Abstract
Catalytic domain variants of activated factor VII (FVIIa) with enhanced hemostatic properties are highly attractive for the treatment of bleeding disorders via gene-based therapy. To explore this in a hemophilic mouse model, we characterized 2 variants of murine activated FVII (mFVIIa-VEAY and mFVIIa-DVQ) with modified catalytic domains, based on recombinant human FVIIa (rhFVIIa) variants. Using purified recombinant proteins, we showed that murine FVIIa (mFVIIa) and variants had comparable binding to human and murine tissue factor (TF) and exhibited similar extrinsic coagulant activity. In vitro in the absence of TF, the variants showed a 6- to 17-fold enhanced proteolytic and coagulant activity relative to mFVIIa, but increased inactivation by antithrombin. Gene delivery of mFVIIa-VEAY resulted in long-term, effective hemostasis at 5-fold lower expression levels relative to mFVIIa in hemophilia A mice or in hemophilia B mice with inhibitors to factor IX. However, expression of mFVIIa-VEAY at 14-fold higher than therapeutic levels resulted in a progressive mortality to 70% within 6 weeks after gene delivery. These results are the first demonstration of the hemostatic efficacy of continuous expression, in the presence or absence of inhibitors, of a high-activity gene-based FVIIa variant in an animal model of hemophilia.
Introduction
Over the past 3 decades, discoveries in multiple disciplines have been instrumental in the molecular dissection of hemophilia. These resulted in the development of novel therapies for the disease, including more effective treatment for the most serious complication in factor replacement, the development of inhibitory antibodies to factor VIII (FVIII) or IX (FIX). In particular, rhFVIIa (commercially known as NovoSeven [Novo Nordisk]) has a proven record of successful treatment for hemophilia patients with inhibitors, by providing localized hemostasis when administered at supraphysiologic doses of 90-110 μg/kg.1 However, its short plasma half-life (2.7 hours) necessitates frequent infusions, resulting in high treatment costs2 and preventing its use as a universal hemostatic agent.
For pharmacologic intervention, efforts to enhance the procoagulant activity of rhFVIIa have focused on chemical modification/formulation3,4 or the rational design of novel, high-specific activity variants of rhFVIIa. This has been achieved by enhancing the phospholipid binding or increasing the catalytic activity of rhFVIIa.5–8 Although data from human subjects suggest that a single-dose of a rhFVIIa variant (rhFVIIa-DVQ, also known as NN1731) with enhanced, TF-independent activity is safe,9 efficacy and long-term safety data in hemophilic patients are limited. Moreover, the majority of its in vivo efficacy data have been derived using nonhomologous animal models in short-term studies, where potential species incompatibilities between human FVIIa and endogenous (animal) TF10 may affect the hemostatic outcomes.11,12
As an alternative to rhFVIIa infusion, we have demonstrated in animal models the efficacy of gene-based FVIIa therapy for inherited bleeding disorders. In a pilot study, we generated a murine factor VII (mFVII) transgene (mFVII-2RKR) that can be secreted in its active form (mFVIIa) and, after continuous ex-pression via viral vector–mediated gene delivery, effect hemostasis in vivo in hemophilic mice.13,14 Moreover, in a multiyear study, we confirmed our approach in hemophilic dogs using canine FVII-2RKR (cFVIIa) as a transgene and demonstrated the complete elimination of spontaneous bleeds, a characteristic disease feature of these animals, without any evidence of thrombosis.15 Although the adeno-associated virus (AAV) serotype 8 (AAV8) vector used in that study was the most efficient choice for expression,16 the relatively high viral vector doses used compared with similar approaches with canine FVIII (1.2- to 12.5-fold17,18) or FIX (12- to 24-fold19) impose a technical barrier for further development of a FVIIa gene-based approach that needs to be addressed.
An avenue to overcome this limitation is to use transgene variants of FVIIa with enhanced activity that can potentially lower minimum required expression levels and translate to lower viral vector dose requirements. Here, we explore this possibility by a comprehensive characterization of 2 murine FVIIa (mFVIIa) variants with amino acid substitutions in their catalytic domains, based on rhFVIIa variants. We demonstrate that (1) mFVIIa variants exhibit increased TF-independent coagulant activity relative to mFVIIa; (2) gene transfer of a high-activity mFVIIa variant can achieve long-term hemostasis at a substantially lower expression level (and lower viral vector doses) compared with mFVIIa; (3) the presence of inhibitors to human factor IX does not alter or reduce hemostatic efficacy in hemophilic mice expressing the mFVIIa variant; and (4) high-level continuous expression of this mFVIIa variant leads to rapid mortality. These results are the first to demonstrate the improved hemostatic efficacy of gene-based FVIIa variants for the treatment of hemophilia but also indicate what are likely to be the major safety considerations for gene-based strategies using enhanced activity coagulation factor(s).
Methods
Plasmid construction and AAV vector production
The plasmid vectors for mFVII, mFVIIa, and the liver-specific, adeno-associated virus (AAV) vector plasmid backbone have been previously described.14 The mFVIIa-DVQ and VEAY variants were constructed by PCR mutagenesis and helper-free, recombinant AAV virus vector production was performed as previously described.14 Vector genome (vg) titering for each construct was performed side-by-side in duplicate and confirmed by slot blot as well as silver staining.
Recombinant proteins and purification
Stable cell lines expressing mFVII, mFVIIa, mFVIIa-DVQ or mFVIIa-VEAY containing a C-terminal epitope tag were established as previously described.14 All murine proteins (except mFVII) contained the Arg-Lys-Arg-Arg-Lys-Arg (RKRRKR [2RKR]) sequence inserted at Arg152-Ile153 of the mature protein. Recombinant proteins were purified using an antibody column, as previously described.14 Human soluble TF (hsTF) and murine soluble TF (msTF) were kind gifts from Dr S. Krishnaswamy (Children's Hospital of Philadelphia) and Dr Mirella Ezban (Måløv, Novo Nordisk, Denmark), respectively. Human FX (hFX) and activated hFX (hFXa) were kind gifts from Dr R. M. Camire (Children's Hospital of Philadelphia).
