Abstract
Background:
Lymph node status is a major determinant of stage and survival in patients with lung cancer; however, little information is available about the expected yield of a mediastinal lymphadenectomy.
Methods:
The American College of Surgeons Oncology Group Z0030 prospective, randomized trial of mediastinal lymph node sampling vs complete mediastinal lymphadenectomy during pulmonary resection enrolled 1,111 patients from July 1999 to February 2004. Data from 524 patients who underwent complete mediastinal lymph node dissection were analyzed to determine the number of lymph nodes obtained.
Results:
The median number of additional lymph nodes harvested from a mediastinal lymphadenectomy following systematic sampling was 18 with a range of one to 72 for right-sided tumors, and 18 with a range of four to 69 for left-sided tumors. The median number of N2 nodes harvested was 11 on the right and 12 on the left. A median of at least six nodes was harvested from at least three stations in 99% of patients, and 90% of patients had at least 10 nodes harvested from three stations. Overall, 21 patients (4%) were found to have occult N2 disease.
Conclusions:
Although high variability exists in the actual number of lymph nodes obtained from various nodal stations, complete mediastinal lymphadenectomy removes one or more lymph nodes from all mediastinal stations. Adequate mediastinal lymphadenectomy should include stations 2R, 4R, 7, 8, and 9 for right-sided cancers and stations 4L, 5, 6, 7, 8, and 9 for left-sided cancers. Six or more nodes were resected in 99% of patients in this study.
Trial registry:
ClinicalTrials.gov; No.: NCT00003831; URL: clinicaltrials.gov
The American College of Surgeons Oncology Group (ACOSOG) Z0030 study is a randomized, multiinstitutional, prospective trial designed to determine whether long-term survival is affected by mediastinal lymph node dissection (MLND) vs lymph node sampling (MLNS) at the time of pulmonary resection for lung cancer. We enrolled 1,023 eligible and /or evaluable patients with stage T1 or T2, N0 or nonhilar N1 lung cancer to either MLNS (n = 498) or complete mediastinal lymphadenectomy (n = 525). After analysis, we concluded that a complete mediastinal lymphadenectomy adds little morbidity to a pulmonary resection for lung cancer and found that the overall operative mortality for pulmonary resection was 1.4% (14/1,023).1 The impact of MLND on long-term survival will be determined once long-term follow-up data of the study are mature.
Because lymph node status is a main determinate of stage, and thus prognosis, complete lymph node dissection has been recommended for all patients undergoing surgical resection of lung cancer. However, what constitutes a complete lymph node dissection is not defined, and different terminology is used to describe various techniques of surgical lymph node assessment. The purpose of this analysis was to determine the expected yield of mediastinal lymphadenectomy following systematic MLNS in terms of the number of lymph nodes examined from each lymph node station, the frequency of obtaining at least one lymph node from a station, and the frequency of occult N2 disease, and to determine if surgical approach affected the yield of lymph node harvest when performing a lymph node dissection.
Materials and Methods
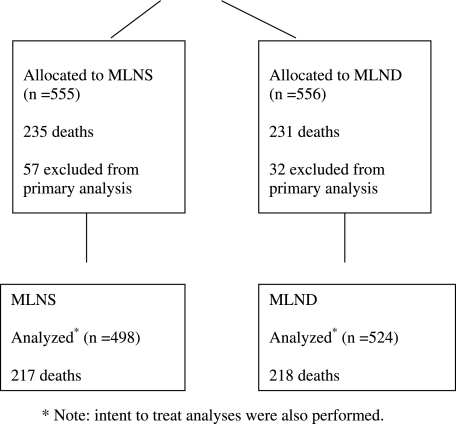
Only eligible patients entered in the Z0030 trial who were randomized to complete lymph node dissection were analyzed for the purpose of this report (Fig 1). The protocol was approved by the central institutional review board and the institutional review board of each institution that enrolled patients. All patients signed informed consent. Eligibility requirements included patients older than 18 years of age with an Eastern Cooperative Oncology Group performance score lower than 3 and a clinically resectable T1 or T2 lung cancer with no evidence of distant metastatic disease. Prior to randomization, histologic or cytologic confirmation of non-small cell lung cancer (squamous cell carcinoma, large cell carcinoma, or adenocarcinoma, including bronchoalveolar carcinoma) was required. After confirming lymph node status as either N0 or nonhilar N1 based on preresection sampling of the mediastinal (N2 ± N3) and hilar (station 10) lymph nodes, patients were randomized intraoperatively to MLNS or to complete lymph node dissection at thoracotomy (or thoracoscopy). Patients randomized to sampling had no further lymph nodes removed other than those required by the protocol prior to randomization.
