidylinositol transfer protein membrane-associated 3 (PITPNM) [13], protein regulating synaptic membrane exocytosis 1 (RIM1) [14], semaphorin 4A (SEMA4A) [15] and Homolog of C. elegans 119 (UNC119) [16].
The previously reported prevalence of the GUCY2D gene mutations around codon 838 in CORD and COD is about 35%. Heterozygous missense mutations at codons 837, 838, or 839 in the GUCY2D gene, which produce a gain of function, have been shown to cause autosomal dominant CORD and COD [12,17-21], whereas homozygous or compound heterozygous mutations, which produce loss of function, cause Leber congenital amaurosis (LCA) [22-24].
The GUCY2D gene is located in 17p13.1 (LCA1/CORD6) [18,25]. It is 16 kb long and encodes a protein 1,103 amino acids long. The 20 exons identified in this gene code for a photoreceptor-specific protein, retinal guanylyl cyclase-1 (RetGC-1), mostly located in the marginal region of the cone’s outer segments. RetGC proteins play an important role in restoring photoreceptor sensitivity due to their involvement in the synthesis of cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP). Light stimulates the degradation of cGMP, causing the closing of cation channels, which results in a reduction of the Na+ and Ca2+ concentration, cell hyperpolarization, and slowing of neurotransmitter release. At a lower concentration, the Ca2+ binds guanylate cyclase activator proteins (GCAPs) and stimulates the RetGCs, and in consequence, the cGMP level is restored. As a result, the cation channels reopen and photosensitivity is restored to the cell [26].
To our knowledge, all GUCY2D mutations identified so far in CORD or COD patients are located at codon 838 or the two adjacent ones (Figure 1) [12,17-21]. The aim of this study was to analyze the prevalence of GUCY2D mutations clustered at codon 838 in Spanish patients with CORD, COD, and autosomal dominant macular dystrophy (adMD).
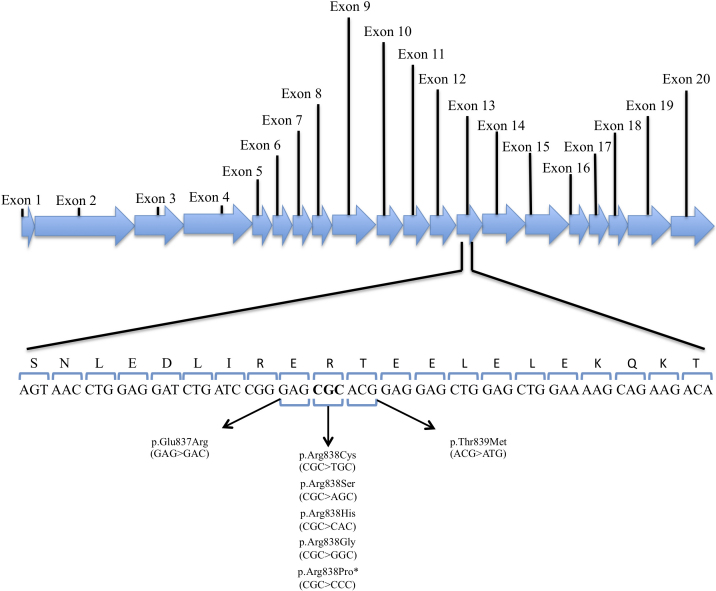
Figure 1.
Reported mutations in a fragment of the primary structure of the retinal guanylyl cyclase-1 (RetGC-1) protein, encoded by exon 13 of Guanylate Cyclase 2D (GUCY2D). This figure shows the different mutations that have been previously described in codons 837, 838, and 839 of the GUCY2D gene. The asterisk indicates the novel p.Arg838Pro mutation described in this manuscript. The shadowed area corresponds to the Hemophilus hemolyticus (HhaI) recognition site.
Methods
Selection of patients
A total of 22 unrelated Spanish patients clinically diagnosed with CORD-COD or adMD and with a family history consistent with an autosomal dominant mode of inheritance were included in this study.
After ophthalmologic examination, adMD patients were classified according to the following criteria: fundus compatible with MD (atrophy and/or hyperpigmentation spots restricted to the macular region, drusen-like fundus, yellow spots), an electroretinogram (ERG) showing reduction of cone signals but normal rod signals, progressive loss of central vision (central scotoma), progressive reduction of visual acuity, and dyschromatopsia.