Peptidyl substrate cleavage and TF binding
Proteins (100nM) were incubated in reaction buffer (20mM HEPES, 150mM NaCl pH 7.4 [HBS], 5mM CaCl2 and 0.1% [wt/vol] PEG 8000 [HBS-PEG-CaCl2]) at 25°C in the presence or absence of soluble TF and 400μM phosphatidylcholine/phosphatidylserine (75%/25%, PCPS). Binding to human or murine soluble TF was performed using a range of concentrations (0-2μM [hsTF] or 0-150nM [msTF]). Spectrozyme FVIIa (American Diagnostica) was added at 375nM immediately before monitoring the A405nm as a function of time using a VMax plate reader (Molecular Devices). Dissociation constant (Kd) determination was performed from the fitted data obtained, essentially as previously described.20 Although we cannot exclude the possibility of phospholipids influencing the FVII/FVIIa interaction with soluble TF (hence the amidolytic activity and Kd), we do not believe that it is likely, based on previous reports with human FVII.20,21
Macromolecular substrate cleavage
Proteins (200nM) were incubated in reaction buffer at 25°C with 100μM PCPS. The reaction was initiated by the addition of 100nM hFX and aliquots of the reaction were quenched at several time points up to 3.5 minutes in HBS with 0.1% [wt/vol] PEG 8000 and 50mM EDTA (HBS-PEG-EDTA). The hFXa chromogenic substrate (Spectrozyme FXa, American Diagnostica) was added at a final concentration of 100μM. Human FXa activity was monitored by measuring the A405nm as a function of time at 25°C. The concentration of hFXa/min (nM hFXa/min) was determined by interpolation from the linear dependence of the initial rate of Spectrozyme FXa hydrolysis on known concentrations of hFXa that were determined separately. The initial steady-state rate of hFXa formation was determined from the slope of plots that documented the linear appearance of hFXa with time, expressed as nM hFXa/min.
Coagulation assays
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) assays on plasma, cell culture supernatants, and purified proteins were determined as previously described,14 using human factor deficient plasmas (George King Bio-Medical, Inc). Clotting activity was determined by performing clotting assays on serial dilutions of samples (in Tris-buffered saline [TBS]-bovine serum albumin [BSA, 0.01%]), using a standard curve with rhFVIIa. Briefly, for PT assays, 25 μL of sample were incubated with 25 μL of human FVII deficient plasma for 1 minute and 100 μL of Innovin (CSL-Behring) were added before measuring the time to clot using a Start4 instrument (Diagnostica Stago). For aPTT assays, 25 μL of sample were incubated with an equal volume of aPTT reagent (Trinity Biotech) and 25 μL of human FVIII or FIX deficient plasma for 3 minutes at 37°C and, after the addition of 25 μL of 25mM CaCl2, time to clot formation was measured using a Start4 instrument. Baseline values for PT assays were: 32.2 ± 1.9 seconds (wild-type C57BL/6 mice); 28.2 ± 4.8 seconds (hemophilia A [HA] mice); 34.1 ± 3.0 (hemophilia B, [HB] mice). Baseline values for aPTT assays were: 32.1 ± 2.4 seconds (wild-type C57BL/6 using human FIX–deficient plasma); 37.9 ± 1.6 seconds (wild-type C57BL/6 using human FVIII–deficient plasma); 57.7 ± 9.7 seconds (HA); 58.8 ± 3.9 seconds (HB). Baseline values were obtained from at least 10 mice.
Animal experiments, in vivo hemostatic challenge assays and histologic analysis
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at The Children's Hospital of Philadelphia. Eight- to 12-week-old hemophilia A22 or B23 mice on a C57BL/6 background were used for all animal experiments. Recombinant AAV delivery and collection of mouse plasma samples were performed as previously described.15 The tail-clip and FeCl3 assay were performed at or after 7 weeks after AAV administration, as previously described.13 Histologic analysis was performed on tissues fixed in 10% formalin overnight at 4°C, as previously described.24 Staining for fibrin(ogen) on paraffin sections was performed as previously described.13 For immunofluorescence staining, paraffin sections were hydrated through xylene and graded alcohol and equilibrated in deionized water. Nonspecific binding was blocked with 3% BSA. They were then incubated with rat anti–mouse CD31 monoclonal antibody (10 μg/mL; catalog no. 558738, BD Pharmingen), and FITC-conjugated anti–Fibrin(ogen) polyclonal antibody (10 μg/mL; catalog no. F0111, Dako) overnight at 4°C; developed with Cy3-conjugated donkey anti–rat IgG (10 μg/mL; catalog no. 712-165-150, Jackson ImmunoResearch Laboratories) and mounted with VectaShield DAPI mounting medium (Vector Laboratories). The sections were analyzed using a Leica TCS-SP2 AOBS confocal laser scanning microscope on a Leica DM-IRE2 inverted microscope stand.
ELISA for mFVIIa
Detection of free mFVIIa in the mouse plasma was performed using an enzyme-linked immunosorbent assay (ELISA), as previously described13 with some modifications. Briefly, samples were diluted 20- or 80-fold in blocking buffer (1% skim milk in phosphate-buffered saline [PBS]) and incubation with the biotinylated Phe-Pro-Arg-chloromethylketone (Biotin-FPR-CMK, 77 μg/mL final concentration; Haematologic Technologies) was performed overnight at 4°C on an orbital shaker (300 rpm). Murine FVII/mFVIIa in the samples was captured using a custom rat anti-mFVII/a monoclonal antibody (Green Mountain Antibodies) coated at 10 μg/mL in 50mM NaHCO3, pH 9.6. This antibody was raised against purified recombinant mFVIIa and maps on the heavy chain of mFVII/mFVIIa (P.M., E.R., unpublished observations, May 2008). Detection of the biotin moiety on the FPR-CMK in the active site of mFVIIa (not mFVII) was by incubation with horseradish peroxidase (HRP) labeled streptavidin (catalog no. 554066, BD Pharmingen) and visualized by addition of o-phenylenediamine dihydrochloride (1 mg/mL in 0.01M sodium citrate, pH 4.5). For quantification, a standard curve mFVIIa or mFVIIa-VEAY in 1:20 or 1:80 diluted untreated HA or HB plasma (in blocking buffer) was used. Because this method essentially detects active site occupancy by the biotinylated FPR-CMK, it detects mFVIIa and not mFVII. Baseline values for mFVIIa in mouse plasma were ∼ 150 ng/mL and were subtracted from the presented data.
Generation of inhibitors to hFIX
Baseline mouse plasma samples were obtained after tail bleeding several days before the start of the experiment. Mice were subsequently injected subcutaneously with 200 μL of an emulsion consisting of complete Freund adjuvant (CFA; Sigma-Aldrich) and 20 μg of human FIX (hFIX; Mononine, CSL-Behring). Four dorsal sites were used, 50 μL per site. Three weeks later, the procedure was repeated substituting CFA with incomplete Freund adjuvant (IFA; Sigma-Aldrich) but injecting 4 different dorsal sites. Plasma samples were obtained after tail bleeding for determination of antibody level (total anti-hFIX IgG) and inhibitor titer (Bethesda assay), performed as previously described.25 Application of this protocol in HA mice or by weekly (for 8 weeks) intravenous administration of 2.5 U recombinant human FVIII (Refacto [Pfizer Inc] or Kogenate [Bayer Healthcare]) did not result in inhibitory antibody formation for reasons that are unclear.
