Abstract
Objective:
To investigate the relationship between cerebral blood flow and dementia in older stroke survivors and subjects with Alzheimer disease (AD).
Methods:
This cohort study used arterial spin labeling MRI at 3 T to examine cerebral blood flow (CBF). We scanned 39 patients 6 years after stroke. They were older than 75 years at the time of stroke and free of dementia 3 months poststroke, with 8 subsequently developing dementia. We also scanned 17 subjects with AD and 29 healthy control subjects. We determined the perfusion in regions of interest (ROIs). Hippocampal volume was also measured using a previously validated automated procedure.
Results:
The gray matter/white matter CBF ratio was reduced globally in the poststroke dementia (PSD) group (1.55 SD = 0.12) relative to control subjects (1.78 SD = 0.18; p = 0.03). The CBF ratio in a parietal ROI was reduced in the AD (1.34 SD = 0.31; p = 0.003), PSD (1.32 SD = 0.22; p = 0.041), and poststroke no-dementia (PSND) (1.44 SD = 0.34; p = 0.014) groups relative to that of control subjects (1.70 SD = 0.32). In subjects without stroke, the best predictor of dementia was hippocampus volume, whereas in the stroke group, it was the global CBF gray matter/white matter ratio. Hippocampus volume was not significantly different between the AD and PSD groups, and both had reduced hippocampi relative to those of control subjects and the PSND group.
Conclusions:
We found evidence for both vascular and AD pathology in PSD, suggesting that both the direct impact of the stroke and subsequent development of AD-type changes play a role in the etiology of PSD.
Dementia is common in people with stroke and may be preexisting in 10% of subjects and a direct consequence of the stroke in a further 15%.1 However, stroke is also a risk factor for dementia in the long term.1,2
Alzheimer pathology may play an important role in the development of poststroke dementia (PSD), and risk factors for Alzheimer disease (AD) overlap with those for stroke.3,4 The incidence of AD increases strongly with age5 and may play a greater role in development of dementia in older stroke survivors.
In AD, there is a well-established pattern of hypoperfusion or hypometabolism in the posterior cingulate, temporoparietal, and prefrontal cortices, with relative sparing of the primary sensory cortices.6
Reduced cerebral blood flow (CBF) has been reported in vascular dementia (VaD) compared with that in control subjects.7–9 Likewise, vascular cognitive decline has been associated with global hypoperfusion8,10 and specific reductions in the thalamus in studies of small vessel disease.7,9
The aim of this study was to measure CBF using arterial spin labeling MRI in older stroke survivors (aged older than 75 years) to compare CBF in those with vs those without subsequent dementia. We also compared them with subjects with AD and healthy control subjects. We hypothesized that the PSD group would have globally low perfusion, with thalamic deficits (possibly associated with subcortical stroke), whereas we expected the AD group to show hypoperfusion in the posterior parietal and prefrontal cortices. We expected medial temporal lobe atrophy to be marked in AD but also to be present in PSD because of the presence of Alzheimer-type pathology in this older group.
METHODS
Subjects.
Stroke patients taking part in this imaging investigation were from a cohort described previously,11 which has been followed longitudinally. These subjects were recruited between 2000 and 2002 from representative hospital-based stroke registers in Tyneside and Wearside (UK) and were older than 75 years at the time of the stroke, defined using the World Health Organization criteria. Clinical and CT scan evidence-based diagnoses of stroke and the Oxfordshire Community Stroke Project (OCSP) classification12 were recorded. Patients were screened at 3 months to exclude dementia. Subjects underwent an annual clinical and cognitive assessment, and all surviving stroke subjects were invited for a MRI scan on average 6.2 (SD 0.86) years after the initial stroke. Of the original 355 stroke patients, 135 were still undergoing regular study follow-up (153 had died and 67 had refused follow-up). Of these 135 subjects, 41 agreed to an MRI scan (49 refused and 45 had contraindications to imaging).
Dementia at the time of imaging was diagnosed in the stroke group using the DSM-III-R criteria, and subjects were classified as either poststroke no dementia (PSND) (n = 33) or poststroke dementia (PSD) (n = 8). For comparison, additional subjects of similar age with AD (n = 26) were recruited from clinical old age psychiatry, geriatric medicine, and neurology services, along with 30 healthy subjects, drawn from friends and spouses of patients and a register of subjects who had previously indicated a willingness to participate in research. All subjects with AD fulfilled the criteria for probable AD according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria.13 No subjects had contraindications for MRI.