Figure 1.
Consort diagram. Note that intent-to-treat analyses were also performed. MLND = mediastinal lymph node dissection; MLNS = mediastinal lymph node sampling.
All surgeons participating in the trial were general thoracic surgeons and diplomats of the American Board of Thoracic Surgery or the equivalent. Patients were enrolled in the trial by 102 different surgeons from 63 institutions. The enrolling surgeon was required to read a detailed description of the technique of MLND, watch a video that demonstrated the technique of a complete MLND, and certify that he or she would perform the MLND as demonstrated.
Complete MLND for tumors on the right involved removing all tissue from an area bounded caudally by the origin of the right upper lobe bronchus, superiorly by the innominate artery, anteriorly by the superior vena cava (SVC), and posteriorly by the trachea. All tissue was removed from this area and, at the completion of the dissection, the trachea, vagus nerve, and SVC were visible. Lymph nodes in the prevascular area, adjacent to the SVC, were removed, as were lymph nodes in the retrotracheal area.
Complete MLND for tumors on the left involved removing all tissue from the area between the phrenic nerve anteriorly and the vagus nerve posteriorly. Superiorly, all tissue was removed to the apex of the triangle between the two nerves. The caudal boundary was the left main stem bronchus. At the completion of the dissection, the aortopulmonary window was free of lymph tissue and the recurrent nerve was preserved.
Regardless of the side on which the tumor was located, all subcarinal, inferior pulmonary ligament, periesophageal, lobar, and intralobar lymph nodes were resected. Complete subcarinal lymph node dissection included removing all tissue caudal to the carina and both left and right mainstem bronchi. All lymph nodes adjacent to the inferior pulmonary ligament and the caudal half of the esophagus were also removed. When the dissection was complete, both mainstem bronchi, the posterior pericardium, and the esophagus were free of all lymph tissue. During resection of the lung, all lobar and interlobar lymph nodes were resected.
Information on the number of lymph nodes examined from each station was collected beginning in January 2002. For patients enrolled between 1999 and 2002, two authors (M. S. A. and G. E. D.) reviewed operative and pathology reports to determine the number of lymph nodes obtained from each station. The ATS lymph node map was used for documentation. For consistency, nonspecific terms used in pathology reports were standardized: multiple was translated into four lymph nodes, aggregate into three, several into three, fragment into three, and numerous into five. Concordance between the two reviews was within one lymph node 90% of the time.
Lymph node data were summarized using mean ± SD, median, and range. The two-sample rank sum test was used to compare lymph node data between groups. Regression analyses were used to assess the association of select variables with the number of lymph nodes resected. In all cases, P values < .05 were considered statistically significant.
Results
Five hundred twenty-four patients were in the complete lymphadenectomy group: 271 men, with a median age of 67.4 years (range 37-87 years). Histology of resected tumors and stage are shown in Tables 1 and 2, respectively. The tumor was located in the right lung in 317 patients and in the left in 207 patients.
Table 1.
—Histology of Resected Tumors
| Histology | No. (%) |
| Squamous cell | 141 (27.1) |
| Adenocarcinoma | 227 (43.6) |
| Large cell | 22 (4.2) |
| Bronchoalveolar | 32 (6.1) |
| Other | 99 (19.0) |
Table 2.
—Tumor Stage
| Pathologic Stage | No. (%) |
| IA | 211 (40.4) |
| IB | 213 (40.8) |
| IIA | 24 (4.6) |
| IIB | 41 (7.9) |
| IIIA | 22 (4.2) |
| IIIB | 11 (2.1) |
The median number of lymph nodes dissected was the same for both right-sided and left-sided cancers: 18 with a range of one to 72 for right-sided tumors and 18 with a range of four to 69 for left-sided tumors (P = .93). All patients had at least one lymph node removed in addition to those removed for preresection sampling (at mediastinoscopy and /or thoracotomy or video-assisted thoracic surgery). The median number of lymph nodes resected from N1 stations 10 to 14 was similar between right-sided and left-sided cancers (five vs six, P = .134). The largest numbers of lymph nodes were resected from stations 7 and 4R, with a median of three and four nodes, respectively (Tables 3, 4). Almost all patients (517 or 98.7%) had at least six lymph nodes examined and 470 (89.7%) had at least 10 lymph nodes examined. Lymph nodes were harvested from at least three stations in 521 patients (99.4%). Combining these data, 516 (98.5%) had at least six lymph nodes examined from three lymph node stations and 469 (89.5%) had at least 10 lymph nodes examined from three stations.
