Abstract
Objectives:
MRI white matter hyperintensity (WMH) volume is associated with cognitive impairment. We hypothesized that specific loci of WMH would correlate with cognition even after accounting for total WMH volume.
Methods:
Subjects were identified from a prospective community-based study: 40 had normal cognition, 94 had mild impairment (defined here as a Clinical Dementia Rating [CDR] score of 0.5 without dementia), and 11 had mild Alzheimer's dementia. Factor analysis of a 22-item neuropsychological battery yielded 4 factors (episodic memory, executive function, spatial skills, and general knowledge). MRI WMH segmentation and analysis was performed using FreeSurfer software.
Results:
Higher WMH volume was independently associated with lower executive function and episodic memory factor scores. Voxel-based general linear models showed loci where WMH was strongly inversely associated with specific cognitive factor scores (p < 0.001), controlling for age, education, sex, APOE genotype, and total WMH volume. For episodic memory, clusters were observed in bilateral temporal-occipital and right parietal periventricular white matter, and the left anterior limb of the internal capsule. For executive function, clusters were observed in bilateral inferior frontal white matter, bilateral temporal-occipital and right parietal periventricular white matter, and the anterior limb of the internal capsule bilaterally.
Conclusions:
Specific WMH loci are closely associated with executive function and episodic memory, independent of total WMH volume. The anatomic locations suggest that WMH may cause cognitive impairment by affecting connections between cortex and subcortical structures, including the thalamus and striatum, or connections between the occipital lobe and frontal or parietal lobes.
There is growing recognition that ischemic brain lesions are a significant contributor to cognitive impairment and that many cases of dementia are mixed, with a cerebrovascular component.1 Ischemic white matter lesions, seen on MRI as white matter hyperintensity (WMH), have previously been associated with decreased performance on neuropsychological testing,2,3 and risk of mild cognitive impairment4 and dementia.5 It has been hypothesized that WMH interfere with cognitive processing by impairing the speed or fidelity of signal transmission through affected areas.6 The direct evidence to support this hypothesis is scant, however.6
We reasoned that if WMH impair white matter function, then clinical impairments should be associated with WMH in discrete loci involving white matter tracts connecting cortical networks serving those clinical functions. For example, WMH in specific occipito-parietal periventricular regions have previously been associated with gait impairment.7 Similarly, specific regions of abnormal fractional anisotropy (FA) have been identified in the frontal white matter that are associated with executive dysfunction in the hereditary small vessel disease cerebral autosomal dominant arteriopathy with stroke and ischemic leukoencephalopathy (CADASIL).8
In order to better understand the relationship between WMH location and cognitive impairment, we determined the correlation between WMH involvement in specific regions and neuropsychological test performance in subjects participating in a prospective study of cognitive decline.
METHODS
Subject population.
Subjects were drawn from a longitudinal prospective cohort study of predictive factors for transition to dementia. The details of study subject recruitment and assessment have been previously published.9 Study subjects were recruited through community advertising seeking older individuals with and without memory impairment. To be included in the study, all participants had to be 65 or older and to have an informant as a collateral source of information. At entry to the parent study, persons with dementia, major vascular risk factors (atrial fibrillation or diabetes mellitus requiring insulin), history of stroke, or clinical diagnosis of cognitive impairment due to medical disorders such as hypothyroidism were excluded.
A semi-structured interview by an experienced clinician was used to evaluate the subjects at study entry and at each subsequent year, as previously described,9,10 to generate a Clinical Dementia Rating (CDR) score.11 For subjects who developed dementia during the study, the underlying disorder was diagnosed by consensus of the investigators using all available study information. Dementia was defined using criteria from the DSM-IV,12 probable Alzheimer disease (AD) was diagnosed using the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria,13 and vascular dementia was diagnosed according to the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche en l'Enseignement en Neurosciences criteria.14
Those with mildly impaired cognition at the time of MRI, defined as a CDR score of 0.5 without dementia, are defined here as mild cognitive impairment (MCI).4,15 The distribution of CDR sum of boxes scores among the mildly impaired subjects was broad (see table 1). At the mild end of the spectrum, many subjects would not meet psychometric cutoffs commonly used to select MCI subjects in epidemiologic studies and clinical trials,16 and could be considered early MCI. The subjects at the more impaired end of the spectrum (i.e., CDR sum of boxes >2) are comparable to MCI subjects recruited in these settings, based on likelihood of progression to a diagnosis of AD.9 We use MCI here to refer to the entire group of mildly impaired subjects.