Statistical analysis and protein percentage identity calculation and homology-based modeling
Data were analyzed using a Mann-Whitney (nonparametric) test. Survival data analysis was performed using the log-rank (Mantel-Cox) test in the Prism Version 5.0 software package (GraphPad Software). In all tests, differences were considered significant at P < .05. Percentage identity for human, mouse and canine proteins was calculated from the linear protein sequence alignment, using the ClustalW function of the MegAlign software (part of the Lasergene software package, DNAStar Inc). Homology-based modeling of the heavy chains of murine FVII and variants VEAY and DVQ was performed with the SWISS-MODEL homology-modeling server from the ExPASys web site (http://swissmodel.expasy.org/workspace/).26–29
Results
Purification, amidolytic and proteolytic activity of mFVIIa, mFVIIa-DVQ and mFVIIa-VEAY
Previous reports have identified key residues in the catalytic domain of the human FVII sequence that can be modified to enhance its catalytic activity, specifically at positions 158 (V = > D), 296 (E = > V) and 298 (M = > Q; mutant DVQ30) as well as 305 (L = > V), 314 (S = > E), 337 (K = > A) and 374 (F = > Y; mutant VEAY7). Because murine FVII exhibits extensive sequence identity to human FVII (70%), we introduced these mutations in the mFVIIa backbone (containing the RKRRKR PACE/furin cleavage site14) and generated mFVIIa-DVQ and mFVIIa-VEAY (Figure 1). Purified recombinant proteins exhibited the expected fragment sizes (Figure 2A). Zymogen mFVII purified under identical conditions was in the single-chain form, indicating that there was no autoactivation during the purification process. Murine FVIIa as well as both mFVIIa variants were present in almost 100% active form (Figure 2A).
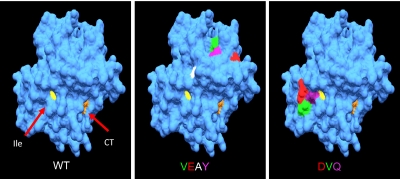
Figure 1.
Spatial localization of the mutations in mFVIIa-VEAY (VEAY) and mFVIIa-DVQ (DVQ) on the heavy chain compared with mFVII wild-type (WT). The crystal structure of the heavy chain of human FVIIa (1DAN31) was used as a template for homology modeling (see “Statistical analysis and protein percentage identity calculation and homology-based modeling”). The catalytic triad (CT, orange [His193, Asp242, Ser194]) and the location of the newly formed N-terminus of the heavy chain of the activated form are shown (Ile153, yellow). The VEAY mutations are: Leu305Val (green); Ala314Glu (red); Lys337Ala (white); Ile374Tyr (magenta). The DVQ mutations are: Val158Asp (red); Glu296Val (green); Met298Gln (purple). Amino acid numbering refers to the mature secreted mFVII.
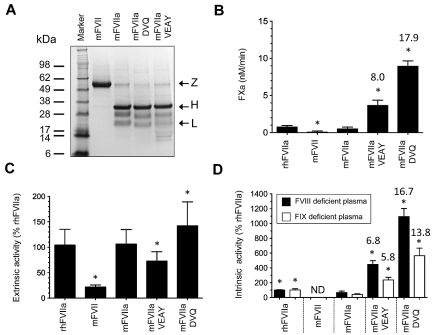
Figure 2.
Characterization of recombinant proteins. (A) Approximately 4 μg of purified protein (mFVII, mFVIIa, mFVIIa-DVQ and mFVIIa-VEAY) was electrophoresed under reducing conditions and stained with Coomassie blue. The molecular sizes of the marker are shown on the left. Arrows indicate the zymogen form (Z), heavy (H) and light (L) chains. The lower-molecular-weight bands (10-16 kDa) are most likely degradation products, as have been previously observed in human recombinant FVIIa preparations.32,33 The differential staining of light and heavy chains with Coomassie blue is commonly found in human32,33 as well as murine FVIIa preparations.10,14 (B) Proteolytic activity of proteins using human FX as a macromolecular substrate in the absence of tissue factor expressed as nM FXa/min. Data were derived from at least 3 independent experiments. *P < .04 compared with mFVIIa. Fold increase is indicated relative to mFVIIa. (C) Extrinsic coagulant activity of recombinant proteins (% of rhFVIIa) in human FVII–deficient plasma. (D) Intrinsic coagulant activity of recombinant proteins (% of rhFVIIa) in human FVIII or FIX deficient plasma. Data for panels C and D were derived from at least 4 experimental points. *P < .03 compared with mFVIIa. ND indicates not detectable. Fold increase in activity in panels C and D is indicated relative to mFVIIa. All data are shown as average ± 1 SD.
We subsequently investigated the binding of recombinant proteins to msTF or hsTF indirectly by measuring cleavage of a small peptidyl substrate (amidolytic activity of the FVIIa-TF complex). Murine FVIIa and its variants were stimulated in the presence of increasing concentrations of msTF or hsTF and showed similar binding compared with each other (Table 1), although binding to hsTF was not as tight as previously reported using surface plasmon resonance.10 Zymogen mFVII showed negligible amidolytic activity. Because the rhFVIIa-DVQ and rhFVIIa-VEAY exhibit enhanced intrinsic activity toward peptidyl and macromolecular substrates,7 we determined the activity of the recombinant mFVII/FVIIa proteins toward macromolecular substrate (FX), in the absence of TF (Figure 2B). Human FX was used as a substitute for murine FX because the latter was not commercially available. Under those experimental conditions, compared with mFVIIa, both mFVIIa-VEAY and mFVIIa-DVQ exhibited enhanced rates of activated hFX (hFXa) generation (P < .03) that ranged from 8-fold (mFVIIa-VEAY) to 17.9-fold (mFVIIa-DVQ). In contrast, rhFVIIa exhibited a rate of hFXa generation similar to mFVIIa (P = .11) and zymogen mFVII showed substantially reduced proteolytic activity (P < .04 vs mFVIIa). Together, these data demonstrate that the specific modifications present in the catalytic site of the mFVIIa variants do not alter binding to TF but substantially enhance their proteolytic activity, compared with mFVIIa.