Applying these criteria and based on clinical examination (ERG and fundus photographs), 10 patients were diagnosed with CORD or COD, whereas 12 patients were classified as having adMD. The first group (CORD or COD) included cases with decreased central vision, abnormal color vision, abnormal ERG, and fundus pigment changes. Ganzfeld electroretinography was recorded according to International Society for Clinical Electrophysiology of Vision (ISCEV) standards, using the UTAS 2000 system (LKC Technology, Gaithersburg, MD) and jet electrodes.
In the family with the novel mutation, seven affected members and four unaffected members were used for segregation analysis and clinical examinations.
In addition, 190 randomly selected DNA samples (380 chromosomes) from a healthy control population were analyzed to assess the frequency of sequence changes in the normal population.
Informed consent was obtained from all persons involved in the study, in accordance with the tenets of the Declaration of Helsinki (Seoul, 2008).
Screening for mutations
DNA was extracted from peripheral blood leukocytes collected in EDTA tubes, in an automated DNA extractor according to manufacturer instructions (BioRobot EZ1; Qiagen, Hilden, Germany).
Exon 13 of the human GUCY2D gene, which includes codon 838 and surrounding codons, was directly amplified from genomic DNA using primers previously described [27] Forward primer: 5′CAG CTT TAC CAG CTT CCT TC 3′, melting temperature=56.9 °C; Reverse primer: 5′ GCA GGC AGT GAG GTC ACC TG 3′, melting temperature=64.4 °C). A sample of genomic DNA (100 ng) from patients or control individuals were used in a 25 µl reaction mixture containing 0.6 µM of forward and reverse primers, 24 µM of each dNTP, 1× PCR buffer, and 1 U of FastStart Taq DNA polymerase (Roche, Indianapolis, IN). After an initial denaturation of 95 °C for 5 min, 30 cycles were performed at 95 °C for 30 s, 63 °C for 20 s, and 74 °C for 50 s, with a final extension step of 74 °C for 5 min.
The PCR products (278 base pairs length) were digested with HhaI according to the manufacturer instructions (New England BioLabs, Beverly, MA) and resolved by electrophoresis in 5% metaphor agarose (Lonza, Rockland, ME). Wild-type samples produce two fragments of 130 bp and 150 bp, but the restriction target site (5′-… GCGC …-3′) in exon 13 of GUCY2D, which lies between the last nucleotide of codon 837 and the last nucleotides of codon 838 (both included), is destroyed by the previously reported mutations at these two codons (Figure 1).
To confirm the results obtained (positive and negative for restriction enzyme digest), PCR products were also sequenced using the BigDye Terminator v. 1.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA) in the ABI 3130xl Genetic Analyzer (Applied Biosystems) and analyzed with the Sequencing Analysis v. 5.2 software package (Applied Biosystems).
Haplotype analysis
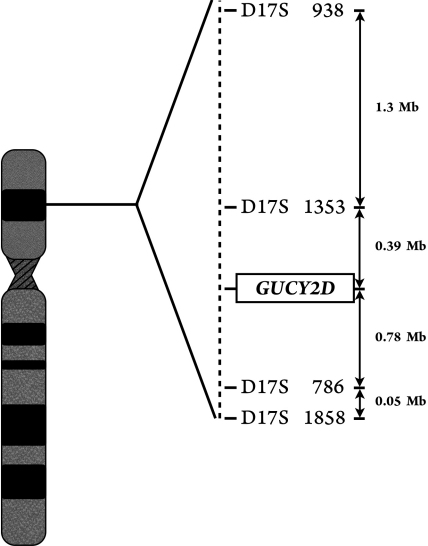
To assess the role of the CORD6 locus, haplotype analysis was performed using four polymorphic markers with a strong link to this locus: D17S938, D17S1353, D17S786, and D17S1858 (Figure 2). For all four markers, each forward PCR primer was fluorescently labeled and separately amplified by PCR in a total volume of 15 µl containing 100 ng of genomic DNA, 125 µM of each dNTP, 10 pmol of each primer (forward and reverse), 1× Taq DNA polymerase buffer (500 mM Tris/HCl, 100 mM KCl, 50 mM [NH4]2SO4, 20 mM MgCl2), and 0.6 U of FastStart Taq DNA Polymerase (Roche). After denaturation at 95 °C for 5 min, PCR was performed in a GeneAmp PCR System 2700 (Applied Biosystems) for 10 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 90 s, and then at 15 cycles at 89 °C for 30 s, 55 °C for 30 s, and 72 °C for 90 s, with a final extension time of 30 min at 72 °C. For the genotyping process, PCR products were electrophoresed in an ABI 3130xl Genetic Analyzer (Applied Biosystems) and analyzed with the GeneMapper v. 4.0 software package (Applied Biosystems).