Table 1.
Study population characteristicsa
Abbreviations: CDR = Clinical Dementia Rating; MCI = mild cognitive impairment; WMHr = total white matter hyperintensity volume, expressed as percentage of intracranial volume.
Values are percentages, mean ± SD, or median (25th, 75th percentile). p Values are by Fisher exact test, testing for significant differences across all 3 groups.
Neuropsychological assessments.
A 22-item neuropsychological battery was administered to all study subjects.17 The median time between MRI and neuropsychological testing was 76 days (interquartile range 19–219 days). Details of the battery, including the individual test items, have been previously described (table e-1 on the Neurology® Web site at www.neurology.org).17,18 A factor analysis of the neuropsychological measures yielded a 4-factor representation of the overall test battery: 1) general knowledge, 2) episodic memory, 3) spatial skill, and 4) executive function.17 The factor scores were standardized to have zero mean and unit variance among the cognitively normal study subjects, adjusted for age and education.
MRI measurements.
Subjects underwent MRI on either of 2 1.5-T scanners (Signa, General Electric Medical Systems, Milwaukee, WI).4 A sagittal localizer, coronal T1-weighted (T1W) spoiled gradient recalled (SPGR), and dual echo sequence, yielding proton density-weighted (PDW) and T2-weighted (T2W) images of the whole head, was performed. T1W SPGR sequence parameters were as follows: repetition time (TR) 35 msec, echo time (TE) 5 msec, flip angle 45 degrees, 1.5-mm slice thickness with no interslice gap. Dual echo sequence parameters were as follows: TR 3,000 msec, TE1 30 msec (for PDW images), TE2 80 msec (for T2W images), and slice thickness 3 mm interleaved. The matrix was 256 × 192, and field of view 24 cm, for all sequences.
Previous work in this study population has shown that total WMH volume was associated with the risk of progression from normal cognition to MCI.4 To take advantage of advances in computer processing, we have revised our methods for WMH segmentation, using custom-designed algorithms implemented in the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). Briefly, this processing includes motion correction, removal of nonbrain tissue using a hybrid watershed/surface deformation procedure, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures, intensity normalization, tessellation of the gray matter white matter boundary, automated topology correction, and surface deformation following intensity gradients to optimally segment borders at the location where the greatest shift in intensity defines the transition to the other tissue class.19–23 The structural segmentations were used to identify regions where WMH was possible while excluding regions where WMH does not occur (that is, cortical and subcortical gray matter structures). Final algorithm-generated WMH maps were visually inspected to identify obvious errors. No manual corrections were necessary; however, 13 scans could not be processed because of motion or other artifact. The accuracy of the automated FreeSurfer algorithm was verified by comparison with 10 scans where WMH was independently manually segmented by an experienced rater blinded to the automated algorithm results; the intraclass correlation coefficient was 0.91, indicating excellent agreement.
There were 160 consecutive subjects who underwent MRI as part of the study and had no history of stroke prior to MRI scanning. Exclusion of 13 subjects whose scan data could not be processed left 147 for analysis. The MRI was performed after study entry in 65/147 (44%); dementia had developed in 11 subjects during the preceding median 4.8 years of follow-up (interquartile range 2.9 to 9.1 years). The cause of dementia was probable AD in all 11 subjects.
Statistical analysis.
Total WMH volume was expressed as a percentage of the intracranial volume (WMHr), as in other studies, to account for differences in subject head size.4 Continuous variables were normally distributed, with the exception of WMH, which was right-skewed and therefore analyzed using nonparametric tests. There were no missing data. Characteristics associated with the neuropsychological factor scores or WMHr were determined by t test, Wilcoxon rank sum test, Pearson correlation coefficient, or Spearman correlation coefficient as appropriate. To determine the independent predictors of each of the factor scores, variables associated with the factor score in univariate analysis (p < 0.20) were entered into a linear regression model with backward elimination of nonsignificant variables.
