Abstract
Alkylating agents have been shown to be very promising for the radiolabelling of oligonucleotides with fluorine-18. In this report we describe the fully automated synthesis of 2-bromo-N-[3-(2-[18F]fluoropyridin-3-yloxy)propyl]acetamide ([18F]FPyBrA) utilizing a modular synthesis unit. Reaction conditions for the coupling of this pyridine-based alkylating agent at the 5′ end of a fully phosphorothioated random 20-mer DNA sequence were optimized to achieve very high radiochemical yields (>90%) and a maximum specific activity of 5–6 GBq/μmoL. The potential for rapid purification by solid phase extraction without need of chromatographic isolation of the radiolabelled oligonucleotide presents an overall benefit for the application of oligonucleotides in preclinical studies and potential clinical applications.
Keywords: Fluorine-18, Oligonucleotides, Alkylation, Automation, Prosthetic group, Modular synthesis unit
1. Introduction
Oligonucleotides (ODN), formulated as antisense ODN (Tamm et al., 2001; Hnatowich, 1999) and aptamers (Brody and Gold, 2000; Perkins and Missailidis, 2007), are very attractive candidates for developing new therapeutic drugs or imaging agents. The antisense technology is based on the specific hybridization of an antisense ODN to its complementary target mRNA, whereas aptamers are ODN designed to bind specifically and with high affinity to a variety of target molecules. ODN can be easily synthesized and the development of the systematic evolution of ligands by exponential enrichment (selex) has accelerated the isolation of ODN sequences capable of recognizing virtually any class of target molecules (Ellington and Szostak, 1990; Tuerk and Gold, 1990). The excellent targeting capacities of ODN make them ideal agents for molecular imaging purposes (Tavitian, 2003), recognizing that there are still many improvements needed for their safe and effective application in vivo (Younes et al., 2002). Various labelling strategies for the synthesis of ODN-based molecular imaging agents have been described for positron emission tomography (PET) (Dollé et al., 1997; Yngve et al., 1999; Hedberg and Langstrom, 1997, 1998; Kuhnast et al., 2000, 2003, 2004; de Vries et al., 2003), single-photon emission tomography (Hjelstuen et al., 1998; Hicke et al., 2006), magnetic resonance imaging (Paunesku et al., 2008), and reflectance-based molecular imaging (Javier et al., 2008). Within the spectrum of non-invasive molecular imaging techniques, PET provides the highest sensitivity and the best capacity for quantitative measurements of physiological processes in vivo at the cellular and molecular level. The short-lived positron emitter fluorine-18 (18F) is a particularly attractive radionuclide for the design and synthesis of PET-radiotracer due to its favourable nuclear properties, high reactivity of the no carrier added [18F]fluoride ion produced by irradiation of 18O-enriched water, and availability of large radioactivity quantities from medical cyclotrons. The low positron energy of 0.64 MeV results in low radiation doses and short tissue range, and the relatively long 109.7 min half-life allows complex and multi-step radiosyntheses and imaging protocols of 4–6 h. However, radiolabelling of biomolecules with 18F presents specific challenges. Direct incorporation of 18F at high specific radioactivity into biomolecules such as peptides, proteins and ODN is hampered by the harsh reaction conditions occurring during labelling reactions. In order to circumvent this obstacle, 18F-labelling of biomolecules including ODN is usually carried out by means of prosthetic groups, also referred to as bifunctional labelling agents (Wester and Schottelius, 2007). Compared to the many prosthetic groups which have been applied to the 18F-labelling of amino acid-based biomolecules like peptides and proteins, only a few prosthetic groups have been used for labelling ODN. The incorporation of 18F into ODN was accomplished via acylation (Hedberg and Langstrom, 1998), alkylation (Dollè et al., 1997; Kuhnast et al., 2000, 2003, 2004; de Vries et al., 2003), photochemical conjugation (Lange et al., 2002), and thiourea formation (Hedberg and Langstrom, 1997). Acylation, photochemical conjugation and thiourea formation are based on the presence of a primary amine group-containing alkyl chain linked to the 5′ position of the ODN, whereas alkylation reactions rely on the reaction with a phosphorothioate group at the 3′ or 5′ end of the ODN backbone. Fig. 1 displays some of the prosthetic groups which have been used for ODN-labelling with 18F; including N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB), 4-([18F]fluoromethyl)phenyl isothiocyanate ([18F]FMPI), 3-azido-5-nitrobenzyl-[18F]fluoride ([18F]ANBF), N-(4-[18F]fluorobenzyl)-2-bromoacetamide ([18F]FBBA) and 2-bromo-N-[3-(2-[18F]fluoropyridin-3-yloxy)propyl]acetamide ([18F]FPyBrA).
Fig. 1.
A selection of prosthetic groups used for ODN-labelling with 18F.