Table 3.
—Lymph Nodes Examined for Right-Sided Cancers
| No. Lymph Nodes |
|||||
| Station | No. Patients (%) | Mean (SD) | Median | Range | Histologically Positive (%) |
| All lymph nodes | 317 (100) | 19.9 (11.1) | 18.0 | 1-72 | … |
| N2 stationsa | 317 (100) | 13.6 (8.4) | 11.0 | 1-49 | … |
| N1 stationsb | 311 (98) | 6.4 (5.0) | 5.0 | 1-37 | … |
| Right-sided cancers: N2 stations | |||||
| 2R | 276 (87) | 3.1 (2.7) | 2.0 | 1-17 | 1 (0.3) |
| 4R | 305 (96) | 5.1 (4.3) | 4.0 | 1-24 | 5 (1.6) |
| 7 | 310 (98) | 4.5 (3.6) | 3.0 | 1-24 | 4 (1.3) |
| 8 | 154 (49) | 1.4 (1.0) | 1.0 | 1-6 | 2 (0.6) |
| 9 | 204 (64) | 1.4 (0.8) | 1.0 | 1-6 | 1 (0.3) |
| Right-sided cancers: N1 stations | |||||
| 10R | 303 (95) | 2.3 (2.1) | 1.0 | 1-14 | 7 (2.2) |
| 11R | 246 (77) | 2.8 (3.0) | 2.0 | 1-28 | 17 (5.4) |
| 12R | 154 (48) | 3.5 (3.4) | 3.0 | 1-28 | 10 (3.2) |
| 13R | 32 (10) | 2.6 (2.0) | 2.0 | 1-8 | 1 (0.3) |
| 14R | 7 (2) | 2.1 (1.3) | 2.0 | 1-4 | 0 |
N2 stations: 2R, 4R, 7, 8, and 9.
N1 stations: 10R, 11R, 12R, 13R, and 14R.
Table 4.
—Lymph Nodes Examined for Left-Sided Cancers
| No. Lymph Nodes |
|||||
| Station | No. Patients (%) | Mean (SD) | Median | Range | Histologically Positive (%) |
| All lymph nodes | 207 (100) | 19.1 (8.9) | 18.0 | 4-69 | … |
| N2 stationsa | 207 (100) | 12.7 (7.1) | 12.0 | 3-52 | … |
| N1 stationsb | 200 (97) | 6.6 (4.1) | 6.0 | 1-22 | … |
| Left-sided cancers: N2 stations | |||||
| 2L | 19 (9) | 2.0 (1.5) | 1.0 | 1-6 | 0 |
| 4L | 102 (49) | 2.7 (3.6) | 2.0 | 1-33 | 0 |
| 5 | 203 (98) | 3.0 (2.4) | 2.0 | 1-18 | 3 (1.5) |
| 6 | 177 (86) | 2.5 (1.7) | 2.0 | 1-11 | 2 (1.0) |
| 7 | 200 (97) | 4.2 (3.7) | 3.0 | 1-25 | 3 (1.5) |
| 8 | 90 (43) | 1.4 (0.9) | 1.0 | 1-7 | 0 |
| 9 | 166 (80) | 1.8 (1.2) | 1.0 | 1-8 | 1 (0.5) |
| Left-sided cancers: N1 stations | |||||
| 10L | 186 (90) | 2.5 (2.2) | 2.0 | 1-12 | 3 (1.5) |
| 11L | 151 (73) | 3.0 (2.6) | 2.0 | 1-17 | 13 (6.3) |
| 12L | 101 (49) | 3.0 (2.2) | 3.0 | 1-13 | 14 (6.9) |
| 13L | 28 (14) | 3.5 (3.0) | 2.0 | 1-12 | 5 (2.5) |
| 14L | 5 (2) | 2.0 (1.7) | 1.0 | 1-5 | 1 (0.5) |
N2 stations: 2L, 4L, 5, 6, 7, 8, and 9.