Regional associations between WMH frequency and neuropsychological factor scores were tested using voxel-wise general linear models, implemented in FreeSurfer v4.0.1. We chose to perform MRI voxel-wise analyses of the executive function and episodic memory factor scores only, because those domains have been most consistently associated with WMH in the previous literature. The spatial skills and general knowledge factor scores were not analyzed further because cognitive function in these domains has been less closely associated with WMH-associated cognitive dysfunction in prior studies, and because these factor scores were not associated with total WMH volume in initial analyses. Univariate associations between neuropsychological factor score (as the dependent variable) and the voxel-specific presence or absence of WMH (as the independent variable) were assessed. Because age and years of education were also associated with neuropsychological test performance, we next constructed whole-brain voxel-wise multivariable models additionally controlling for these variables. Finally, in order to explore whether the association of WMH regions with neuropsychological test performance was independent of total WMH volume, final fully adjusted models were created that additionally controlled for WMHr as a covariate.24 In all models we adjusted for multiple comparisons using the False Discovery Rate method with an α of 0.05.25
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board of Massachusetts General Hospital. Written informed consent was obtained from all study participants.
RESULTS
There were 147 subjects who met inclusion criteria and were included in the analysis. There were 40 subjects with normal cognition (CDR = 0), 96 subjects with MCI (CDR 0.5 without dementia), and 11 subjects with mild dementia due to probable AD (all had CDR 1.0). Characteristics according to clinical diagnosis are given in table 1. There was a trend toward higher WMHr in those with MCI or mild dementia (p = 0.07). Age (p = 0.001) and hypertension (p = 0.01) were the only other characteristics associated with WMHr.
Univariate analyses showed that worse scores for executive function and episodic memory were associated with increased age (p ≤ 0.04 for both comparisons), fewer years of education (p < 0.005 for both comparisons), and the presence of 1 or 2 APOE ϵ4 alleles (p ≤ 0.03 for both comparisons). Female sex was associated with higher scores in episodic memory (p = 0.001). Higher WMHr was correlated with lower scores on episodic memory (r = −0.28, p = 0.001) and executive function (r = −0.28, p = 0.001). Fully adjusted models of the relationship between factor scores and WMHr are given in table 2.
Table 2.
Relationship between total WMH volume, expressed as percentage of intracranial volume, in fully adjusted multivariable modelsa
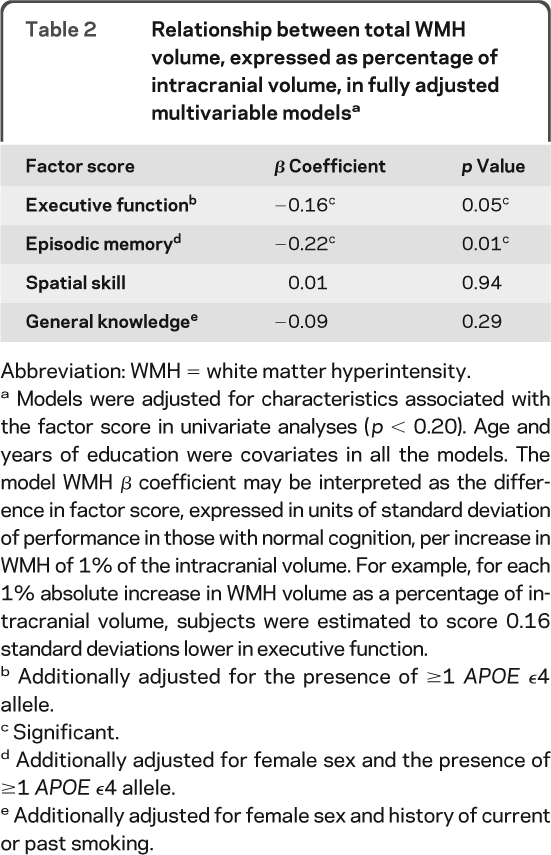
Abbreviation: WMH = white matter hyperintensity.