Conjugation of the prosthetic groups [18F]SFB (acylation), [18F]FMPI (thiourea formation) and [18F]ANBF (photochemistry) to ODN (15-mer, 18-mer and 20-mer) containing a hexylamine linker afforded only low to moderate radiochemical yields (RCY) in the range of 7–23% (Hedberg and Langstrom, 1997, 1998; Lange et al., 2002). Significantly improved RCY could be achieved by exploiting the alkylation reactions of ODN (9-mer and 18-mer) containing a phosphorothioate moiety with [18F]FBBA and [18F]FPyBrA, respectively. The conjugation reaction proceeded rapidly under mild conditions in RCY of 45–75% (Kuhnast et al., 2000, 2004). The advantage of alkylation reaction for ODN-labelling was further confirmed by a comparative study of various alkylating agents in a model reaction with adenosine 5′-O-thiomonophosphate (de Vries et al., 2003).
In this report we describe the automated modular synthesis of [18F]FPyBrA based on the robotic synthesis described by Kuhnast et al. (2004) using a commercially available modular synthesis unit. We further describe optimization strategies for the conjugation of this alkylating agent to the 5′ terminus of an ODN, using a fully phosphorothioated random 20-mer ODN as a model. Reaction conditions were optimized to achieve high radiolabelling yields with a minimum amount of ODN, obtaining the radiolabelled agent at high specific activity and without need of purification.
2. Materials and methods
2.1. General
All chemicals obtained commercially were of analytic or HPLC grade and were used without further purification unless otherwise stated.
The N-Boc-protected nitro-precursor, [3-(2-nitropyridin-3-yloxy)propyl] carbamic acid tert-butyl ester (1) was synthesized according to Kuhnast et al. (2004). The non-radioactive, fluorinated reference compound 2-bromo-N-[3-(2-[19F]fluoropyridin-3-yloxy)propyl]acetamide ([19F]FPyBrA) was kindly provided by Frédéric Dollé (Service Hospitalier Frédéric Joliot, Institut d’Imagerie Biomédicale — CEA, Orsay, France). [18F]fluoride was produced on a TR-19/9 cyclotron (Advanced Cyclotron Systems, Burnaby, Canada) by the 18O (p, n)18F nuclear reaction.
A 20-mer oligodeoxynucleotide with phosphorothioate backbone and a phosphorothioate monoester at the 5′ end (PS-ODN) with the sequence 5′-CCG-GTG-AAC-GAG-CGA-GCA-CA-3′ (Tian et al., 2000) and purity >90% as analyzed by high performance liquid chromatography and mass spectrometry was purchased from the University Core DNA Services (Calgary, Canada) and used to evaluate the radiolabelling performance of [18F]FPyBrA.
2.2. Analytical methods
Thin layer chromatography (TLC). Radio-TLC (MK6F Silica Gel 60 Å, size 2.5×7.5 cm, layer thickness 250 μm, Whatman, Maidstone, UK) with ethyl acetate (EtOAc) was used to monitor the RCY of the different reaction steps during the optimization of the automated synthesis. The radioactivity distribution on the plate was analyzed with a TLC-scanner (Mini-Scan, Bioscan, Washington DC, USA) and the retention factor (Rf) was calculated. All compounds were additionally localized with UV illumination at 254 nm. The final radiochemical purity (RCP) of [18F]FPyBrA after purification was analyzed by radio-TLC co-spotted with the non-radioactive fluorinated reference compound (Rf 0.7). The decay-corrected and non-decay-corrected RCY was calculated based on starting [18F]fluoride.
High performance liquid chromatography (HPLC). [18F]FPyBrA ([18F]-4) was purified by normal phase HPLC on a Knaur chromatography system (Berlin, Germany), equipped with a Waters Prep Nova-Pak® HR Silica column (Semi/Prep; 300×7.8 mm; porosity 6 μm), integrated radioactivity detection and UV detection at 254 nm (fixed wavelength detector K-200, Knaur, Berlin, Germany), using isocratic elution with dichloromethane (CH2Cl2)/EtOAc (1/1, v/v) and flow rate 5 mL/min (HPLC method 1). The radioactive peak corresponding to [18F]-4 with retention time (Rt) 5 min was collected by remote control. The RCP of [18F]-4 and the radiolabelled PS-ODN was analyzed by reversed phase HPLC on a Beckman Coulter chromatography system with a Model 126 analytical dual pump, Model 168 Diode Array variable UV detector and ACE Mate™ Single Channel Analyzer (Ortec, TN) radiometric detection, equipped with a Phenomenex Luna C18 column (Semi/Prep; 250×10.0 mm; porosity 10 μm) using a gradient system of 0.1 M aqueous triethylammonium acetate pH 7 and acetonitrile (ACN) with flow rate 3 mL/min and increasing concentrations of ACN: 0–20 min 10–50%, 20–21 min 50–70%, 21–25 min 70%, 25–26 min 70–10%, 26–30 min 10% (HPLC method 2).