Standard protocol approvals, registrations, and patient consents.
All participants gave written informed consent for the study. The appropriate local research ethics committees had granted ethical approval for the study.
Neuropsychologic methods.
To evaluate global cognitive performance, we used the Cambridge Cognitive Examination–Revised, Section B (CAMCOG-R),14 which is a standardized paper-and-pencil test for global cognition (maximum score, 105). It has subscores for several cognitive domains including executive function and includes the Mini-Mental State Examination (MMSE). Memory was assessed with the Rey Auditory Verbal Learning Test15 and the Boston Naming Test16 was given as a measure of language function.
MRI acquisition and processing.
MRI was obtained on a 3-T whole-body Philips Achieva System (Philips Medical Systems, Best, the Netherlands). A T1-weighted anatomic volume with 1-mm isotropic resolution and fluid-attenuated inversion recovery (FLAIR) image with 3-mm slice thickness were collected using a standard clinical protocol. CBF images were acquired from an arterial spin labeling sequence17 (flow-sensitive alternating inversion recovery) with 4 contiguous slices (6-mm-thick, in-plane resolution, 4 × 4 mm).
The MRI scans were processed with SPM software to identify gray matter (GM) and white matter (WM) and CSF components on the image. We then calculated total brain volume (TBV) as the sum of GM and WM volume (in mL) and intracranial volume (ICV) as the sum of GM and WM plus CSF. The TBV/ICV ratio was then calculated as an estimate of brain atrophy. The hippocampus volume was also determined. Total white matter hyperintensity (WMH) volume was calculated from the FLAIR images. Analysis was performed blind to subject group. CBF was calculated in GM within regions of interest (ROIs) (figure) and throughout the imaged brain and also within a WM mask. To avoid brain atrophy causing artificially low CBF values, we only measured CBF within voxels with at least 50% GM or WM. We then calculated the ratio of GM CBF to WM CBF in the whole brain and these ROIs (GM/WM CBF). We used a ratio of CBF in GM to CBF in WM because there is considerable between-individual variability in global CBF due to physiologic factors.18 Accounting for this variability by normalizing to a reference region decreases between-subject variance. A previous study found that using a GM/WM CBF ratio allowed subjects with AD to be detected with a greater degree of sensitivity,19 and the WM has been shown to be a good reference region in older subjects.20
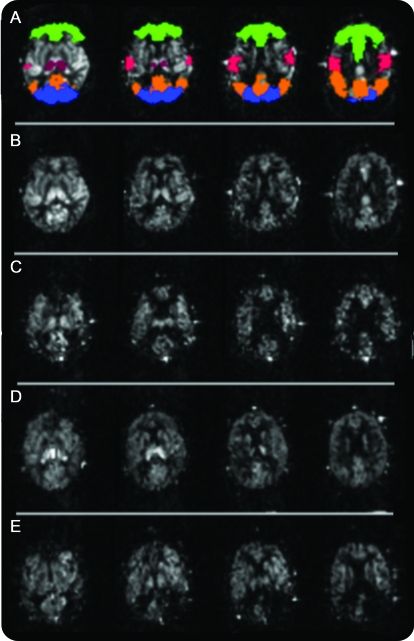
Figure. Typical perfusion images from a subject in each group.
(A) Normal subject with region map overlaid. Green, frontal; orange, parietal; red, central gyrus; blue, occipital; dark red, thalamus. (B) Normal subject (80-year-old woman). (C) Subject with Alzheimer disease (AD) (84-year-old woman). (D) Poststroke no dementia subject (80-year-old woman). (E) Poststroke dementia (PSD) subject (81-year-old woman). Note parietal hypoperfusion in the subject with AD and asymmetric perfusion in PSD due to a large infarct.
Full details of the MRI acquisition and processing are found in appendix e-1 on the Neurology® Web site at www.neurology.org.
Statistics.