Models were adjusted for characteristics associated with the factor score in univariate analyses (p < 0.20). Age and years of education were covariates in all the models. The model WMH β coefficient may be interpreted as the difference in factor score, expressed in units of standard deviation of performance in those with normal cognition, per increase in WMH of 1% of the intracranial volume. For example, for each 1% absolute increase in WMH volume as a percentage of intracranial volume, subjects were estimated to score 0.16 standard deviations lower in executive function.
Additionally adjusted for the presence of ≥1 APOE ϵ4 allele.
Significant.
Additionally adjusted for female sex and the presence of ≥1 APOE ϵ4 allele.
Additionally adjusted for female sex and history of current or past smoking.
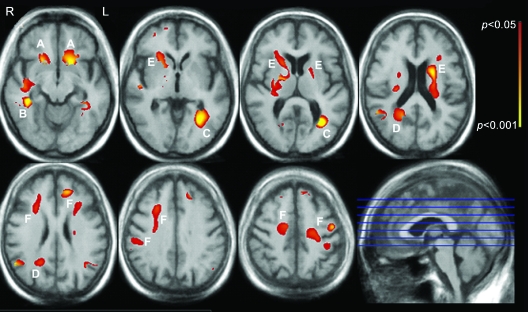
Whole-brain voxel-based analyses were then performed to determine relationships between WMH frequency and neuropsychological factor score at each voxel. Figure 1 shows white matter regions where WMH frequency was associated with the executive function factor score in fully adjusted voxel-wise general linear models that included total WMHr as a covariate. The most strongly correlated clusters of significant voxels were seen in the bilateral inferior frontal white matter (figure 1, label A), temporal-occipital periventricular white matter in the left (figure 1, label B) and right (figure 1, label C) hemispheres, right parietal periventricular white matter (figure 1, label D), and the anterior limb of the internal capsule bilaterally (figure 1, label E), as well as scattered more peripheral clusters in the prefrontal white matter (figure 1, label F).
Figure 1. Regions where white matter hyperintensity (WMH) is associated with executive function.
White matter regions where WMH frequency is independently associated with executive function are color-coded red to yellow according to the strength of the multivariable-adjusted p value (see color bar). Associations are adjusted for age, years of education, APOE genotype, and total WMH volume, and are adjusted for multiple comparisons (see text).
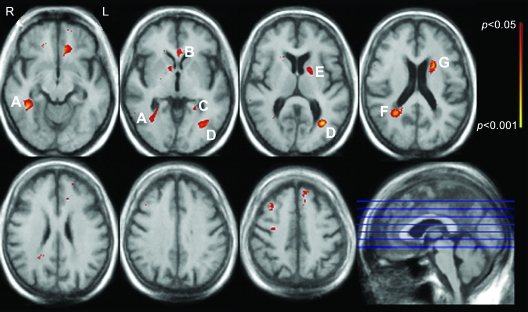
Figure 2 shows white matter regions where WMH frequency was associated with the episodic memory factor score in fully adjusted voxel-wise general linear models. Clusters of significant voxels were seen in the right inferior temporal-occipital white matter (figure 2, label A), left temporal-occipital periventricular white matter (figure 2, labels C and D), right parietal periventricular white matter (figure 2, label F), and the anterior limb of the internal capsule on the left (figure 2, labels E and G).
Figure 2. Regions where white matter hyperintensity (WMH) is associated with episodic memory.
White matter regions where WMH frequency is independently associated with episodic memory are color-coded red to yellow according to the strength of the multivariable-adjusted p value (see color bar). Associations are adjusted for age, years of education, APOE genotype, and total WMH volume, and are adjusted for multiple comparisons (see text).
In a sensitivity analysis, the results were essentially unchanged when restricting the analyzed population to the 96 subjects with MCI (data not shown).