Table 1.
Binding of recombinant proteins to human or murine soluble TF derived from at least 2 independent experiments
| Cofactor | Protein |
||||
|---|---|---|---|---|---|
| mFVII | mFVIIa | mFVIIa-DVQ | mFVIIa-VEAY | rhFVIIa | |
| hsTF | ND | 86.7 ± 22.8 | 42.4 ± 20.7 | 71.7 ± 22.2 | 12.1 ± 6.0 |
| msTF | ND | 2.0 ± 0.5 | 2.9 ± 0.8 | 3.2 ± 1.9 | ND |
Values, stated as Kd (nM) were derived from amidolytic activity assays.
ND indicates not determined (data fitting was not possible).
mFVIIa variants exhibit enhanced in vitro coagulant activity
Next, we examined the coagulant activity of recombinant proteins using extrinsic (PT) and intrinsic (aPTT) coagulation pathway assays, compared with rhFVIIa (100%). As expected,14 mFVIIa had similar extrinsic activity to rhFVIIa (91%), whereas mFVII had approximately 22% extrinsic activity (Figure 2C). Compared with mFVIIa, the mFVIIa-VEAY and mFVIIa-DVQ variants exhibited modestly reduced (P = .01) and elevated (P = .03) extrinsic activity, respectively (Figure 2C). However, in the intrinsic (TF-independent) clotting assay using human FVIII or FIX deficient plasmas, both mFVIIa variants showed increased activity in both plasmas (P < .002 vs mFVIIa; Figure 2D). The fold enhancement was not statistically different between FVIII and FIX deficient plasma (P > .07). Zymogen mFVII had minimal activity (< 1%) and none of the recombinant proteins exhibited appreciable coagulant activity in hFX-deficient plasma (data not shown). Similar results were obtained using mouse HA or HB plasma (data not shown). Both variants displayed enhanced inactivation in HA or HB mouse plasma in the order of mFVIIa-VEAY ≫ mFVIIa-DVQ > mFVIIa, that was antithrombin-dependent (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), similar to previous observations with their human counterparts.7 These data confirm the observed enhancement in proteolytic activity (see “Purification, amidolytic and proteolytic activity of mFVIIa, mFVIIa-DVQ and mFVIIa-VEAY”) and indicate that mFVIIa-VEAY and mFVIIa-DVQ had essentially unaltered TF-dependent but substantially enhanced TF-independent coagulant activity compared with mFVIIa.
mFVIIa-VEAY normalizes the hemophilia A phenotype at greatly reduced AAV vector doses and expression levels
The inactivation profiles of the mFVIIa variants suggest that their improved hemostatic properties in vitro may not necessarily translate to enhanced hemostatic efficacy in vivo in a gene transfer setting. This is particularly relevant for mFVIIa-VEAY, which exhibited the fastest inactivation. We chose the mFVIIa-VEAY variant to demonstrate whether improved hemostatic effects can be observed with enhanced activity FVIIa variants even with unfavorable inactivation profiles.
We constructed an AAV serotype 8 vector carrying either the mFVIIa or mFVIIa-VEAY transgene under the control of a liver-specific promoter/enhancer, as previously described.14 Preliminary dose-escalation studies in HA mice demonstrated that normalization of the hemophilic aPTT was achieved using tail vein administration of 1.2 × 1012 vector genomes (vg)/mouse for mFVIIa (P = .31 vs wild-type) and a substantially lower dose (40- to 100-fold) for AAV-mFVIIa-VEAY (mFVIIa-VEAY [L], P = .08 vs wild-type; Figure 3A). A dose of AAV-mFVIIa-VEAY comparable to AAV-mFVIIa resulted in superphysiologic reduction of the aPTT (mFVIIa-VEAY [H], P = .007 vs wild-type; Figure 3A). We thus determined the long-term efficacy (in vitro and in vivo) using AAV in 2 cohorts of HA mice, as follows: mice treated with AAV-mFVIIa (1.2 × 1012 vg/mouse) and mice treated with 40- to 100-fold lower doses of AAV-mFVIIa-VEAY (1.2-3 × 1010 vg/mouse, AAV-mFVIIa-VEAY [L]).
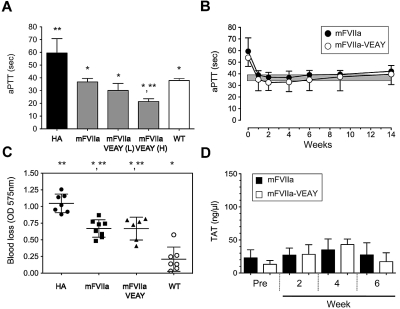
Figure 3.
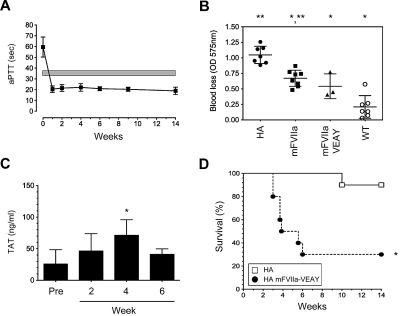
Hemostatic efficacy of mFVIIa-VEAY after gene transfer in hemophilia A mice. (A) Dose escalation study in HA mice receiving 1.2 × 1012 vg of AAV-mFVIIa, 1.2-3 × 1010 vg of AAV-mFVIIa-VEAY (low dose, mFVIIa-VEAY [L]) or 0.6-0.3 × 1012 vg of AAV-mFVIIa-VEAY (high dose, mFVIIa-VEAY [H]). Activated partial thromboplastin time (aPTT) values are shown for samples assayed at 6 weeks post AAV administration. Wild-type (WT) and HA aPTTs are also shown. Each cohort consisted of at least 3 mice. *P < .009 vs HA; **P < .007 vs WT. All AAV vectors were of serotype 8. (B) aPTT in HA mice after delivery of AAV-mFVIIa (1.2 × 1012 vg/mouse) or AAV-mFVIIa-VEAY (1.2-3 × 1010 vg/mouse). Each cohort consisted of 10 mice. The gray box indicates the range of aPTT values using human FVIII deficient plasma in hemostatically normal mice (37.9 ± 1.6 seconds, n = 10). (C) Blood loss after a tail clip injury in AAV-treated HA mice compared with wild-type (WT) and HA controls. *P < .002 compared with HA; **P < .001 vs WT. (D) TAT levels compared with baseline (Pre) in HA mice after gene transfer. All data are shown as average ± 1 SD.