Ultraviolet–visible (UV) spectroscopy. The concentration of ODN was determined by UV spectroscopy at 260 nm using a Beckman DU 7400 spectrophotometer.
2.3. Automated radiosynthesis of [18F]FPyBrA
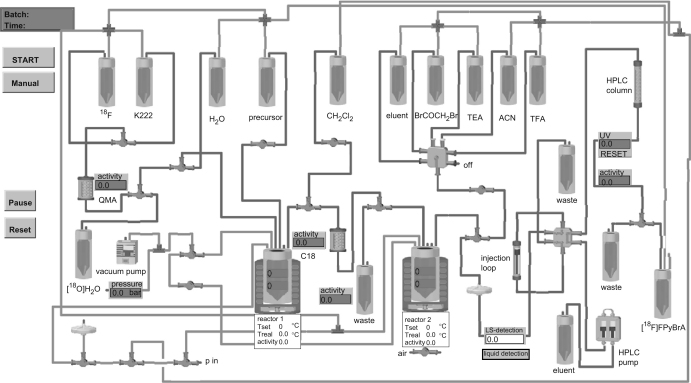
A commercially available modular synthesis system (Modular Lab, Eckert & Ziegler, Berlin, Germany) was used for the fully automated radiosynthesis. The modular system included two reactor modules, a variety of valve modules, a vacuum pump and a compact HPLC module (K-120, Knaur, Berlin, Germany) and was PC controlled through the programming of the Modular-Lab software. A schematic diagram of the synthesis setup is shown in Scheme 1.
Scheme 1.
Schematic diagram of the automated synthesis set-up for [18F]FPyBrA production.
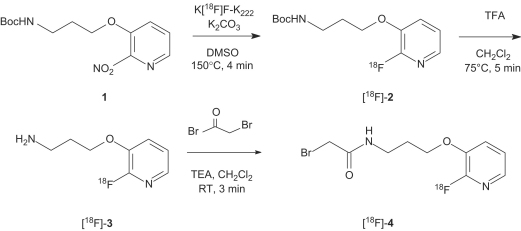
The radiosynthesis of [18F]FPyBrA according to Kuhnast et al. (2004) was carried out in three synthesis steps (see Fig. 2): (1) introduction of 18F into the pyridine ring by nucleophilic heteroaromatic substitution, (2) removal of the N-Boc protective group and (3) amide formation with 2-bromoacetyl bromide. Each synthesis step was evaluated and optimized over 60 separate preparations as described in the following sections:
Fig. 2.
Reaction scheme for the synthesis of [18F]FPyBrA.
Synthesis of [3-(2-[18F]fluoropyridin-3-yloxy)propyl] carbamic acid tert-butyl ester ([18F]-2). Aqueous [18F]fluoride was introduced into the synthesis unit by applying a nitrogen stream and trapped on an anion-exchange resin (Sep-Pak® Light QMA cartridge, Waters, Milford, MA), preconditioned with 10 mL of 0.5 M potassium carbonate (K2CO3) and 20 mL of water. The target [18O]H2O was recovered and [18F]fluoride was eluted into reactor one using a 0.8 mL mixture of 2.1 mg/mL K2CO3 in water and 11.8 mg/mL 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane (Kryptofix 222) in ACN (15/85, v/v). The resulting solution was concentrated to dryness under reduced pressure and nitrogen stream at 85–100 °C to obtain the no carrier added K[18F]-K222 complex. The temperature was reduced to 50 °C and 5 mg of 1 in 0.6 mL anhydrous dimethylsulfoxide (DMSO) was added and allowed to react with the K[18F]-K222 complex at 150 °C for 4 min with stirring (silica gel TLC, eluent EtOAc, Rf 0.9). The temperature was reduced again to 50 °C and the reaction mixture was diluted with 5 mL of water and transferred onto a C-18 cartridge (Sep-Pak® C-18 Plus, Waters, Milford, MA), preconditioned with 5 mL of ethanol and 10 mL of water. The cartridge was washed with an additional portion of 5 mL water to separate unreacted [18F]fluoride and the C-18 cartridge was partially dried by applying a nitrogen stream for 30 s to minimize residual moisture. [18F]-2 was eluted into reactor two with 3.5 mL of CH2Cl2.