N1 stations: 10L, 11L, 12L, 13L, and 14L.
In terms of individual stations for the 317 right-sided cancers, the median yield from station 2R was two lymph nodes; for station 4R it was four; station 7, three; station 8, one; station 9, one; and station 10R, one (Table 3). For the 207 left-sided cancers, the median yield from station 2L was one; for station 4L it was two; station 5, two; station 6, two; station 7, three; station 8, one; station 9, one; and station 10L, two (Table 5).
Table 5.
—N2 Patients
| Patient | Stage | T Stage | N Stage | Histology | Location | Positive Stations |
| 1 | IIIA | T2 | N2 | Squamous | LUL | 5 |
| 2 | IIIA | T2 | N2 | Adenocarcinoma | LLL | 9 |
| 3 | IIIA | T2 | N2 | Adenocarcinoma | LUL | 5, 11L |
| 4 | IIIA | T2 | N2 | Squamous | LUL | 7 |
| 5 | IIIA | T2 | N2 | Bronchoalveolar | LLL | 7, 12L |
| 6 | IIIA | T2 | N2 | Other NSCLCa | LUL, LLL | 11Lb |
| 7 | IIIA | T1 | N2 | Adenocarcinoma | LUL | 6, 12L |
| 8 | IIIA | T1 | N2 | Adenocarcinoma | LLL | 6, 7, 11L |
| 9 | IIIA | T1 | N2 | Other NSCLCa | LUL | 5, 12L |
| 10 | IIIA | T2 | N2 | Adenocarcinoma | RLL | 7, 11R |
| 11 | IIIB | T4 | N2 | Adenocarcinoma | RUL | 2R, 4R |
| 12 | IIIA | T3 | N2 | Adenocarcinoma | RLL | 4R |
| 13 | IIIA | T2 | N2 | Adenocarcinoma | RUL | 4R |
| 14 | IIIA | T2 | N2 | Other NSCLCa | RLL | 11Rb |
| 15 | IIIA | T2 | N2 | Other NSCLCa | RLL | 7 |
| 16 | IIIA | T2 | N2 | Squamous | RLL | 7, 11R |
| 17 | IIIA | T2 | N2 | Adenocarcinoma | RLL | 8,9 |
| 18 | IIIA | T1 | N2 | Adenocarcinoma | RUL | 4R, 10R |
| 19 | IIIB | T4 | N2 | Squamous | RHIL | b |
| 20 | IIIA | T2 | N2 | Squamous | RUL | 4R, 10R |
| 21 | IIIA | T2 | N2 | Other NSCLCa | RLL | b |
LLL = left lower lobe; LUL = left upper lobe; NSCLC = non-small cell lung cancer; RLL = right lower lobe; RUL = right upper lobe.
Other NSCLC not otherwise specified.
Positive N2 nodes not specified.
Twenty-one patients (4%) had occult N2 disease identified by mediastinal lymphadenectomy that was not found by the rigorous prerandomization systematic sampling used in this protocol. Overall, 63 patients had N1 disease, and 21 patients had N2 disease. However, of the 21 patients with N2 disease, only 11 had positive N1 nodes. Patients with positive station 2 nodes also had positive nodes in station 4 (Table 5). kThe reasons for designation of stage IIIA/B (non-N2) in the MLND arm included another cancer in the same lobe, tumor < 2 cm from carina, involvement of the phrenic nerve, and tracheal involvement.
Most patients were resected using an open thoracotomy, but 29 were resected using video-assisted thoracic surgery. There was no significant difference between the two approaches with respect to the number of lymph nodes dissected (Table 6). There was no association between tumor histology, patient age, and gender and the number of nodes harvested, but increasing N stage was associated with increased lymph node harvest (N0, 19.2 ± 10.1; N1, 22.8 ± 10.9; N2, 24.5 ± 10.8; P = .043).
Table 6.