Figure 2.
Figure shows a map of the CORD6 region. Distance between markers and the locus CORD6 are indicated in bp.
Results
We identified the mutation associated with the disease in two of the 22 (9.09%) unrelated Spanish families that were included in this study because of their suspected clinical diagnosis. In all cases, the additional sequencing analysis of exon 13 of the human GUCY2D gene confirmed the results obtained using restriction analysis.
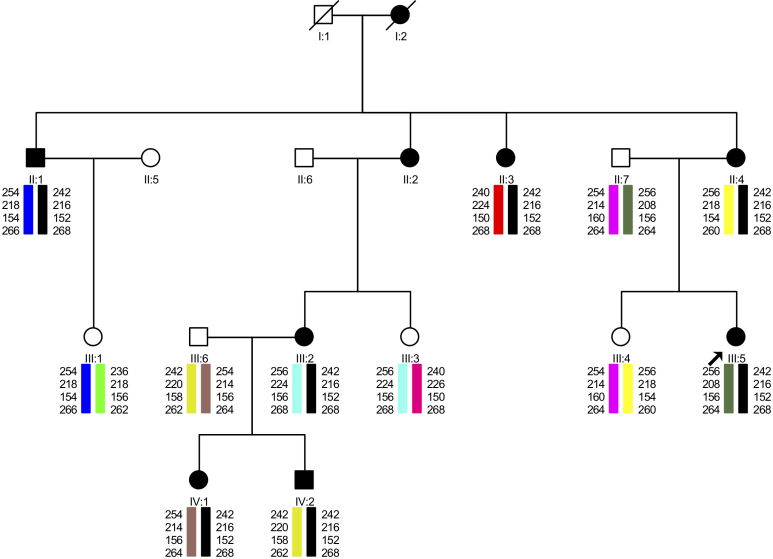
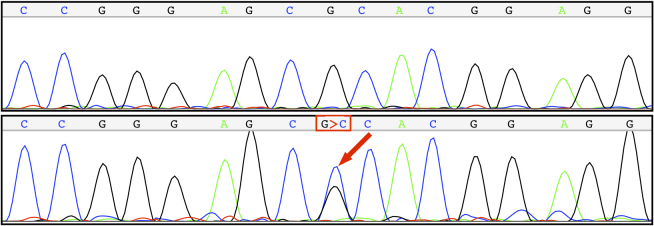
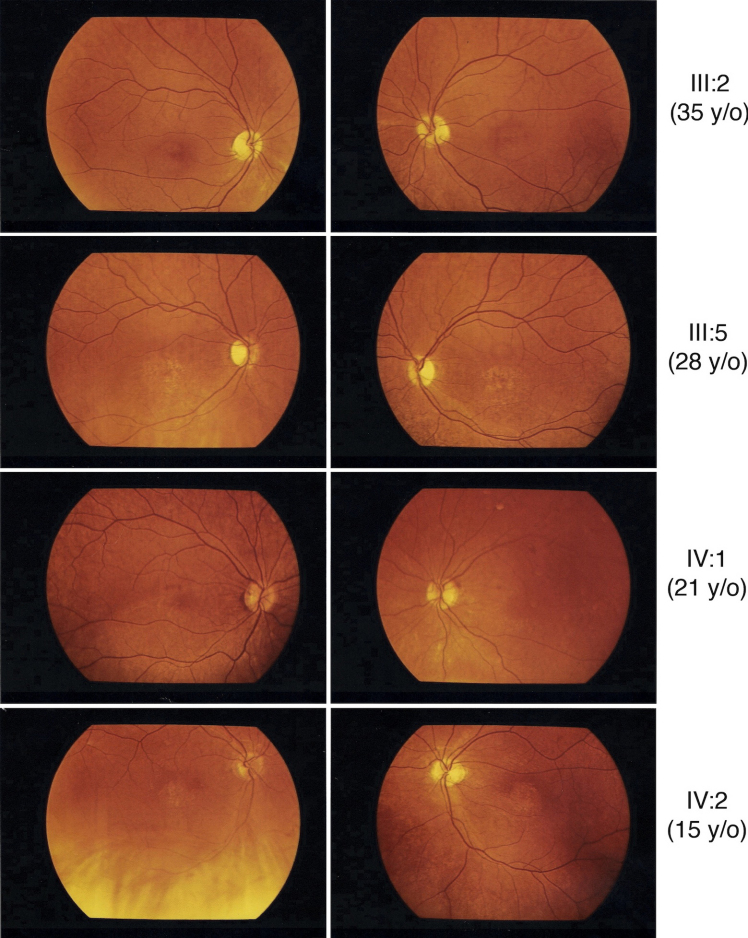
One proband carried a novel c.2513G>C (p.R838P) mutation that segregated with the disease in this family, as all affected members presented this change which was absent in his family (Figure 3) and was absent in 380 control chromosomes (Figure 4). In the family with the novel mutation (p.R838P), the clinical phenotype associated with the disease was characterized both by its onset in the first decade of life and by the detection of a central scotoma. Nystagmus and reduced visual acuity were noticed in all affected family members, although no color vision abnormality or increased glare sensitivity were reported (Table 1). Within this family, older patients typically had a more severe phenotype than did the younger patients (Figure 5).
Figure 3.
Results of the haplotype analysis performed in a family with the p.R838P mutation in Guanylate Cyclase 2D (GUCY2D). The figure also shows the status of genotyped individuals for mutation p.R838P, where “+” is used for normal alleles and “–” is used for mutated alleles.
Figure 4.
A heterozygous mutation (Guanine-to-Citosine; G>C) results in an arginine-to-proline mutation at codon 838 of the Guanylate Cyclase 2D (GUCY2D) gene.
Table 1. Clinical features of the families with the p.R838P mutation in GUCY2D.
| Individual | Age | Visual acuity (RE/ LE) | Fundus findings | Central scotoma | Color vision | Nystagmus |
|---|---|---|---|---|---|---|
| II:1 |
70 |
0.01/0.01 |
Macular degeneration |