We used the Kolmogorov-Smirnov test to assess normality of the data. CAMCOG-R, MMSE, and Rey scores were not normally distributed, and hence we used the Kruskal-Wallis test to compare all groups, followed by Mann-Whitney post hoc tests to compare control vs PSND and AD vs PSD groups. The Fisher exact test was used to compare sex between groups. WMH volumes were normally distributed after a log transform. All other MRI variables were normally distributed. We compared values between groups using analysis of variance, followed by Gabriel post hoc tests. When MRI scans were missing or not usable (due to movement), subjects were excluded from analysis. For data missing for individual variables, the subject was only excluded from the relevant analysis.
RESULTS
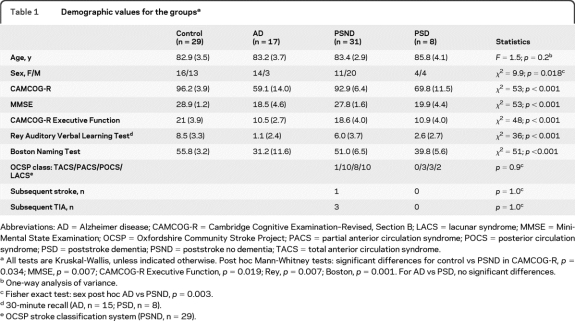
Demographic details are shown in table 1. There were no significant differences in the numbers of men and women between stroke with dementia vs without dementia or between AD and PSD or control groups, although the AD group did have more women than the PSND group did. Cognitive measures did not differ between the PSD and AD groups; however, cognitive measures were slightly lower in the PSND group than for control subjects. Baseline stroke classification was 28 PSND and 6 PSD with ischemic infarction, one PSD and one PSND with hemorrhagic infarct, one PSD with intracerebral hemorrhage, and 3 PSND with transient ischemic attacks.
Table 1.
Demographic values for the groupsa
Abbreviations: AD = Alzheimer disease; CAMCOG-R = Cambridge Cognitive Examination–Revised, Section B; LACS = lacunar syndrome; MMSE = Mini-Mental State Examination; OCSP = Oxfordshire Community Stroke Project; PACS = partial anterior circulation syndrome; POCS = posterior circulation syndrome; PSD = poststroke dementia; PSND = poststroke no dementia; TACS = total anterior circulation syndrome.
All tests are Kruskal-Wallis, unless indicated otherwise. Post hoc Mann-Whitney tests: significant differences for control vs PSND in CAMCOG-R, p = 0.034; MMSE, p = 0.007; CAMCOG-R Executive Function, p = 0.019; Rey, p = 0.007; Boston, p = 0.001. For AD vs PSD, no significant differences.
One-way analysis of variance.
Fisher exact test: sex post hoc AD vs PSND, p = 0.003.
30-minute recall (AD, n = 15; PSD, n = 8).
OCSP stroke classification system (PSND, n = 29).
To investigate any bias arising from patient participation, we analyzed demographics across the whole stroke patient cohort. In those patients who declined to participate, the incidence of dementia (14 of 49) was not significantly different from that of the group who underwent MRI (8 of 41) (Fisher exact test, p = 0.5). Of those with contraindications, 19 of 45 had dementia, a higher proportion than the 8 of 41 in the imaged group (p = 0.038). In the group without MRI, the numbers of reported further strokes (5 of 94) and TIAs (16 of 94) were not significantly different from those of the group with MRI (stroke, 1 of 41; TIA, 3 of 41). There was a difference (Fisher exact test, p = 0.015) in OCSP classification between the original cohort (lacunar syndrome/partial anterior circulation syndrome/total anterior circulation syndrome/posterior circulation syndrome = 104/132/18/36) vs those with MRI (lacunar syndrome/partial anterior/circulation syndrome/total anterior circulation syndrome/posterior circulation syndrome = 12/13/1/13) with a greater proportion of posterior vs anterior syndrome in the MRI group. Six subjects (all with AD) did not have T1-weighted anatomic scans because they did not tolerate the whole scan protocol and were excluded from the analysis. Perfusion data were not usable in one control subject, 4 subjects with AD, and 2 PSND subjects because of uncorrectable motion artifacts.