—Thoracotomy vs VATS
| No. Lymph Nodes | ||||
| Approach | No. Patients | Mean (SD) | Median | Range |
| Thoracotomy | 488 | 20.3 (10.7) | 19.0 | 1-83 |
| VATS | 29 | 17.6 (8.6) | 15.0 | 5-48 |
P = .17 (two-sample rank sum test). Note that intent-to-treat analyses were also performed. VATS = video-assisted thoracic surgery.
Discussion
It is well established that adequate lymph node assessment is important in the staging of non-small cell lung cancer.2,3 Despite this, in a recent pattern of care study, only 57.3% of patients had mediastinal lymph nodes removed at the time of pulmonary resection.4 In the ACOSOG Z0030 study, which was conducted by both community and academic thoracic surgeons, 99% of patients had at least six nodes removed, and a median of 18 lymph nodes were examined with MLND. Overall, two-thirds of the lymph nodes examined were N2 lymph nodes. Although the overall lymph node harvest was variable in our study, all patients had at least one node assessed in addition to those sampled prior to resection and > 90% had at least two N2 stations (right, 4R/7; left, 5/7) and one N1 (station 10) examined. In terms of individual lymph node stations, the number of nodes found and removed was also variable, depending on the station. Occasionally, few or even no nodes were found in some stations, such as stations 8 and 9, despite exploration of these stations as documented by the operating surgeon. Station 4L is often not routinely dissected for left-sided tumors and was not part of the mandatory dissection in the study protocol, hence the lower yield for this station in our results.
It has been recommended that the minimum requirements for accurate nodal staging include the removal of at least six lymph nodes from hilar and mediastinal stations, at least one of which must be subcarinal.5,6 Gajra et al7 also reported that assessment of six nodes provided accurate staging. However, others recommend examination of a minimum of 10 lymph nodes and at least three lymph node stations.8,9 These recommendations are derived either from consensus conferences or by retrospective analysis of staging accuracy based on lymph nodes harvested. Given this standard, clearly the majority of patients in the ACOSOG Z0030 study had sufficient nodes examined for staging accuracy. The ACOSOG Z0030 study provides prospective data as to what can be expected in terms of adequate lymph node harvest in the conduct of pulmonary resection for non-small cell lung cancer. For those treating non-small cell lung cancer, these data provide a benchmark for determining whether an adequate lymph node assessment has been performed.
Although MLNS and MLND appear to be comparable in terms of staging accuracy, nonsystematic sampling is less reliable. MLND is reported to be more accurate in determining multilevel N2 disease and skip metastases.10-14 Because two to five lymph nodes were resected from each N2 station in the majority of patients in our study, it is likely that occult N2 disease would be discovered if present and, despite rigorous systematic sampling prior to randomization, 21 patients were found to have occult N2 disease with MLND.
Based on our median lymph node harvest of 18 nodes with two-thirds being N2 nodes, the ACOSOG Z0030 study group recommends that the number of lymph nodes resected during MLND be ≥ 12, with nodes removed from stations 2R, 4R, 7, 8, 9, and 10R on the right, and stations 4L, 5, 6, 7, 8, 9, and 10L on the left.
Whether MLND offers any therapeutic benefit remains to be determined. Reports in the literature suggest that MLND offers some survival advantage, but this is largely related to stage migration. Several authors report improved survival with harvest of > 10 lymph nodes, but that removal of > 16 nodes did not confer any further improvement in survival.8,9 In a population-based study of 16,800 patients, multivariate analysis showed maximum survival for all-cause mortality (hazard ratio, 0.78; 95% CI, 0.68-0.90) and lung-cancer-specific mortality (hazard ratio, 0.74; 95% CI, 0.62- 0.89) in patients who had resection of 13 to 16 lymph nodes.15 Similar results were reported by Ou and Zell16 and Varlotto et al.17 Mature results of the ACOSOG Z0030 trial will provide level 1 evidence addressing the question of survival benefit.
Conclusions
The ACOSOG Z0030 study demonstrates that MLND can be performed reliably and safely by thoracic surgeons in both community and academic settings. A minimum of six lymph nodes were resected in 99% and > 10 lymph nodes in 90% of patients. Overall, a median of 18 lymph nodes were resected per patient. In the majority of patients, dissection of N2 stations provided a median of one to four lymph nodes per station. The ACOSOG Z0030 study provides a benchmark for assessment of the adequacy of lymph node harvest in the conduct of pulmonary resection for non-small cell lung cancer. This study demonstrates that the minimum requirements for lymph node staging can be provided in both community and academic centers and should be expected for optimum care of the patient with resectable non-small cell lung cancer.