Synthesis of 3-(2-[18F]fluoropyridin-3-yloxy)propylamine ([18F]-3). Trifluoroacetic acid (TFA; 100 μL in 0.5 mL CH2Cl2) was added to reactor two to carry out N-Boc deprotection and the solvent was evaporated at 75 °C for 5 min (silica gel TLC, eluent EtOAc, Rf 0.0). After evaporation of the solvents an additional drying step with 1 mL ACN at 85 °C for 2.5 min under reduced pressure and nitrogen stream was performed to reduce the TFA content and residual moisture. Before continuing with the last reaction step reactor two was cooled to 37 °C.
Synthesis of [18F]FPyBrA ([18F]-4). The residue containing [18F]-3 was redissolved with 40 μL triethylamine (TEA) in 0.5 mL CH2Cl2 followed by addition of 20 μL of 2-bromoacetyl bromide (BrCOCH2Br) in 0.5 mL CH2Cl2. The reaction mixture was reacted for 3 min at 37 °C to obtain the final product (silica gel TLC, eluent EtOAc, Rf 0.7). For purification on normal phase HPLC the final reaction mixture was diluted with 1 mL CH2Cl2/EtOAc (1/1, v/v) and passed through a GHP Acrodisc® GF 25 mm, 0.45 μm filter (Waters, Milford, MA) before injection into the HPLC system. Online normal phase HPLC purification was performed with a Knaur HPLC-module (Berlin, Germany) using HPLC method 1 (Rt 5 min). The whole synthesis including purification was performed within 65 min affording pure [18F]-4 in 5 mL HPLC solvent. The solvents were evaporated and [18F]-4 was redissolved in a minimum amount of methanol (MeOH).
2.4. Oligonucleotide labelling
The PS-ODN dissolved in 0.1 M phosphate buffered saline (PBS) pH 7 at a concentration of 1 μg/μL was conjugated with [18F]-4 in varying concentrations of PBS/MeOH at 120 °C for 15–30 min in a sealed V-vial. The influence of the pH, MeOH concentration and PS-ODN concentration on the radiolabelling performance was evaluated.
The RCP of the different reaction mixtures was analyzed by HPLC method 2. The overall recovery of labelled ODN from the HPLC system was monitored and the formation of radiocolloids was analyzed by solid phase extraction on a Sep-Pak® C-18 Light cartridge (Waters, Milford, MA). The concentration of PS-ODN was confirmed by UV spectroscopy at 260 nm before and after radiolabelling.
3. Results and discussion
The preparation of 18F-labelled imaging probes is a complex process often involving multiple reaction steps requiring purification of the intermediates and the final product and is more easily accomplished in automated synthesis units (Satyamurthy et al., 1999). The commercially available synthesis units, however, are generally designed for the optimized preparation of a specific radiopharmaceutical, most prominently 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG). There are several reports on performing a variety of other one-pot labelling strategies in commercially available FDG modules (Reischl et al., 2005; Hamacher and Coenen, 2002; Ryzhikov et al., 2005; Oh et al., 2005; Kryza et al., 2008). We have recently described the automated radiosynthesis of [18F]FBBA in a TRACERLab FXFDG system (GE Medical Systems) (Koslowsky et al., 2008). The development of novel multistage synthesis procedures for 18F-labelled radiotracers within these systems is not always possible or is complicated by the requirement for extensive software and hardware modifications (Marik and Sutcliffe, 2007).
Recently several modular automated radiosynthesis units have become available. These units can be configured to permit user-designed, multi-step reactions to be performed (Krasikova, 2007). In this study we used such a modular system for the fully automated radiosynthesis and remote-controlled semi-preparative HPLC purification of [18F]FPyBrA. The synthesis unit was readily configured for the three-step synthesis according to Kuhnast et al. (2004) starting from the nitro-precursor (see Scheme 1 and Fig. 2). The PC-controlled synthesis was optimized during the initial synthesis evaluation by programming of the controlling software. Minor modifications of the synthesis allowed improvements of the RCY in each reaction step. The reagents loaded into the system for the optimized synthesis of [18F]FPyBrA are presented in Table 1.
Table 1.
Reagent list for the automated radiosynthesis of [18F]FPyBrA (Scheme 1).
| Filling of the synthesis unit |
|---|
| 1–2 mL aqueous [18F]fluoride |
| 0.8 mL K222/K2CO3 mixture |
| 5 mg nitro-precursor in 0.6 mL DMSO |
| 10 mL water for dilution and washing steps |
| 3.5 mL CH2Cl2 for SepPak elution |
| 0.5 mL CH2Cl2 containing 100 μL TFA |
| 1 mL ACN to minimize TFA content |
| 0.5 mL CH2Cl2 containing 40 μL TEA |
| 0.5 mL CH2Cl2 containing 20 μL BrCOCH2Br |
| 1 mL HPLC eluent |
During the optimization of the automated synthesis a total of 60 batches of [18F]FPyBrA were produced. An overview of the radiolabelling performance is given in Table 2. [18F]fluoride was efficiently desorbed (⩾94%) from the QMA cartridge using a 2:1 molar ratio of K222 and K2CO3 as recommended previously (Gomzina et al., 2002) in 0.8 mL of solvent. A single drying sequence without additional azeotropic drying steps with ACN proved effective. The average precursor amount of 5.9 mg (20 μmol) resulted in an almost equimolar K222 to 1 ratio. This ratio together with a slightly increased reaction temperature of 150 °C (Tewson and Watkins, 2001) resulted in an optimized yield as determined by TLC. The presence of undissolved K[18F]-K222 complex (Block et al., 1986) was noted in some reactions resulting in lower yields. This loss was reduced by stirring the contents during the first reaction step.