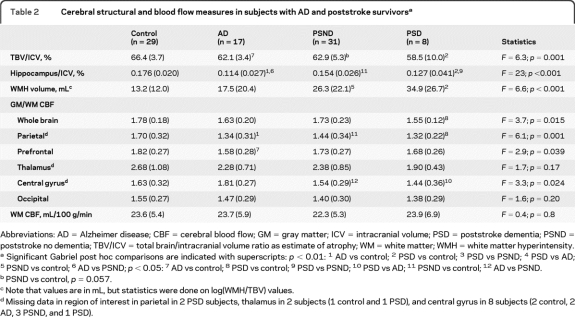
The figure shows examples of perfusion images. Table 2 shows magnetic resonance variables compared between groups. Total brain volume was reduced in the AD and PSD groups compared with that in control subjects. Hippocampus volume was reduced in all 3 patient groups compared with that in control subjects and in the PSD compared with the PSND group. WMH volumes, as expected, were increased in both stroke groups, but not in the AD group.
Table 2.
Cerebral structural and blood flow measures in subjects with AD and poststroke survivorsa
Abbreviations: AD = Alzheimer disease; CBF = cerebral blood flow; GM = gray matter; ICV = intracranial volume; PSD = poststroke dementia; PSND = poststroke no dementia; TBV/ICV = total brain/intracranial volume ratio as estimate of atrophy; WM = white matter; WMH = white matter hyperintensity.
Significant Gabriel post hoc comparisons are indicated with superscripts: p < 0.01: 1 AD vs control; 2 PSD vs control; 3 PSD vs PSND; 4 PSD vs AD; 5 PSND vs control; 6 AD vs PSND; p < 0.05: 7 AD vs control; 8 PSD vs control; 9 PSD vs PSND; 10 PSD vs AD; 11 PSND vs control; 12 AD vs PSND.
PSND vs control, p = 0.057.
Note that values are in mL, but statistics were done on log(WMH/TBV) values.
Missing data in region of interest in parietal in 2 PSD subjects, thalamus in 2 subjects (1 control and 1 PSD), and central gyrus in 8 subjects (2 control, 2 AD, 3 PSND, and 1 PSD).
Whole-brain GM/WM CBF was reduced in the PSD group compared with that in the control group. Parietal perfusion was reduced in all 3 patient groups, whereas prefrontal perfusion was reduced in the AD group and central gyrus perfusion was reduced in the PSND and PSD groups relative to that in the AD group. There were no significant differences in thalamic perfusion between any of the groups. We also investigated CBF in the stroke group according to the OCSP classification and found no CBF differences between the different classifications, either in the entire stroke group or in the PSD and PSND groups separately (appendix e-2). In the PSD group, CBF did not correlate with time of dementia onset.
We next divided the subjects into stroke (PSD and PSND) and nonstroke (control and AD) groups and performed a binary logistic regression of predictors of dementia (AD or PSD) with fixed independent variables of age and sex and stepwise variables of TBV, WMH volume, hippocampus volume, and global GM/WM CBF. We found in the stroke group that CBF (Wald = 3.9; p = 0.047) and in the nonstroke group that hippocampus volume (Wald = 7.9, p = 0.005) predicted dementia. Again, by analyzing the stroke and nonstroke groups separately, a linear regression of predictors of CAMCOG-R score with fixed independent variables of age and sex and stepwise variables of TBV, WMH volume, hippocampus volume, and global GM/WM CBF found in the stroke group that age (b = −0.37; p = 0.016) and CBF (b = 0.36; p = 0.020) and in the nonstroke group that hippocampus volume (b = 0.76; p < 0.001) and female sex (b = 0.202; p = 0.044) predicted a low CAMCOG-R score. By adding the 3-month poststroke CAMCOG-R score to the regression for the stroke patients, predictors of CAMCOG-R score at the time of imaging were 3-month CAMCOG-R score (b = 0.60; p = 0.001) and hippocampus volume (b = 0.33; p = 0.023).
A stepwise regression model (with variables of TBV, WMH volume, hippocampus volume, age, and sex) found that the best predictor of reduced global CBF was hippocampus volume in both the stroke (b = 0.38; p = 0.017) and nonstroke (b = 0.58; p < 0.001) groups.
For 16 stroke subjects (2 with dementia), we had a visual rating of medial temporal lobe atrophy (MTA) from an MRI scan obtained at baseline.21 The MTA rating at baseline correlated with hippocampus volume at a follow-up scan (r = −0.70; p = 0.003) and with parietal CBF (r = −0.51; p = 0.041) but not with global CBF or any other regional CBF.