+++ |
Normal |
+ |
| II: 3 |
61 |
0.3/0.2 |
Macular degeneration |
N/A |
Normal |
+ |
| II:4 |
59 |
0.01/0.01 |
Macular degeneration Salt-and-pepper fundus appearance in the posterior pole |
++ |
Normal |
+ |
| III:2 |
35 |
0.2/0.2 |
Tapetoretinal degeneration
Salt-and-pepper fundus appearance |
++ |
Normal |
+ |
| III:5 |
28 |
0.3/0.1 |
Discreet salt-and-pepper fundus appearance |
++ |
Normal |
+ |
| IV:1 |
21 |
N/A |
Salt-and-pepper fundus appearance |
++ |
Normal |
+ |
| IV:2 | 15 | 0.1/0.1 | Poorly distinguishable macula | + | Normal | + |
Figure 5.
Fundus photograph of five different patients of the families with p.R838P mutation in GUCY2D. Older patients had a more severe phenotype compared to the younger generation, and to the Goldmann kinetic perimetric fields of patients III:5 and IV:2. Fundus description for these five patients has been summarized in Table 1.
In addition, another affected patient was heterozygous for the mutation c.2513G>A (p.R838H), previously reported by Weigell-Weber et al. [19]. This affected patient had gross central macular degeneration in both eyes, with bilateral central scotoma. The best-corrected visual acuity was 0.1 in each eye at the age of 59 years.
Discussion
Since the aim of our study was to evaluate the prevalence of mutations at codon 838, we used a restriction enzyme digestion (RE) that could detect all possible mutations at codon 838 and also at codon 837, but not at the adjacent codon 839. Additional sequencing of exon 13 of GUCY2D confirmed that only the two mutations previously detected at codon 838 by using RE (c.2513G>C and c.2513G>A) were associated with the disease in our cohort of patients. For that reason, we proposed the use of RE as a quick first step to analyze our families.
Although a previous study reported mutations around codon 838 of GUCY2D associated with CORD or COD in more than a third of the patients [17], the prevalence of GUCY2D mutations around this codon was lower (9.09%) in our group of Spanish families.