Table 2.
Overview of the radiolabelling performance in the synthesis of [18F]FPyBrA.
| Radiolabelling performance | Min | Max | Mean±sd | (n) |
|---|---|---|---|---|
| Nitro-precursor (mg) | 3.6 | 9.1 | 5.9±1.2 | (60) |
| Starting [18F]fluoride (MBq) | 85 | 4212 | 780±1021 | (60) |
| Activity in reactor one (%)a | 50 | 100 | 81±10 | (40) |
| Activity in reactor two (%)a | 21 | 51 | 38±9 | (40) |
| Activity in the waste (%)a | 22 | 48 | 34±7 | (40) |
| Unrecovered activity on the QMA catridge (%)a | 1 | 4 | 2±1 | (20) |
| Unrecovered activity on the SepPak catridge (%)a | 1 | 10 | 4±2 | (40) |
| Yield of nucleophilic substitution (% RCY)b | 48 | 89 | 74±11 | (40) |
| Yield of N-Boc-deprotection (% RCY)b | 88 | 100 | 96±4 | (20) |
| Yield of bromoacetylation (% RCY)b | 31 | 78 | 63±10 | (20) |
| Activity recovery during HPLC purification | 60 | 87 | 73±7 | (10) |
| Decay-corrected yielda | 11 | 24 | 17±5 | (10) |
| Non-decay-corrected yield | 7 | 16 | 11±3 | (10) |
Decay-corrected based on starting [18F]fluoride.
According to TLC.
A mean of 38% of the starting 18F was present in reactor two after elution of [18F]-2 from the C-18 cartridge due to unavoidable losses during transfer although no activity losses due to wall absorption on the reaction vessel were observed (Block et al., 1987). Activity loss due to retention of [18F]-2 on the cartridge was reduced by incubating the cartridge with CH2Cl2 for 30 s before continuing the elution into reactor two. Despite drying of the C-18 cartridge prior to CH2Cl2 elution by applying a nitrogen stream for up to 5 min, residual moisture was always present in the eluate.
As already described by Schildan et al. (2007) no deprotection was achieved by addition of TFA and reaction at room temperature, whereas heating at moderate temperatures of 50–60 °C resulted in N-Boc-deprotection of greater than 80% and evaporation of the solvents at 75 °C under reduced pressure and nitrogen stream resulted in virtually quantitative deprotection (>95%) as determined by TLC. Residual moisture and TFA were successfully removed by an additional drying step with ACN at 85 °C. No significant losses of radioactivity were observed in this step of the synthesis.
The reaction between the intermediate propylamine ([18F]-3) and BrCOCH2Br proceeded readily to form [18F]FPyBrA ([18F]-4) with optimized labelling yields obtained at room temperature after 3 min with TEA concentration being a significant determinant of RCY (>30% with 20 μL TEA; >60% with 40 μL TEA). A similar improvement was reported also by Viel et al. (2007). HPLC purification of the crude product resulted in a mean recovery of 73% of pure [18F]-4.
For the complete three step reaction to form [18F]-4 under optimized radiolabelling conditions, a maximum overall decay-corrected RCY of 24% based on starting [18F]fluoride, corresponding to a non-decay-corrected RCY of 16% was achieved with a 65 min synthesis time.
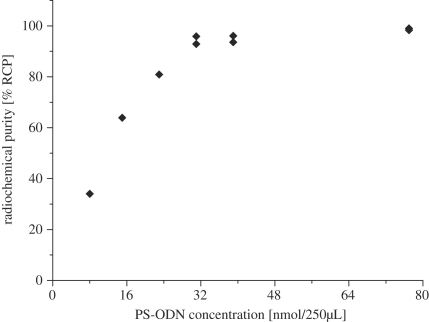
The final phase of this study involved the coupling of the 18F-labelled prosthetic group ([18F]-4) at the 5′ end of a model 20-mer PS-ODN. The reaction was optimized to a very high yield (>90%) by adjusting the reaction parameters of temperature (120 °C), time (30 min) and pH (7.4). The reaction solvent of MeOH and 0.1 M PBS was found to play a major role in the RCY with a concentration of 20% MeOH resulting in the highest yields. The minimum amount of PS-ODN for quantitative alkylation under these conditions was evaluated (see Fig. 3), achieving incorporation >90% with 200 μg (31 nmol) in a 250 μL reaction volume. The improved labelling conditions appear to be generally applicable to bromoacetamide alkylation of PS-ODN since we obtained similar yields using [18F]FBBA to produce [18F]FBBA-PS-ODN (data not shown).