Both cohorts exhibited long-term normalization of the hemophilic aPTT (Figure 3B; P > .05 for AAV-mFVIIa-VEAY [L] vs AAV-mFVIIa at all timepoints). However, this was achieved at ∼ 5-fold lower circulating protein levels for mFVIIa-VEAY compared with mFVIIa (0.6 ± 0.25 μg/mL vs 3.0 ± 0.75 μg/mL at the expression plateau [4 weeks after AAV administration], respectively; P < .002). To further confirm this observation, we determined relative mRNA levels in the transduced mouse livers for each cohort (harvested after 14 weeks after AAV administration). Both cohorts exhibited increased mFVII/mFVIIa transcript levels compared with untreated mice but AAV-mFVIIa-VEAY (L) treated mice had ∼ 10-fold lower levels than the AAV-mFVIIa treated mice (supplemental Figure 2).
We subsequently investigated whether hemostatic efficacy with mFVIIa-VEAY can be achieved at these greatly reduced vector doses/expression levels compared with mFVIIa, using 2 in vivo hemostatic challenges. In the tail clip assay, both AAV-mFVIIa and AAV-mFVIIa-VEAY (L) mice exhibited a similar (P = .98) reduction in blood loss compared with untreated HA mice (P < .002 for either cohorts vs HA) although not at levels seen with hemostatically normal mice (Figure 3C), similar to our previous observations.13,14 In another hemostatic challenge (FeCl3 carotid artery injury), both AAV-mFVIIa and AAV-mFVIIa-VEAY (L) mice exhibited stable vessel occlusion within 5 minutes in 33% of the cases after FeCl3 application, although partial occlusion was also observed in 2 of 9 AAV-mFVIIa treated mice (Table 2). In terms of safety, we did not observe any difference in levels of thrombin-antithrombin (TAT) complex for either cohort (Figure 3D).
Table 2.
FeCl3 vessel occlusion within 5 minutes of FeCl3 application in AAV-mFVIIa, AAV-mFVIIa-VEAY or untreated HA mice
| Mouse group | Mice with full occlusion (%) within 5 minutes | Mice with no occlusion (%) |
|---|---|---|
| AAV-mFVIIa* (n = 9) | 3 (33%) | 4 (45%) |
| AAV-mFVIIa-VEAY (n = 9) | 3 (33%) | 6 (67%) |
| HA, untreated (n = 11) | 0 (0%) | 11 (100%) |
Number of mice (n) is indicated for each group.
Two mice showed partial occlusion at 5-6 minutes after FeCl3 application.
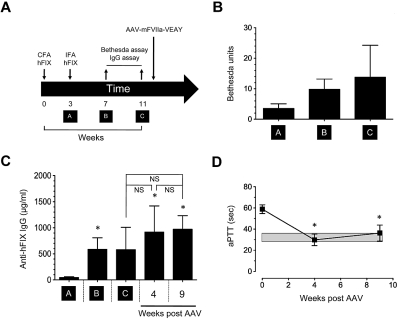
To further investigate hemostatic efficacy of mFVIIa-VEAY in a setting of hemophilic inhibitors, we used a protocol (Figure 4A) to generate inhibitors to human FIX (hFIX) in HB mice. This resulted in induction of neutralizing antibodies as measured by a Bethesda assay (range, 6-26 Bethesda units [BU], Figure 4B) as well as total anti-hFIX IgG (Figure 4C, timepoints A-C). We subsequently administered low-dose AAV-mFVIIa-VEAY (3 × 1010 vg/mouse), similar to those administered in HA mice (see “mFVIIa-VEAY normalizes the hemophilia A phenotype at greatly reduced AAV vector doses and expression levels”). Because the expressed mFVIIa-VEAY transgene product would result in erroneous Bethesda titer (because it is a clotting-based assay), we measured total anti-hFIX IgG after gene transfer as a surrogate test. We found that this was similar to pre-AAV infusion levels (weeks 4 and 9 vs timepoint C, P ≥ .2; Figure 4C). This indicates that the immune response to hFIX remained stable over the course of the experiment, after AAV administration. More importantly, AAV-mFVIIa-VEAY administration resulted in expression of mFVIIa-VEAY at ∼ 1 μg/mL (assayed at 4 or 9 weeks after AAV administration) and near normalization of the aPTT as seen in HA mice (compare Figure 4D with Figure 3B). This suggests that the presence of inhibitory antibodies does not change the hemostatic effects of mFVIIa-VEAY. This was also observed using AAV-mFVIIa injected at 1.2 × 1012 vg/mouse (P.M., A.F., unpublished observations, July 2009).
Figure 4.
Continuous expression of mFVIIa-VEAY in hemophilia B mice with inhibitors. (A) Scheme for generating anti–human FIX inhibitory antibodies in HB mice. Downward arrows indicate injection of complete or incomplete Freund adjuvant (CFA and IFA, respectively) complexed with 20 μg of hFIX or administration of AAV-expressing mFVIIa-VEAY. Upwards arrows indicate collection of tail blood to assay Bethesda titer and total anti-hFIX IgG. Timepoints A-C are indicated. (B) Bethesda titers of HB mice before administration of AAV-mFVIIa-VEAY. (C) Total anti-hFIX IgG in HB mice prior (timepoints A-C) and after administration of AAV-mFVIIa-VEAY. *P < .05 vs timepoint A. Nonsignificant (NS) differences are indicated. (D) aPTT in HB mice with inhibitors to hFIX after administration of AAV-mFVIIa-VEAY. *P < .05 vs baseline (week 0). A gray box indicates the range in hemostatically normal mice using human FIX–deficient plasma (32.1 ± 2.4 seconds, n = 10). All data shown as average ± 1 SD.
Together, these data suggest that, in agreement with the in vitro enhancement of intrinsic activity seen for mFVIIa-VEAY, in vivo hemostatic efficacy similar to mFVIIa can be achieved by lower level of expression of mFVIIa-VEAY and reduced AAV vector doses.