DISCUSSION
We found that the PSD group has more evidence of vascular pathology indicated by WMH volumes and lower cortical perfusion. In the AD group, there was reduced perfusion specifically in the parietal and prefrontal areas, consistent with previous studies,6 but no increase in WMH volume.
Differences between the PSD and AD groups are that, relative to control subjects, the stroke group had lower perfusion throughout the whole GM and specifically reduced perfusion relative to that in the AD group in the central gyrus region, which is typically spared in AD. WMH volume, typical of small vessel disease, was increased in stroke but not in AD, and the PSND group had a total brain volume of the same size as that of the AD group (and almost significantly different from that of the control group) (table 2), whereas their hippocampus size, although smaller than that for the control group, was larger than that in the AD group.
The PSND group showed subtle changes in cognitive function, with low CBF rather than hippocampus volume predicting a low CAMCOG-R score in the stroke group. The data in the stroke group, i.e., the presence of WMH, lower brain volume, and decreased cognitive ability, are in accord with the suggestion2 that stroke increases the risk of dementia because vascular damage increases the susceptibility of the brain to further insult. Hence, either a second stroke, which markedly increases dementia incidence,1 or development of Alzheimer pathology will lead to earlier dementia or cognitive decline than would otherwise occur. None of the PSD group and only one of the PSND group reported a subsequent stroke, and hence in this cohort, development of AD seems a more likely cause of dementia or cognitive decline.
Hippocampus atrophy has been found to be a good surrogate of AD pathology in postmortem studies.22 In the PSND group, there was evidence of mild hippocampus atrophy and hypoperfusion in the parietal cortex, both typical of early AD.6,22 MTA just after the stroke was correlated specifically with parietal hypoperfusion at 6 years, again supporting the idea that the atrophy is related to AD. Furthermore, the addition of baseline cognition to the regression changed the imaging predictor of CAMCOG-R score from CBF to hippocampus, perhaps suggesting that stroke caused a simultaneous lowering in perfusion and cognitive ability and that subsequent cognitive decline is related to hippocampus atrophy. Some of the cognitive decline in the stroke group is likely to be due to AD-type changes, with the probability that the ischemic changes (increased WMH volume and tendency to reduced perfusion) has reduced the cognitive reserve, making the brain more sensitive to the additional pathology. The best predictor of low global CBF in the stroke group was hippocampus volume, again suggesting that presence of AD-type pathology may play a causal role in CBF reduction. Incidence of AD shows a stronger increase with age than does incidence of VaD,23 and this may explain the relatively strong presence of Alzheimer-type neuroimaging features in the stroke group of this old age group. The strong correlation of baseline CAMCOG-R score with 6-year CAMCOG-R score in the stroke group suggests that baseline cognitive testing shortly after stroke may be a good predictor of relative cognitive performance a number of years later.
Research on the relationship of CBF to dementia in vascular disease has mostly been done in subcortical ischemic vascular disease (SIVD). Methodology has varied, with voxel-based analysis being used to find a reduction in the thalamus7,9 and parietal and temporal lobes.9 An ROI study found reductions in parietal, temporal, and frontal lobes in SIVD similar to those in AD compared with those in control subjects8 with a slightly greater magnitude of reduction in parietal CBF in SIVD. A study of a larger cohort of stroke subjects on average 1 year after stroke looked at whole-hemisphere CBF and showed that bilateral CBF reduction was associated with cognitive decline.10 Our study is one of the few to examine CBF in elderly stroke survivors long term after stroke, and its findings are broadly consistent with these previous studies, with parietal and global deficits in CBF related to dementia, and in the cognitively normal stroke group, there are CBF deficits 6 years poststroke.
A number of studies have linked perfusion or metabolic changes to subcortical GM structures, including the thalamus in VaD.7,9,24,25 We did not find significant differences in thalamic perfusion between groups; however, the thalamic perfusion was lowest in the PSD group. There may be some variation in perfusion deficits according to different stroke type (the 2 poststroke studies that found thalamic perfusion reductions were of small vessel disease); however, not all studies examined the thalamus. We did not find any differences in thalamic perfusion between the different stroke classifications albeit with relatively low numbers in each group. It may be that the heterogeneity of our stroke group reduced the significance of any thalamic perfusion changes.