DISCUSSION
In this study, we demonstrate that poor executive function and episodic memory are associated with specific locations of WMH in the cerebral white matter, independent of the total volume of WMH. These observations support the hypothesis that WMH causes vascular cognitive impairment by disrupting cortical connections mediated by specific white matter tracts. We find that WMH are associated with memory impairment as well as executive dysfunction, which has also been observed in other studies.2
Most previous studies have correlated cognitive performance with global WMH volume. Relatively few studies have examined relationships between the topography of WMH frequency and the resulting cognitive deficits, and these clinical-anatomic correlation studies have generally divided the white matter into large regions of interest, such as entire lobes or periventricular vs subcortical regions.2,6,26–30 This approach does not take into account the fact that such large areas will contain multiple white matter tracts coursing to many destinations, however. Despite this limitation, some previous studies have demonstrated clinical-anatomic correlations between WMH and cognitive function. In general, periventricular WMH are more closely associated with cognitive impairment or cognitive decline, possibly suggesting a role for dysfunction of long association tracts.2,26–28 One study of stroke patients suggests that frontal WMH is associated with executive dysfunction and temporal WMH is associated with memory impairment.29 Another study found that left dorsolateral prefrontal cortex WMH is associated with decreased performance on a working memory task.6 In a study using a visual rating scale to grade severity of WMH along a white matter pathway of interest, lesions in cholinergic pathways were shown to be associated with memory impairment.30
An advantage of the present study is the use of smaller regions of interest, namely the individual MRI voxels, to perform regional clinical-anatomic correlations with higher spatial resolution. Using this technique we were able to show strong regional correlations between WMH frequency and dysfunction in specific cognitive domains. We also controlled for total WMH volume when determining regions of correlation, allowing us to determine that these regional correlations are specific to that anatomic area and are independent of total lesion volume. Because the frequency of WMH at any location is partly correlated with total lesion volume, relationships between regional WMH frequency and cognition are confounded by total lesion volume, and may not be valid unless this confounding is considered. By covarying for total lesion volume in our analyses, we can conclude with reasonable certainty that the observed relationships between regional WMH frequency and cognitive functions are equally true in those with low total WMH volume and high total WMH volume.
In this study, we were unable to directly identify the white matter tracts traveling though the regions where associations were seen between WMH frequency and cognition. We did not perform diffusion tensor imaging, which can be used to map white matter tracts. This notwithstanding, we believe it is reasonable to speculate on which tracts might be involved based on tract-tracing studies in monkeys31 and diffusion tensor imaging studies in monkeys32 and humans33–35 that provide insights into the anatomic locations of cerebral white matter tracts. The labeled WMH regions associated with impaired executive function in figure 1 may involve the following fiber tracts: the uncinate fasciculus, linking rostral temporal with orbital and medial prefrontal cortices (figure 1, label A); the inferior longitudinal fasciculus, linking rostral and caudal regions of the ventral visual steam (figure 1, labels B and C); fronto-occipital fibers (figure 1, label C); superior longitudinal fasciculus that links parietal and temporal regions with the prefrontal cortex and that is thought to play a role in spatial processing and spatial attention (figure 1, label C); cingulum bundle, relevant for motivation and behavioral control (figure 1, label D); anterior limb of the internal capsule and, on the left, genu of the internal capsule and striatal fibers coursing in the bundle of Muratoff to the caudate nucleus (figure 1, label E); and multiple types of fibers entering and leaving the cortex (figure 1, label F). The involvement of the anterior limb of the internal capsule is particularly interesting because it conveys information between prefrontal cortex and the medial dorsal and anterior thalamic nuclei, and the prefrontopontine fibers destined for the cerebellar posterior lobe—subcortical areas now known to be relevant for executive function.36,37 The labeled WMH regions associated with impaired episodic memory (figure 2) may involve the following fiber tracts: fronto-occipital fibers and the inferior longitudinal fasciculus (figure 2, labels A and D); rostral and caudal limbs of the arc of the cingulum bundle (figure 2, labels B, C, and F); anterior limb and genu of the internal capsule (figure 2, label E); and bundle of Muratoff (figure 2, label G). The cingulum bundle is implicated in the motivational and emotional aspects of behavior, and it is also thought to contribute to different aspects of mnemonic processing by virtue of its connections with the hippocampus, parahippocampal regions, and retrosplenial cortex, in addition to its connections with the parietal and frontal lobes.31 The finding that WMH in the left anterior limb of the internal capsule, but not the right, was associated with worse episodic memory may reflect the predominant weighting of verbal memory, as opposed to visuospatial memory, in the factor score.