Supplementary Material
Acknowledgments
Author contributions: Dr Darling: contributed to trial design, protocol development, protocol adherence, acquisition of data, data review, and drafting, revision, and approval of the manuscript.
Dr Allen: contributed to trial design, protocol development, protocol adherence, acquisition of data, data review, and revisions and approval of the manuscript.
Mr Decker: contributed to data quality and analysis, statistical review, and approval of the manuscript.
Dr Ballman: contributed to data analysis, statistical review, and approval of the manuscript.
Dr Malthaner: contributed to acquisition and review of data and approval of the manuscript.
Dr Inculet: contributed to acquisition and review of data and approval of the manuscript.
Dr Jones: contributed to review of data and approval of the manuscript.
Dr McKenna: contributed to acquisition and review of data and approval of the manuscript.
Dr Landreneau: contributed to data collection and review and approval of the manuscript.
Dr Putnam: contributed to acquisition of data, review of data, and approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This study was coordinated by the American College of Surgeons Oncology Group. The authors express their appreciation to the late Robert J. Ginsberg, MD, for his valuable leadership in designing this trial.
Additional information: The e-Appendix can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/139/5/1124/suppl/DC1.
Abbreviations
- ACOSOG
American College of Surgeons Oncology Group
- MLND
mediastinal lymph node dissection
- MLNS
mediastinal lymph node sampling
- SVC
superior vena cava
Footnotes
A complete list of study participants is located in e-Appendix 1.
Funding/Support: This study is supported by funding from the US National Cancer Institute to the American College of Surgeons Oncology Group [Grant U10 CA 76001].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Allen MS, Darling GE, Pechet TT, et al. ACOSOG Z0030 Study Group Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81(3):1013–1020. doi: 10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 2.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109(1):120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 3.Naruke T, Goya T, Tsuchiya R, Suemasu K. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg. 1988;96(3):440–447. [PubMed] [Google Scholar]
- 4.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80(6):2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. discussion 2056. [DOI] [PubMed] [Google Scholar]
- 5.Goldstraw P. Report on the international workshop on intrathoracic staging. London October 1996. Lung Cancer. 1997;18(1):107–111. [Google Scholar]
- 6.De Leyn P, Lardinois D, Van Schil P, et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol. 2007;2(4):357–361. doi: 10.1097/01.JTO.0000263722.22686.1c. [DOI] [PubMed] [Google Scholar]
- 7.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21(6):1029–1034. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg. 2007;84(3):1059–1065. doi: 10.1016/j.athoracsur.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 9.Zhong W, Yang X, Bai J, Yang J, Manegold C, Wu Y. Complete mediastinal lymphadenectomy: the core component of the multidisciplinary therapy in resectable non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;34(1):187–195. doi: 10.1016/j.ejcts.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 10.Massard G, Ducrocq X, Kochetkova EA, Porhanov VA, Riquet M. Sampling or node dissection for intraoperative staging of lung cancer: a multicentric cross-sectional study. Eur J Cardiothorac Surg. 2006;30(1):164–167. doi: 10.1016/j.ejcts.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Keller SM, Adak S, Wagner H, Johnson DH. Mediastinal lymph node dissection improves survival in patients with stages II and IIIA non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg. 2000;70(2):358–365. doi: 10.1016/s0003-4975(00)01673-8. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Huang ZF, Wang SY, Yang XN, Ou W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36(1):1–6. doi: 10.1016/s0169-5002(01)00445-7. [DOI] [PubMed] [Google Scholar]
- 13.Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg. 2005;27(4):680–685. doi: 10.1016/j.ejcts.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Izbicki JR, Passlick B, Karg O, et al. Impact of radical systematic mediastinal lymphadenectomy on tumor staging in lung cancer. Ann Thorac Surg. 1995;59(1):209–214. doi: 10.1016/0003-4975(94)00717-L. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig MS, Goodman M, Miller DL, Johnstone PAS. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128(3):1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 16.Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol. 2008;3(8):880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 17.Varlotto JM, Recht A, Nikolov M, Flickinger JC, Decamp MM. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115(4):851–858. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.