Fig. 3.
PS-ODN concentration-dependent incorporation of [18F]FPyBrA.
Reverse phase HPLC is an effective tool for analysis and purification of the labelled and unlabelled ODN as shown in Fig. 4. [18F]FPyBrA-PS-ODN eluted from the column with a shift of ∼1 min in comparison with unlabelled PS-ODN (Fig. 4A,C), whereas free [18F]FPyBrA eluted with a shift of >10 min (Fig. 4B). The integrity of the PS-ODN was confirmed by HPLC analysis of the radiolabelling mixture coinjected with unlabelled PS-ODN (Fig. 4D). The recovery of the HPLC system was 99% and the formation of radiocolloids (as determined by solid phase extraction) was <2%. The virtually quantitative yield obtained in the coupling reaction found in this study raises the possibility of using the labelled ODN products without employing HPLC purification, either directly from the synthesis or by a straightforward solid phase extraction method to remove minor impurities or incompatible solvents. For many imaging applications of radiolabelled ODN very high specific activities will be required. In our preliminary labelling studies we used up to 170 MBq [18F]FPyBrA, achieving a maximum specific activity of 5–6 GBq/μmol. This specific activity could be increased by: (a) higher starting activities of 18F, (b) finding reaction conditions requiring reduced starting ODN amount and (c) separation of labelled and unlabelled ODN. In general the isolation of labelled ODN from the bulk of unlabelled material is not possible due to the small change in physiochemical properties following labelling. In the present study the ∼1 min shift in HPLC retention time for the [18F]FPyBrA-PS-ODN relative to the unlabelled ODN should allow the isolation of a very high specific activity product.
Fig. 4.
HPLC profiles: (A) UV trace of the unlabelled PS-ODN; (B) radiochromatogram of [18F]FPyBrA; (C) UV trace (dots) and radiochromatogram (solid line) of [18F]FPyBrA-PS-ODN; (D) UV trace (dots) and radiochromatogram (solid line) of [18F]FPyBrA-PS-ODN coinjected with unlabelled PS-ODN (HPLC method 2).
4. Conclusion
By the application of modular automated chemistry [18F]FPyBrA can be obtained within 65 min in overall decay-corrected yields of 17±5%. The straightforward and reliable synthesis of [18F]FPyBrA in a remotely controlled synthesis unit allows the convenient preparation of sufficient amounts of [18F]FPyBrA for labelling PS-ODN and other thiol group containing biomolecules. The very high yield conjugation of [18F]FPyBrA to ODN provides the radiolabelled probe in a short synthesis time and at relatively high specific activity. To our knowledge this is the first report on almost quantitative 18F-labelling of an ODN using prosthetic group coupling chemistry. The high overall yield in the coupling reaction and the potential for rapid purification without chromatographic isolation of the labelled ODN presents an overall benefit for the application of ODN in preclinical studies and potential clinical applications.
Acknowledgements
The authors wish to thank the radiochemistry staff at the Edmonton PET Center and Sanjay Sharma (Edmonton Radiopharmaceutical Centre) for the synthesis and analysis of the nitro-precursor. We also thank Frédéric Dollé (Service Hospitalier Frédéric Joliot, Institut d’Imagerie Biomédicale — CEA, Orsay, France) for kindly providing the non-radioactive reference standard [19F]FPyBrA. Elisabeth von Guggenberg was funded by an Erwin Schrödinger Fellowship of the Austrian Science Fund (FWF). Research funding from the Alberta Cancer Board and the Alberta Cancer Foundation are gratefully acknowledged.