A limitation is that our findings were derived from a population that mostly consisted of subjects with mildly impaired cognition and may not be valid in other populations with different distributions of cognitive impairment. The distribution of cognitive impairment of our subjects reflects the original design of the study, with intentional enrichment of the study population for subjects with MCI.9 A sensitivity analysis showed that the cluster locations were essentially unchanged when the analyzed population was restricted to those with MCI, suggesting that the study findings are robust for this group. Further studies with larger sample sizes will be needed to confirm whether our findings are relevant to patients with overall normal cognition or dementia. Another limitation is that our analysis of the neuropsychological data did not permit investigation of components of memory such as encoding or retrieval, which may depend on different anatomic pathways; future studies will be needed to address this.
Our findings suggest that WMH location should be considered in subsequent studies that attempt to determine the relevance of WMH to cognitive function. Such studies should attempt to confirm the clinical relevance of WMH in the regions identified in our work. Future studies could also incorporate quantitative imaging markers of tissue microstructure such as measures of water proton diffusion, which seems to offer additional clinically relevant information beyond that conferred by the presence or absence of T2 hyperintensity.38 White matter diffusivity reflects microstructural changes that can be caused by processes other than WMH, and has been observed in AD (presumably as a consequence of neurodegeneration with secondary alteration of white matter integrity)39 as well as in normal aging.40 The optimal way to integrate and interpret WMH and diffusion information is a critical subject for future studies. Ultimately, a better understanding of WMH location and its significance could inform the individual clinical evaluation of patients with cognitive impairment, by allowing the discrimination of clinically relevant patterns of WMH from relatively “benign” patterns of WMH sometimes seen in cognitively normal individuals.
Supplementary Material
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Eric Smith and Dr. David Salat.
- AD
- Alzheimer disease
- CADASIL
- cerebral autosomal dominant arteriopathy with stroke and ischemic leukoencephalopathy
- CDR
- Clinical Dementia Rating
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FA
- fractional anisotropy
- MCI
- mild cognitive impairment
- PDW
- proton density-weighted
- SPGR
- spoiled gradient recalled
- T1W
- T1-weighted
- T2W
- T2-weighted
- TE
- echo time
- TR
- repetition time
- WMH
- white matter hyperintensity
Supplemental data at www.neurology.org.
DISCLOSURE
Dr. Smith has received speaker honoraria from the BMJ Group and QuantiaMD; has received an honorarium from the Canadian Conference on Dementia; has served on a scientific advisory board for Genentech, Inc.; serves as an Assistant Editor for Stroke; and receives research support from the NIH, the Alberta Heritage Foundation for Medical Research, Canadian Institutes for Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, and the Hotchkiss Brain Institute. Dr. Salat receives research support from the NIH/NINR. J. Jeng and Dr. McCreary report no disclosures. Dr. Fischl serves on the editorial board of NeuroImage; has served as a consultant for Cephalon, Inc.; and receives research support from the NIH (NCRR/NIBIB/NIA/NINDS) and the Ellison Medical Foundation. Dr. Schmahmann serves on the editorial board of The Cerebellum; is listed as author on a pending patent re: Transcranial magnetic stimulation applied to the cerebellum; receives publishing royalties for The Cerebellum and Cognition (Academic Press, 1997), MRI Atlas of the Human Cerebellum (Academic Press, 2000), and Fiber Pathways of the Brain (Oxford University Press, 2006); and receives research support from the NIH, the Birmingham Foundation, MINDlink Foundation, and the Sidney R. Baer, Jr. Foundation; and his sibling serves as legal counsel to Johnson & Johnson. Dr. Dickerson serves on the editorial board of Hippocampus; and receives research support from the NIH/NIA and the Alzheimer's Association. Dr. Viswanathan has served as a consultant for Athena Diagnostics, Inc.; and receives research support from the NIH/NIA. Dr. Albert serves as a consultant for Genentech, Inc., Eli Lilly and Company, and the Dana Foundation; and receives research support from Pfizer Inc, GE Healthcare, the NIH (NIA/NHLBI), and the Alzheimer's Association. Dr. Blacker serves on the editorial board of Experimental Gerontology; serves as a consultant for ClinicalTrials.gov; and receives research support from the NIH (NINDS/ NIA), the Alzheimer's Association, Cure Alzheimer's Foundation, and Anonymous Foundation. Dr. Greenberg serves on a data safety monitoring board for the NIH/NINDS; has received speaker honoraria from Esteve, Medtronics, Inc., and Pfizer Inc; serves on the editorial boards of Neurology®, Stroke, Cerebrovascular Disease, and the Journal of Alzheimer's Disease and Other Dementias; has served as a consultant for Roche, Janssen Alzheimer Immunotherapy, and Bristol-Myers Squibb; and has received/receives research support from the NIH (NIA/NINDS) and the Alzheimer's Association.