Overexpression of mFVIIa-VEAY in hemophilic mice results in rapid and increased mortality
Our previous observations of enhanced mortality in hemophilic mice overexpressing mFVIIa13 set a precedence for investigating the safety profile of FVIIa gene therapy. To establish this for mFVIIa-VEAY, we treated a cohort of HA mice with AAV-mFVIIa-VEAY at an identical (high) vector dose to AAV-mFVIIa (1.2 × 1012 vg/mouse, AAV-mFVIIa-VEAY [H]). In agreement with its enhanced intrinsic activity, the AAV-mFVIIa-VEAY (H) mice exhibited a sustained, supraphysiologic reduction of the aPTT (∼ 20 seconds; Figure 5A), well below levels observed for the other 2 cohorts (P < .001, compare to Figure 3B). Steady-state expression of mFVIIa-VEAY in this cohort was ∼ 8 μg/mL (at 4 weeks after AAV) and reduction in blood loss after a tail clip assay was similar to the mFVIIa cohort (Figure 5B, P = .28). Compared with hemostatically normal mice, the observed blood loss was not statistically different (Figure 5B, P = .07). However, we observed a time-dependent increase in TAT complex formation (Figure 5C), and a rapid decrease in survival to 30% within the first 6 weeks after vector administration. This was in contrast to untreated HA mice (Figure 5D, P < .004) or the other cohorts (AAV-mFVIIa and AAV-mFVIIa-VEAY [L]) that showed nearly 100% survival (data not shown) within the same experimental time period.
Figure 5.
Hemostatic efficacy of mFVIIa-VEAY after high-dose gene transfer in hemophilia A mice. (A) aPTT in HA mice after delivery of AAV-mFVIIa-VEAY (1.2 × 1012 vg/mouse). The initial cohort consisted of 10 mice. The gray box indicates the range of aPTT values using human FVIII-deficient plasma in hemostatically normal mice (37.9 ± 1.6 seconds, n = 10). (B) Blood loss after a tail clip assay, compared with AAV-mFVIIa–treated HA mice, or untreated HA and wild-type (WT) mice, whose data are depicted in Figure 3C and are repeated here for comparison. *P < .02 vs HA; **P < .002 vs WT. (C) TAT levels in AAV-mFVIIa-VEAY–treated mice as a function of time. *P < .03 vs baseline (Pre). (D) Survival of HA mice treated with high-dose AAV-mFVIIa-VEAY (AAV-mFVIIa-VEAY [H], ●), relative to untreated HA controls (□). All cohorts initially consisted of 10 mice. *P < .004 between HA-mFVIIa-VEAY (H) survival compared with untreated HA. All data (except in panel D) shown as average ± 1 SD.
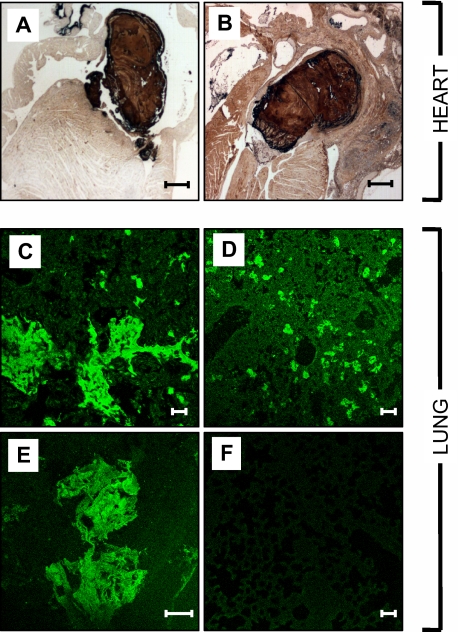
Histopathologic examination was used to further investigate the cause of death in 6 mice in the AAV-mFVIIa-VEAY (H) cohort. Necropsy revealed no gross abnormalities and histologic analyses in the spleen, liver or kidneys were normal compared with 6 untreated HA mice (data not shown). Major pathologic findings were confined to the heart and lungs. We observed visible thrombi in the heart (6 of 6 mice; Figure 6A-B), predominantly in the atria, as well as fibrosis (4 of 6 mice) and occasional inflammatory cell infiltrates in the myocardium (2 of 6 mice; data not shown). In the lungs, we observed hemorrhage (5 of 6 mice) as well as fibrin deposition that extended into the lung parenchyma (Figure 6C-E), that was absent in untreated HA controls (Figure 6E). These findings are similar to those observed previously in transgenic mice overexpressing mFVIIa13 and, together with the elevated levels of TAT, pulmonary hemorrhage and fibrin deposition, are indicative of ongoing clot formation in the microcirculation as the most likely cause of death in these mice.
Figure 6.
Histologic analysis of hearts and lungs of HA mice overexpressing mFVIIa-VEAY. Fibrin(ogen) immunohistochemical staining in the heart (A-B) showing visible thrombi in the atria. (C-F) Immunofluorescent staining for fibrin(ogen) in the lungs of AAV-mFVIIa-VEAY HA–treated mice (C-E) showing fibrin(ogen) deposition in the lung parenchyma in contrast to untreated HA control mice (F). Scale bars, 500 μm (A-B) and 50 μm (C-F).
Discussion
The development of inhibitory antibodies against FVIII or FIX remains the most serious and most common complication of the current treatment of hemophilia by protein replacement.34 Although treatment of inhibitor patients with bolus infusion of rhFVIIa is effective, frequent and high dose administration is necessary. As an alternative to bolus dosing, we have previously used gene delivery to continuously express an engineered activated FVII transgene and shown efficacy in small (murine) and large (canine) hemophilic models.14,15 In particular, multiyear expression of gene-based cFVIIa in hemophilic dogs was safe and resulted in elimination of spontaneous bleeds15 that are characteristic in that model.35 However, that study also underscored that improvements aimed to lower the hemostatically effective viral vector dose will be necessary before a human application.
We hypothesized that enhancement of the catalytic function of our FVIIa transgene would confer hemostatic efficacy at lower expression levels, and hence, reduce the vector dose. The hemophilia mouse model, having well-established in vivo hemostatic endpoints, offers a convenient platform to systematically test this hypothesis. Here, we generated and characterized 2 murine FVIIa variants with enhanced intrinsic activity, that harbored corresponding mutations from enhanced activity human FVIIa variants with catalytic domain modifications.7 A series of in vitro assays using purified recombinant proteins demonstrated that the murine FVIIa variants model their human counterparts in terms of enhanced activity as well as faster inhibition kinetics compared with the parent mFVIIa molecule. Although these opposing attributes may limit efficacy in a protein infusion setting, continuous expression of such FVIIa variants via gene delivery results in more favorable pharmacokinetic parameters. In the latter setting, hemostatic efficacy will be governed by the balance of expression level, rate of inhibition/secretion, clearance and extravascular dissemination of the expressed transgene. Our in vivo data using mFVIIa-VEAY clearly demonstrate that continuous expression can overcome its relatively unfavorable inactivation profile, and result in long-term hemostatic efficacy at lower expression levels and vector doses relative to the parent mFVIIa transgene. This was evident using 2 in vivo hemostatic challenge assays that demonstrated similar responses between HA mice expressing mFVIIa (∼ 3 μg/mL) or mFVIIa-VEAY (∼ 0.6 μg/mL). In addition, our data show that the presence of inhibitors to FIX did not appear to change the hemostatic effect of mFVIIa-VEAY. This is the first report for liver-directed FVIIa gene transfer in a hemophilia in vivo model complicated by inhibitors and lends further support for the therapeutic application of this approach. Clearly, the use of mFVIIa-DVQ or other variants with substantially enhanced activity would be expected to have more pronounced effects than mFVIIa-VEAY shown here.