References
- Block D., Coenen H.H., Stöcklin G. The N.C.A. nucleophilic 18F-fluorination of 1,N-disubstituted alkanes as fluoroalkylation agents. J. Labelled Compd. Radiopharm. 1987;24(9):1029–1042. [Google Scholar]
- Block D., Klatte B., Knöchel A., Beckmann R., Holm U. N.C.A. [18F]-labelling of aliphatic compounds in high yields via aminopolyether-supported nucleophilic substitution. J. Labelled Compd. Radiopharm. 1986;23(5):467–477. [Google Scholar]
- Brody E.N., Gold L. Aptamers as therapeutic and diagnostic agents. J. Biotechnol. 2000;74(1):5–13. doi: 10.1016/s1389-0352(99)00004-5. [DOI] [PubMed] [Google Scholar]
- de Vries E.F., Vroegh J., Elsinga P.H., Vaalburg W. Evaluation of fluorine-18-labeled alkylating agents as potential synthons for the labeling of oligonucleotides. Appl. Radiat. Isotopes. 2003;58(4):469–476. doi: 10.1016/s0969-8043(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Dollé F., Hinnen F., Vaufrey F., Tavitian B., Crouzel C. A general method for labeling oligodeoxynucleotides with 18F for in vivo PET imaging. J. Labelled Compd. Radiopharm. 1997;39(4):319–330. [Google Scholar]
- Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Gomzina N.G., Vasil’ev D.A., Krasikova R.N. Optimization of automated synthesis of 2-[F]Fluoro-2-deoxy-d-glucose involving base hydrolysis. Radiochemistry. 2002;44(4):366–372. [Google Scholar]
- Hamacher K., Coenen H.H. Efficient routine production of the 18F-labelled amino acid O-(2-18F fluoroethyl)-L-tyrosine. Appl. Radiat. Isotopes. 2002;57(6):853–856. doi: 10.1016/s0969-8043(02)00225-7. [DOI] [PubMed] [Google Scholar]
- Hedberg E., Langstrom B. Synthesis of 4-([18F]Fluoromethyl)phenyl isothiocyanate and its use in labelling oligonucleotides. Acta. Chim. Scand. 1997;51:1236–1240. [Google Scholar]
- Hedberg E., Langstrom B. 18F-Labeling of Oligonucleotides Using Succinimido 4-[18F]Fluorobenzoate. Acta. Chim. Scand. 1998;52:1034–1039. [Google Scholar]
- Hicke B.J., Stephens A.W., Gould T., Chang Y.F., Lynott C.K., Heil J., Borkowski S., Hilger C.S., Cook G., Warren S., Schmidt P.G. Tumor targeting by an aptamer. J. Nucl. Med. 2006;47(4):668–678. [PubMed] [Google Scholar]
- Hjelstuen O.K., Tønnesen H.H., Bremer P.O., Verbruggen A.M. 3′-99mTc-labeling and biodistribution of a CAPL antisense oligodeoxynucleotide. Nucl. Med. Biol. 1998;25(7):651–657. doi: 10.1016/s0969-8051(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Hnatowich D.J. Antisense and nuclear medicine. J. Nucl. Med. 1999;40(4):693–703. [PubMed] [Google Scholar]
- Javier D.J., Nitin N., Levy M., Ellington A., Richards-Kortum R. Aptamer-targeted gold nanoparticles as molecular-specific contrast agents for reflectance imaging. Bioconjugate Chem. 2008;19(6):1309–1312. doi: 10.1021/bc8001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowsky I., Shahhosseini S., Wilson J., Mercer J. Automated radiosynthesis of N-[4-[18F]fluorobenzyl]-2-bromoacetamide: an F-18-labeled reagent for the prosthetic radiolabeling of oligonucleotides. J. Labelled Compd. Radiopharm. 2008;51(10):352–356. [Google Scholar]
- Krasikova R. Synthesis modules and automation in F-18 labeling. Ernst. Schering. Res. Found. Workshop. 2007;62:289–316. doi: 10.1007/978-3-540-49527-7_11. [DOI] [PubMed] [Google Scholar]
- Kryza D., Tadino V., Filannino M.A., Villeret G., Lemoucheux L. Fully automated [18F]fluorocholine synthesis in the TracerLab MX FDG Coincidence synthesizer. Nucl. Med. Biol. 2008;35(2):255–260. doi: 10.1016/j.nucmedbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Kuhnast B., de Bruin B., Hinnen F., Tavitian B., Dollé F. Design and synthesis of a new [18F]fluoropyridine-based haloacetamide reagent for the labeling of oligonucleotides: 2-bromo-N-[3-(2-[18F]fluoropyridin-3-yloxy)propyl]acetamide. Bioconjugate Chem. 2004;15(3):617–627. doi: 10.1021/bc049979u. [DOI] [PubMed] [Google Scholar]
- Kuhnast B., Dollé F., Terrazzino S., Rousseau B., Loc′h C., Vaufrey F., Hinnen F., Doignon I., Pillon F., David C., Crouzel C., Tavitian B. General method to label antisense oligonucleotides with radioactive halogens for pharmacological and imaging studies. Bioconjugate Chem. 2000;11(5):627–636. doi: 10.1021/bc990183i. [DOI] [PubMed] [Google Scholar]