REFERENCES
- 1. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204 [DOI] [PubMed] [Google Scholar]
- 2. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000;47:145–151 [DOI] [PubMed] [Google Scholar]
- 3. Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol 2006;63:246–250 [DOI] [PubMed] [Google Scholar]
- 4. Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol 2008;65:94–100 [DOI] [PubMed] [Google Scholar]
- 5. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 6. Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci 2006;18:418–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology 2000;54:1277–1283 [DOI] [PubMed] [Google Scholar]
- 8. O'Sullivan M, Barrick TR, Morris RG, Clark CA, Markus HS. Damage within a network of white matter regions underlies executive dysfunction in CADASIL. Neurology 2005;65:1584–1590 [DOI] [PubMed] [Google Scholar]
- 9. Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol 2000;57:675–680 [DOI] [PubMed] [Google Scholar]
- 10. Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 2001;7:631–639 [DOI] [PubMed] [Google Scholar]
- 11. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 12. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 14. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260 [DOI] [PubMed] [Google Scholar]
- 15. DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004;63:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–2388 [DOI] [PubMed] [Google Scholar]
- 17. Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology 2007;21:158–169 [DOI] [PubMed] [Google Scholar]
- 18. Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol 2007;64:862–871 [DOI] [PubMed] [Google Scholar]
- 19. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I: segmentation and surface reconstruction. Neuroimage 1999;9:179–194 [DOI] [PubMed] [Google Scholar]
- 20. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207 [DOI] [PubMed] [Google Scholar]
- 21. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355 [DOI] [PubMed] [Google Scholar]
- 22. Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004;23(suppl 1):S69–S84 [DOI] [PubMed] [Google Scholar]
- 23. Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 2007;26:518–529 [DOI] [PubMed] [Google Scholar]
- 24. Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke 2008;39:1127–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15:870–878 [DOI] [PubMed] [Google Scholar]
- 26. Debette S, Bombois S, Bruandet A, et al. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 2007;38:2924–2930 [DOI] [PubMed] [Google Scholar]
- 27. van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry 2006;77:149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soderlund H, Nilsson LG, Berger K, et al. Cerebral changes on MRI and cognitive function: the CASCADE study. Neurobiol Aging 2006;27:16–23 [DOI] [PubMed] [Google Scholar]
- 29. Burton EJ, Kenny RA, O'Brien J, et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke 2004;35:1270–1275 [DOI] [PubMed] [Google Scholar]
- 30. Bocti C, Swartz RH, Gao FQ, Sahlas DJ, Behl P, Black SE. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke 2005;36:2126–2131 [DOI] [PubMed] [Google Scholar]
- 31. Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- 32. Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 2007;130:630–653 [DOI] [PubMed] [Google Scholar]
- 33. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008;40:570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008;44:1105–1132 [DOI] [PubMed] [Google Scholar]
- 35. Makris N, Worth AJ, Sorensen AG, et al. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol 1997;42:951–962 [DOI] [PubMed] [Google Scholar]
- 36. Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003;34:2264–2278 [DOI] [PubMed] [Google Scholar]
- 37. Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008;44:1037–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viswanathan A, Patel P, Rahman R, et al. Tissue microstructural changes are independently associated with cognitive impairment in cerebral amyloid angiopathy. Stroke 2008;39:1988–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salat DH, Tuch DS, van der Kouwe AJ, et al. White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging 2008;31:244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 2005;26:1215–1227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.