We have previously shown that expression of mFVIIa in the range of 3-4 μg/mL in hemophilia B mice transgenic for mFVIIa results in mortality within months from birth that was positively associated with FX activity levels.13 Here, expression of ∼ 8 μg/mL of mFVIIa-VEAY in hemophilia A mice, nearly 14-fold higher than therapeutic for this variant (∼ 0.6 μg/mL), resulted in increased mortality but at a substantially shorter timeframe than previously seen (weeks vs months13). This observation thus identifies the enzymatic activity of the mFVIIa transgene as a risk factor, in addition to expression levels. In contrast, hemophilia A mice expressing ∼ 0.6 μg/mL (mFVIIa-VEAY) did not show evidence of a prothrombotic phenotype within the duration of this study, suggestive of a relatively wide therapeutic window of effecting hemostasis with this enhanced variant of mFVIIa.
Earlier clinical studies demonstrated an upper limit to doses of AAV serotype 2 vector that could be infused systemically into humans without triggering an immune response that destroyed the transduced cells.36,37 Thus the ability to achieve hemostasis at lower vector doses is a key step in successful translation to clinical studies for AAV-mediated gene transfer. The data presented here suggest a possible strategy to attain this goal with gene-based FVIIa therapy. Clearly, the issue of safety is of prime importance when using enhanced activity FVIIa variants in this setting. However, there are 2 experimental observations that limit the potential for thrombotic complications: (1) reduced-level expression of mFVIIa-VEAY (∼ 0.6 μg/mL vs ∼ 3 μg/mL for mFVIIa) in hemophilia A mice is hemostatically effective without thrombotic manifestations, as shown here; (2) long-term expression of cFVIIa at levels of ∼ 2 μg/mL is efficacious and not associated with thrombosis in hemophilic dogs, a large animal model of hemophilia.15 Based on these preclinical data, it seems unlikely that high expression levels of high-activity FVIIa transgene(s) will be necessary for hemostatic efficacy, thus minimizing the overall thrombotic risk. Using inducible expression cassettes under the control of exogenously supplied activator drugs can further reduce this. Because of the high conservation between canine and human FVII (75% identity), our results also suggest that variants of cFVIIa with properties analogous to those described here can be generated. Further gene-transfer experiments in hemophilic dogs with such variants will address the issue of improvements in hemostatic efficacy as well as safety in a clinically relevant animal model of hemophilia.
In addition to on-demand therapy, daily treatment of inhibitor patients prophylactically with rhFVIIa at pharmacologic doses for 3 months has been shown to reduce the number of bleeds, an effect that surprisingly extended in the 3-month follow-up.38 The precise mechanism behind these extended hemostatic effects in the absence of administered rhFVIIa is unclear; it may involve the presence of rhFVIIa in the extravascular space39 as has been shown in mice.40,41 Remarkably, we have previously shown that portal vein administration of AAV-cFVIIa at a subtherapeutic vector dose in a hemophilia B dog resulted in elimination of spontaneous bleeding episodes.15 Although more confirmatory experiments are necessary, this suggests that continuous, low-level expression of FVIIa, as facilitated by gene transfer, may have long-term clinical benefits similar to a prophylactic rhFVIIa regimen. Within this context, the ability of enhanced activity FVIIa variants to effect hemostasis at reduced AAV vector doses, represents an additional improvement for this application that can be tested further in hemophilic dogs.
In conclusion, we have applied modifications in the catalytic domain of mFVIIa that resulted in mFVIIa variants with increased, TF-independent activity in vitro. After gene transfer in a mouse model of hemophilia, our data using the mFVIa-VEAY variant underscore the following: (1) low-level expression of mFVIIa-VEAY (∼ 0.6 μg/mL) is safe and hemostatically effective even in the presence of inhibitors, having a 5-fold advantage compared with mFVIIa; and (2) expression of this variant at ∼ 14-fold higher (∼ 8 μg/mL) levels in hemophilic mice results in rapid mortality. The data are thus suggestive of a relatively wide therapeutic window for this approach that can overcome the limitation of the high AAV-FVIIa vector doses previously shown as necessary for hemostatic efficacy in hemophilic dogs.15 However, as variants of other coagulation factors (eg, FIX-Padua42 or FIX-K5A/V10K and FIX-R338A43) may offer improved hemostatic properties in gene transfer settings for hemophilia, the data presented here also indicate that careful consideration should be given along the axis of hemostasis (efficacy) and thrombosis (safety) using such variants.
Supplementary Material
Acknowledgments
The authors thank Drs R. M. Camire, R. Toso, V. R. Arruda, U. Hedner, M. Ezban, and E. Persson for helpful discussions; Drs S. Krishnaswamy and M. Ezban for the preparation of human and murine soluble TF, respectively; Dr R. M. Camire for the preparation of human FX/FXa, and Daniel Martinez at the Children's Hospital of Philadelphia Pathology Core Facility.
The authors also thank the Howard Hughes Medical Institute and the Pennsylvania Department of Health for financial support. The Pennsylvania Department of Health specifically disclaims responsibility for any analysis, interpretations, or conclusions.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M. designed and performed research, analyzed data and wrote the manuscript; E.R. designed and performed research and analyzed data; A.F, H.D.D., L.I., and G.P. performed research; S.Z. provided the AAV vector; R.M.B. performed histopathologic analysis; and K.A.H. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Paris Margaritis, 3501 Civic Center Blvd, 5000 Colket Translational Research Bldg, The Children's Hospital of Philadelphia, Philadelphia, PA 19104; e-mail: margaritis@e-mail.chop.edu.