Limitations of the study include the few stroke subjects with dementia. Given the heterogeneity of stroke, this means that the PSD group is not necessarily representative of patients with PSD as a whole. Because this study investigated poststroke delayed dementia, imaging was, of necessity, performed 6 years after the initial stroke. There is thus a survivor effect, with those subjects experiencing ill health being more likely to have dropped out. This may have biased the profile of the remaining subjects with dementia to a more Alzheimer type, because those with severe vascular disease dropped out. Unfortunately, we did not have any postmortem or Pittsburgh compound B amyloid imaging confirmation of Alzheimer-type pathology. The MRI group had a relatively large proportion of subjects with posterior syndrome stroke compared with the original cohort, which may also have biased the results toward a more posterior deficit in CBF. Although we tried to recruit all the surviving stroke subjects, a number of them were too ill to tolerate imaging, and incidence of dementia was higher in this group. It is likely that this group with poorer health and greater dementia incidence may have had a lower CBF than those with imaging, and hence our findings probably underestimate the degree of hypoperfusion in poststroke survivors.
Strengths of the study include a well-characterized group of older poststroke survivors who did not have dementia immediately after stroke and well-matched comparison groups of subjects with AD and healthy control subjects. We used information from the anatomic image to identify GM pixels in the perfusion image, and thus changes in perfusion values should represent changes in CBF rather than brain atrophy.
We found evidence of both vascular and AD-type changes in the PSD group, suggesting that both the direct impact of the stroke and, more importantly, the subsequent development of AD in a compromised brain play a role in the etiology of PSD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and families for their cooperation in the investigation of this study; Michelle Widdrington, Carein Todd, and Jean Scott for assistance in managing and screening the cohort; and the North East Dementias and Neurodegenerative Diseases Research Network (NEDeNDRoN) staff for help in recruiting the subjects with AD and control subjects.
Supplemental data at www.neurology.org
- AD
- Alzheimer disease
- CAMCOG-R
- Cambridge Cognitive Examination–Revised, Section B
- CBF
- cerebral blood flow
- DSM-III-R
- Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- FLAIR
- fluid-attenuated inversion recovery
- GM
- gray matter
- ICV
- intracranial volume
- MMSE
- Mini-Mental State Examination
- MTA
- medial temporal lobe atrophy
- OCSP
- Oxfordshire Community Stroke Project
- PSD
- poststroke dementia
- PSND
- poststroke no dementia
- ROI
- region of interest
- SIVD
- subcortical ischemic vascular disease
- TBV
- total brain volume
- VaD
- vascular dementia
- WM
- white matter
- WMH
- white matter hyperintensity
DISCLOSURE
Dr. Firbank and Dr. He report no disclosures. Dr. Blamire receives research support from the Medical Research Council UK, EPSRC, Cancer Research UK, British Heart Foundation, and Sir Jules Thorn Charitable Trust. Dr. Singh and P. Danson report no disclosures. Prof. Kalaria has served on a scientific advisory board for the Alzheimer's Research Trust UK; serves on editorial advisory boards for Alzheimer's Disease and Associated Disorders, European Neurology, NeuroReport, and Behavioral and Brain Functions; has received speaker honoraria from Pfizer Inc; and receives research support from the Alzheimer's Research Trust. Prof. O'Brien serves on scientific advisory boards for GE Healthcare, Servier, and Bayer Schering Pharma; has received speaker honoraria from Pfizer Inc, GE Healthcare, Eisai Inc., Shire plc, Lundbeck Inc., Eli Lilly & Company, and Novartis; serves on the editorial board for Psychological Medicine and as Deputy Editor of International Psychogeriatrics and served on the editorial board of the American Journal of Geriatric Psychiatry; receives publishing royalties for Dementia, 3rd ed. (Hodder Arnold, 2005), Dementia, 4th ed. (Hodder Arnold, 2010), and Dementia with Lewy Bodies and Parkinson's Disease Dementia (Taylor and Francis, 2006); serves as a consultant for GE Healthcare, Servier, and Bayer Schering Pharma; and receives research support from the Medical Research Council UK, the Parkinson's Disease Society, the Alzheimer's Research Trust, NHS Trust, Sir Jules Thorn Charitable Trust, Wellcome Trust, and NIHR.