Four different mutations have previously been reported as affecting codon 838: p.R838C [12], p.R838H [19], p.R838S [19], and p.R838G [17]. We report a new mutation at this particular locus, (p.R838P), which further indicates the importance of this codon and leads us to confirm that it is a mutational hotspot in the GUCY2D gene, associated with CORD, COD, and adMD disease.
At a lower concentration, the Ca2+ binding GCAPs stimulate the RetGC proteins. Activation of RetGC1 by GCAP1 involved dimerization of two RetGC1 monomers. The dimerization domain of RetGC-1 extends from amino acid 817 to 857, and this region is likely to adopt a coiled-coil structure [28]. Mutations at position 838 have been described as increasing the stability of the coiled-coil; hence, the mutant protein retains residual catalytic activity even at high calcium levels [27]. These mutations increase the affinity of RetGC-1 for GCAP1 and alter the Ca2+ sensitivity of the GCAP1 response, allowing the mutant to be stimulated by GCAP1 at higher Ca2+ concentrations than is the wild type [28]. This gain of function results in the maintenance of GMP levels, and consequently in a persistently high intracellular Ca+2 level. It is known that a persistent elevated calcium level in the cell tends to disrupt the membrane potential of the mitochondrial inner membrane, leading to the release of cytochrome C, with subsequent caspase activation and apoptosis [23]. This may be the mechanism, resulting from GUCY2D mutations, of photoreceptor degeneration in CORD or COD, and in adMD. While, heterozygous mutations in GUCY2D associated with CORD or COD have been described that cause a gain of function, individuals harbouring either homozygous or compound heterozygous GUCY2D loss-of-function mutations present a more severe disease, such as LCA is associated with being homozygous or compound heterozygous for GUCY2D loss-of-function mutations [12,22].
The phenotypes of the families with mutations at codons 837, 838, or 839 of GUCY2D appear to vary, depending on the specific mutation and the presence or absence of multiple mutations [17,27,29]. In our family with the novel mutation p.R838P, no color vision abnormalities or increased glare sensitivity were reported, though affected members showed reduced visual acuity and a central scotoma.
We believe that this report highlights the importance of codon 838 of GUCY2D as a mutation hotspot associated with CORD or COD and with adMD. In addition, genetic studies of families clinically diagnosed as having CORD, COD, or adMD are important for performing correct genetic counseling.
Moreover, knowledge of the molecular mechanism of these diseases would permit the development of new potential therapies, such as gene therapy. Mutations at codon 838 of GUCY2D are related to autosomal dominant disease; therefore, silencing the mutant allele using allele-specific interference RNA could be used in this case to partially rescue the protein function [30-32].
Acknowledgments
We wish to thank FIS (PI06/0027, PI07/90285, PI09/90047 and PS09/00459), ONCE and CIBER-ER (06/07/0036) for their support. We thank ONCE and Dr. Benavides for sending us their patients.
References
- 1.Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merin S. Inherited Eye Diseases: Diagnosis and Management. 2nd ed. CRC Press (NY): Taylor & Francis Group; 2005. [Google Scholar]
- 3.Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol. 2006;51:232–58. doi: 10.1016/j.survophthal.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Szlyk JP, Fishman GA, Alexander KR. Clinical subtypes of cone-rod dystrophy. Arch Ophthalmol. 1993;111:781–8. doi: 10.1001/archopht.1993.01090060069025. [DOI] [PubMed] [Google Scholar]