- Kuhnast B., Klussmann S., Hinnen F., Boisgard R., Rousseau B., Furste J.P., Tavitian B., Dollé F. Fluorine-18- and iodine-125-labelling of spiegelmers. J. Labelled Compd. Radiopharm. 2003;46(13):1205–1219. [Google Scholar]
- Lange C.W., VanBrocklin H.F., Taylor S.E. Photoconjugation of 3-azido-5-nitrobenzyl-[18F]fluoride to an oligonucleotide aptamer. J. Labelled Compd. Radiopharm. 2002;45(3):257–268. [Google Scholar]
- Marik J., Sutcliffe J.L. Fully automated preparation of n.c.a. 4-[18F]fluorobenzoic acid and N-succinimidyl 4-[18F]fluorobenzoate using a Siemens/CTI chemistry process control unit [CPCU] Appl. Radiat. Isotopes. 2007;65(2):199–203. doi: 10.1016/j.apradiso.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Oh S.J., Chi D.Y., Mosdzianowski C., Kim J.Y., Gil H.S., Kang S.H., Ryu J.S., Moon D.H. Fully automated synthesis of [18F]fluoromisonidazole using a conventional [18F]FDG module. Nucl. Med. Biol. 2005;32(8):899–905. doi: 10.1016/j.nucmedbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Paunesku T., Ke T., Dharmakumar R., Mascheri N., Wu A., Lai B., Vogt S., Maser J., Thurn K., Szolc-Kowalska B., Larson A., Bergan R.C., Omary R., Li D., Lu Z.R., Woloschak G.E. Gadolinium-conjugated TiO2-DNA oligonucleotide nanoconjugates show prolonged intracellular retention period and T1-weighted contrast enhancement in magnetic resonance images. Nanomedicine. 2008;4(3):201–207. doi: 10.1016/j.nano.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A.C., Missailidis S. Radiolabelled aptamers for tumour imaging and therapy. Q. J. Nucl. Med. Mol. Imaging. 2007;51(4):292–296. [PubMed] [Google Scholar]
- Reischl G., Ehrlichmann W., Bieg C., Solbach C., Kumar P., Wiebe L.I., Machulla H.J. Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation. Appl. Radiat. Isotopes. 2005;62(6):897–901. doi: 10.1016/j.apradiso.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Ryzhikov N.N., Seneca N., Krasikova R.N., Gomzina N.A., Shchukin E., Fedorova O.S., Vassiliev D.A., Gulyás B., Hall H., Savic I., Halldin C. Preparation of highly specific radioactivity [18F]flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nucl. Med. Biol. 2005;32(2):109–116. doi: 10.1016/j.nucmedbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Satyamurthy N., Phelps M.E., Barrio J.R. Electronic generators for the production of positron-emitter labeled radiopharmaceuticals. Where would PET be without them? Clin. Positron Imaging. 1999;2(5):233–253. doi: 10.1016/s1095-0397(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Schildan A., Patt M., Sabri O. Synthesis procedure for routine production of 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]F-A-85380) Appl. Radiat. Isotopes. 2007;65(11):1244–1248. doi: 10.1016/j.apradiso.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Tamm I., Dörken B., Hartmann G. Antisense therapy in oncology: new hope for an old idea? Lancet. 2001;358(9280):489–497. doi: 10.1016/S0140-6736(01)05629-X. [DOI] [PubMed] [Google Scholar]
- Tavitian B. In vivo imaging with oligonucleotides for diagnosis and drug development. Gut. 2003;52(Suppl IV):iv40–iv47. doi: 10.1136/gut.52.suppl_4.iv40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewson T., Watkins G.L. Tweaking the FLT synthesis: modifications that improve the yield. J. Nucl. Med. 2001;42(5):S257. [Google Scholar]
- Tian H., Wittmack E.K., Jorgensen T.J. p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer. Res. 2000;60(3):679–684. [PubMed] [Google Scholar]
- Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Viel T., Kuhnast B., Hinnen F., Boisgard R., Tavitian B., Dollé F. Fluorine-18 labelling of small interfering RNAs (siRNAs) for PET imaging. J. Labelled Compd. Radiopharm. 2007;50(13):1159–1168. [Google Scholar]
- Wester H.J., Schottelius M. Fluorine-18 labeling of peptides and proteins. Ernst. Schering. Res. Found. Workshop. 2007;62:79–111. doi: 10.1007/978-3-540-49527-7_4. [DOI] [PubMed] [Google Scholar]
- Yngve U., Hedberg E., Lovqvist A., Tolmachev V., Langstrom B. Synthesis of N-succinimidyl 4-[76Br]bromobenzoate and its use in conjugation labeling of macromolecules. Acta. Chim. Scand. 1999;53:508–512. [Google Scholar]
- Younes C.K., Boisgard R., Tavitian B. Labelled oligonucleotides as radiopharmaceuticals: pitfalls, problems and perspectives. Curr. Pharm. Des. 2002;8(16):1451–1466. doi: 10.2174/1381612023394467. [DOI] [PubMed] [Google Scholar]