REFERENCES
- 1. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurology 2009; 8: 1006– 1018 [DOI] [PubMed] [Google Scholar]
- 2. Sawa GM, Stephan BCM. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke 2010; 41: e41– e46 [DOI] [PubMed] [Google Scholar]
- 3. de la Torre JC. Vascular risk factor detection and control may prevent Alzheimer's disease. Ageing Res Rev 2010; 9: 218– 225 [DOI] [PubMed] [Google Scholar]
- 4. Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev 2010; 68 (suppl 2): S74– S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore longitudinal study of aging. Neurology 2000; 54: 2072– 2077 [DOI] [PubMed] [Google Scholar]
- 6. Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicentre FDG PET. Neuroimage 2002; 17: 302– 316 [DOI] [PubMed] [Google Scholar]
- 7. Kato H, Yoshikawa T, Oku N, et al. Statistical parametric analysis of cerebral blood flow in vascular dementia with small-vessel disease using Tc-HMPAO SPECT. Cerebrovasc Dis 2008; 26: 556– 562 [DOI] [PubMed] [Google Scholar]
- 8. Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 2009; 5: 454– 462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shim YS, Yang DW, Kim BS, Shon YM, Chung YA. Comparison of regional cerebral blood flow in two subsets of subcortical ischemic vascular dementia: statistical parametric mapping analysis of SPECT. J Neurol Sci 2006; 250: 85– 91 [DOI] [PubMed] [Google Scholar]
- 10. Mori S, Sadoshima S, Ibayashi S, Lino K, Fujishima M. Relation of cerebral blood flow to motor and cognitive functions in chronic stroke patients. Stroke 1994; 25: 309– 317 [DOI] [PubMed] [Google Scholar]
- 11. Stephens S, Kenny RA, Rowan E, et al. Association between mild vascular cognitive impairment and impaired activities of daily living in older stroke survivors without dementia. J Am Geriatr Soc 2005; 53: 103– 107 [DOI] [PubMed] [Google Scholar]
- 12. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable sub type of cerebral infarction. Lancet 1991; 337: 1521– 1526 [DOI] [PubMed] [Google Scholar]
- 13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34: 939– 944 [DOI] [PubMed] [Google Scholar]
- 14. Roth M, Tym E, Mountjoy CQ, et al. CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986; 149: 698– 709 [DOI] [PubMed] [Google Scholar]
- 15. Rey A. l'Examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 16. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 17. Kim SG, Tsekos NV. Perfusion imaging by a flow-sensitive alternating inversion recovery (FAIR) technique: application to functional imaging. Magn Reson Med 1997; 37: 425– 435 [DOI] [PubMed] [Google Scholar]
- 18. Wu WC, Edlow BL, Elliot MA, Wang JJ, Detre JA. Physiological modulations in arterial spin labeling perfusion magnetic resonance imaging. IEEE Trans Med Imaging 2009; 28: 703– 709 [DOI] [PubMed] [Google Scholar]
- 19. Yoshiura T, Hiwatashi A, Noguchi T, et al. Arterial spin labelling at 3-T MR imaging for detection of individuals with Alzheimer's disease. Eur Radiol 2009; 19: 2819– 2825 [DOI] [PubMed] [Google Scholar]
- 20. Borghammer P, Jonsdottir KY, Cumming P, et al. Normalization in PET group comparison studies: the importance of a valid reference region. Neuroimage 2008; 40: 529– 540 [DOI] [PubMed] [Google Scholar]
- 21. Firbank MJ, Burton EJ, Barber R, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging 2007; 28: 1664– 1669 [DOI] [PubMed] [Google Scholar]
- 22. Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology 2002; 58: 1476– 1482 [DOI] [PubMed] [Google Scholar]
- 23. Brayne C, Gill C, Huppert FA, et al. Incidence of clinically diagnosed subtypes of dementia in an elderly population: Cambridge Project for Later Life. Br J Psychiatry 1995; 167: 255– 262 [DOI] [PubMed] [Google Scholar]
- 24. Kerrouche N, Herholz K, Mielke R, Holthoff V, Baron JC. 18FDG PET in vascular dementia: differentiation from Alzheimer's disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab 2006; 26: 1213– 1221 [DOI] [PubMed] [Google Scholar]
- 25. Seo SW, Cho SS, Park A, Chin J, Na DL. Subcortical vascular versus amnestic mild cognitive impairment: comparison of cerebral glucose metabolism. J Neuroimaging 2009; 19: 213– 219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.