- 5.Yagasaki K, Jacobson SG. Cone-rod dystrophy. Phenotypic diversity by retinal function testing. Arch Ophthalmol. 1989;107:701–8. doi: 10.1001/archopht.1989.01070010719034. [DOI] [PubMed] [Google Scholar]
- 6.Moore AT. Cone and cone-rod dystrophies. J Med Genet. 1992;29:289–90. doi: 10.1136/jmg.29.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kylstra JA, Aylsworth AS. Cone-rod retinal dystrophy in a patient with neurofibromatosis type 1. Can J Ophthalmol. 1993;28:79–80. [PubMed] [Google Scholar]
- 8.Tranebjaerg L, Sjo O, Warburg M. Retinal cone dysfunction and mental retardation associated with a de novo balanced translocation 1;6(q44;q27). Ophthalmic Paediatr Genet. 1986;7:167–73. doi: 10.3109/13816818609004134. [DOI] [PubMed] [Google Scholar]
- 9.Sohocki MM, Perrault I, Leroy BP, Payne AM, Dharmaraj S, Bhattacharya SS, Kaplan J, Maumenee IH, Koenekoop R, Meire FM, Birch DG, Heckenlively JR, Daiger SP. Prevalence of AIPL1 mutations in inherited retinal degenerative disease. Mol Genet Metab. 2000;70:142–50. doi: 10.1006/mgme.2000.3001. [DOI] [PubMed] [Google Scholar]
- 10.Freund CL, Gregory-Evans CY, Furukawa T, Papaioannou M, Looser J, Ploder L, Bellingham J, Ng D, Herbrick JA, Duncan A, Scherer SW, Tsui LC, Loutradis-Anagnostou A, Jacobson SG, Cepko CL, Bhattacharya SS, McInnes RR. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997;91:543–53. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 11.Payne AM, Downes SM, Bessant DA, Taylor R, Holder GE, Warren MJ, Bird AC, Bhattacharya SS. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum Mol Genet. 1998;7:273–7. doi: 10.1093/hmg/7.2.273. [DOI] [PubMed] [Google Scholar]
- 12.Kelsell RE, Gregory-Evans K, Payne AM, Perrault I, Kaplan J, Yang RB, Garbers DL, Bird AC, Moore AT, Hunt DM. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum Mol Genet. 1998;7:1179–84. doi: 10.1093/hmg/7.7.1179. [DOI] [PubMed] [Google Scholar]
- 13.Kohn L, Kadzhaev K, Burstedt MSI, Haraldsson S, Hallberg B, Sandgren O, Golovleva I. Mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in two Swedish families. Eur J Hum Genet. 2007;15:664–71. doi: 10.1038/sj.ejhg.5201817. [DOI] [PubMed] [Google Scholar]
- 14.Johnson S, Halford S, Morris AG, Patel RJ, Wilkie SE, Hardcastle AJ, Moore AT, Zhang K, Hunt DM. Genomic organisation and alternative splicing of human RIM1, a gene implicated in autosomal dominant cone-rod dystrophy (CORD7). Genomics. 2003;81:304–14. doi: 10.1016/s0888-7543(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 15.Abid A, Ismail M, Mehdi SQ, Khaliq S. Identification of novel mutations in the SEMA4A gene associated with retinal degenerative diseases. J Med Genet. 2006;43:378–81. doi: 10.1136/jmg.2005.035055. Letter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi A, Higashide T, Hamasaki D, Kubota S, Sakuma H, An W, Fujimaki T, McLaren MJ, Weleber RG, Inana G. HRG4 (UNC119) mutation found in cone-rod dystrophy causes retinal degeneration in a transgenic model. Invest Ophthalmol Vis Sci. 2000;41:3268–77. [PubMed] [Google Scholar]
- 17.Kitiratschky VB, Wilke R, Renner AB, Kellner U, Vadalà M, Birch DG, Wissinger B, Zrenner E, Kohl S. Mutation analysis identifies GUCY2D as the major gene responsible for autosomal dominant progressive cone degeneration. Invest Ophthalmol Vis Sci. 2008;49:5015–23. doi: 10.1167/iovs.08-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrault I, Rozet JM, Gerber S, Kelsell RE, Souied E, Cabot A, Hunt DM, Munnich A, Kaplan J. A retGC-1 mutation in autosomal dominant cone-rod dystrophy. Am J Hum Genet. 1998;63:651–4. doi: 10.1086/301985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigell-Weber M, Fokstuen S, Török B, Niemeyer G, Schinzel A, Hergersberg M. Codons 837 and 838 in the retinal guanylate cyclase gene on chromosome 17p: hot spots for mutations in autosomal dominant cone-rod dystrophy? Arch Ophthalmol. 2000;118:300. doi: 10.1001/archopht.118.2.300. [DOI] [PubMed] [Google Scholar]
- 20.Van Ghelue M, Eriksen HL, Ponjavic V, Fagerheim T, Andréasson S, Forsman-Semb K, Sandgren O, Holmgren G, Tranebjaerg L. Autosomal dominant cone-rod dystrophy due to a missense mutation (R838C) in the guanylate cyclase 2D gene (GUCY2D) with preserved rod function in one branch of the family. Ophthalmic Genet. 2000;21:197–209. [PubMed] [Google Scholar]
- 21.Udar N, Yelchits S, Chalukya M, Yellore V, Nusinowitz S, Silva-Garcia R, Vrabec T, Hussles Maumenee I, Donoso L, Small KW. Identification of GUCY2D gene mutations in CORD5 families and evidence of incomplete penetrance. Hum Mutat. 2003;21:170–1. doi: 10.1002/humu.9109. [DOI] [PubMed] [Google Scholar]
- 22.Perrault I, Rozet JM, Calvas P, Gerber S, Camuzat A, Dollfus H, Châtelin S, Souied E, Ghazi I, Leowski C, Bonnemaison M, Le Paslier D, Frézal J, Dufier JL, Pittler S, Munnich A, Kaplan J. Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nat Genet. 1996;14:461–4. doi: 10.1038/ng1296-461. [DOI] [PubMed] [Google Scholar]
- 23.Rozet JM, Perrault I, Gerber S, Hanein S, Barbet F, Ducroq D, Souied E, Munnich A. Kaplan. Complete abolition of the retinal-specific guanylyl cyclase (retGC-1) catalytic ability consistently leads to Leber congenital amaurosis (LCA). Invest Ophthalmol Vis Sci. 2001;42:1190–2. [PubMed] [Google Scholar]
- 24.den Hollander AI, Roepman R, Koenekoop RK, Cremers FPM. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Kelsell RE, Evans K, Gregory CY, Moore AT, Bird AC, Hunt DM. Localisation of a gene for dominant cone-rod dystrophy (CORD6) to chromosome 17p. Hum Mol Genet. 1997;6:597–600. doi: 10.1093/hmg/6.4.597. [DOI] [PubMed] [Google Scholar]
- 26.Wilkie SE, Newbold RJ, Deery E, Walker CE, Stinton I, Ramamurthy V, Hurley JB, Bhattacharya SS, Warren MJ, Hunt DM. Functional characterization of missense mutations at codon 838 in retinal guanylate cyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum Mol Genet. 2000;9:3065–73. doi: 10.1093/hmg/9.20.3065. [DOI] [PubMed] [Google Scholar]
- 27.Smith M, Whittock N, Searle A, Croft M, Brewer C, Cole M. Phenotype of autosomal dominant cone-rod dystrophy due to the R838C mutation of the GUCY2D gene encoding retinal guanylate cyclase-1. Eye. 2007;21:1220–5. doi: 10.1038/sj.eye.6702612. [DOI] [PubMed] [Google Scholar]
- 28.Tucker CL, Woodcock SC, Kelsell RE, Ramamurthy V, Hunt DM, Hurley JB. Biochemical analysis of a dimerization domain mutation in RetGC-1 associated with dominant cone-rod dystrophy. Proc Natl Acad Sci USA. 1999;96:9039–44. doi: 10.1073/pnas.96.16.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downes SM, Payne AM, Kelsell RE, Fitzke FW, Holder GE, Hunt DM, Moore AT, Bird AC. Autosomal dominant cone-rod dystrophy with mutations in the guanylate cyclase 2D gene encoding retinal guanylate cyclase-1. Arch Ophthalmol. 2001;119:1667–73. doi: 10.1001/archopht.119.11.1667. [DOI] [PubMed] [Google Scholar]
- 30.Campochiaro PA. Potential applications for RNAi to probe pathogenesis and develop new treatments for ocular disorders. Gene Ther. 2006;13:559–62. doi: 10.1038/sj.gt.3302653. [DOI] [PubMed] [Google Scholar]
- 31.O'Reilly M, Palfi A, Chadderton N, Millington-Ward S, Ader M, Cronin T, Tuohy T, Auricchio A, Hildinger M, Tivnan A, McNally N, Humphries MM, Kiang AS, Humphries P, Kenna PF, Farrar GJ. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–35. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam LC, Kiang AS, Kennan A, Kenna PF, Chadderton N, Ader M, Palfi A, Aherne A, Ayuso C, Campbell M, Reynolds A, McKee A, Humphries MM, Farrar GJ, Humphries P. Therapeutic benefit derived from RNAi-mediated ablation of IMPDH1 transcripts in a murine model of autosomal dominant retinitis pigmentosa (RP10). Hum Mol Genet. 2008;17:2084–100. doi: 10.1093/hmg/ddn107. [DOI] [PubMed] [Google Scholar]