References
- 1.Hedner U. Recombinant factor VIIa: its background, development and clinical use. Curr Opin Hematol. 2007;14(3):225–229. doi: 10.1097/MOH.0b013e3280dce57b. [DOI] [PubMed] [Google Scholar]
- 2.Hedner U. Recombinant factor VIIa (Novoseven) as a hemostatic agent. Semin Hematol. 2001;38(4 Suppl 12):43–47. doi: 10.1016/s0037-1963(01)90147-7. [DOI] [PubMed] [Google Scholar]
- 3.Weimer T, Wormsbacher W, Kronthaler U, Lang W, Liebing U, Schulte S. Prolonged in-vivo half-life of factor VIIa by fusion to albumin. Thromb Haemost. 2008;99(4):659–667. doi: 10.1160/TH07-08-0525. [DOI] [PubMed] [Google Scholar]
- 4.Yatuv R, Dayan I, Carmel-Goren L, et al. Enhancement of factor VIIa haemostatic efficacy by formulation with PEGylated liposomes. Haemophilia. 2008 doi: 10.1111/j.1365-2516.2008.01741.x. [DOI] [PubMed] [Google Scholar]
- 5.Harvey SB, Stone MD, Martinez MB, Nelsestuen GL. Mutagenesis of the gamma-carboxyglutamic acid domain of human factor VII to generate maximum enhancement of the membrane contact site. J Biol Chem. 2003;278(10):8363–8369. doi: 10.1074/jbc.M211629200. [DOI] [PubMed] [Google Scholar]
- 6.Nelsestuen GL, Stone M, Martinez MB, Harvey SB, Foster D, Kisiel W. Elevated function of blood clotting factor VIIa mutants that have enhanced affinity for membranes. Behavior in a diffusion-limited reaction. J Biol Chem. 2001;276(43):39825–39831. doi: 10.1074/jbc.M104896200. [DOI] [PubMed] [Google Scholar]
- 7.Persson E, Bak H, Ostergaard A, Olsen OH. Augmented intrinsic activity of Factor VIIa by replacement of residues 305, 314, 337 and 374: evidence of two unique mutational mechanisms of activity enhancement. Biochem J. 2004;379(Pt 2):497–503. doi: 10.1042/BJ20031596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persson E, Kjalke M, Olsen OH. Rational design of coagulation factor VIIa variants with substantially increased intrinsic activity. Proc Natl Acad Sci U S A. 2001;98(24):13583–13588. doi: 10.1073/pnas.241339498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss J, Scharling B, Ezban M, Sorensen TM. Evaluation of the safety and pharmacokinetics of a fast-acting recombinant FVIIa analogue, NN1731, in healthy male subjects. J Thromb Haemost. 2008 doi: 10.1111/j.1538-7836.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- 10.Petersen LC, Norby PL, Branner S, et al. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: a human-murine species compatibility study. Thromb Res. 2005;116(1):75–85. doi: 10.1016/j.thromres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Lauritzen B, Tranholm M, Ezban M. rFVIIa and a new enhanced rFVIIa-analogue, NN1731, reduce bleeding in clopidogrel-treated and in thrombocytopenic rats. J Thromb Haemost. 2009 doi: 10.1111/j.1538-7836.2009.03287.x. [DOI] [PubMed] [Google Scholar]
- 12.Tranholm M, Kristensen K, Kristensen AT, Pyke C, Rojkjaer R, Persson E. Improved hemostasis with superactive analogs of factor VIIa in a mouse model of hemophilia A. Blood. 2003;102(10):3615–3620. doi: 10.1182/blood-2003-05-1369. [DOI] [PubMed] [Google Scholar]
- 13.Aljamali MN, Margaritis P, Schlachterman A, et al. Long-term expression of murine activated factor VII is safe, but elevated levels cause premature mortality. J Clin Invest. 2008;118(5):1825–1834. doi: 10.1172/JCI32878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margaritis P, Arruda VR, Aljamali M, Camire RM, Schlachterman A, High KA. Novel therapeutic approach for hemophilia using gene delivery of an engineered secreted activated Factor VII. J Clin Invest. 2004;113(7):1025–1031. doi: 10.1172/JCI20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margaritis P, Roy E, Aljamali MN, et al. Successful treatment of canine hemophilia by continuous expression of canine FVIIa. Blood. 2009;113(16):3682–3689. doi: 10.1182/blood-2008-07-168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Wang H, Bell P, et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther. 2010;18(1):118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Lillicrap D, Patarroyo-White S, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108(1):107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 18.Sabatino DE, Lange AM, Altynova ES, et al. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther. 2011;19(3):442–449. doi: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Calcedo R, Nichols TC, et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105(8):3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 20.Toso R, Bernardi F, Tidd T, et al. Factor VII mutant V154G models a zymogen-like form of factor VIIa. Biochem J. 2003;369(Pt 3):563–571. doi: 10.1042/BJ20020888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruf W, Rehemtulla A, Morrissey JH, Edgington TS. Phospholipid-independent and -dependent interactions required for tissue factor receptor and cofactor function. J Biol Chem. 1991;266(4):2158–2166. [PubMed] [Google Scholar]
- 22.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10(1):119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 23.Lin HF, Maeda N, Smithies O, Straight DL, Stafford DW. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90(10):3962–3966. [PubMed] [Google Scholar]
- 24.Tai SJ, Herzog RW, Margaritis P, et al. A viable mouse model of factor X deficiency provides evidence for maternal transfer of factor X. J Thromb Haemost. 2008;6(2):339–345. doi: 10.1111/j.1538-7836.2008.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields PA, Arruda VR, Armstrong E, et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4(3):201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 26.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 27.Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 30.Persson E, Olsen OH. Assignment of molecular properties of a superactive coagulation factor VIIa variant to individual amino acid changes. Eur J Biochem. 2002;269(23):5950–5955. doi: 10.1046/j.1432-1033.2002.03323.x. [DOI] [PubMed] [Google Scholar]
- 31.Banner DW, D'Arcy A, Chene C, et al. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380(6569):41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 32.Nicolaisen EM, Thim L, Jacobsen JK, et al. FVIIa derivatives obtained by autolytic and controlled cathepsin G mediated cleavage. FEBS Lett. 1993;317(3):245–249. doi: 10.1016/0014-5793(93)81285-8. [DOI] [PubMed] [Google Scholar]
- 33.Toso R, Pinotti M, High KA, Pollak ES, Bernardi F. A frequent human coagulation Factor VII mutation (A294V, c152) in loop 140s affects the interaction with activators, tissue factor and substrates. Biochem J. 2002;363(Pt 2):411–416. doi: 10.1042/0264-6021:3630411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedner U. History of rFVIIa therapy. Thromb Res. 2010;125(Suppl 1):S4–S6. doi: 10.1016/j.thromres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Russell KE, Olsen EH, Raymer RA, et al. Reduced bleeding events with subcutaneous administration of recombinant human factor IX in immune-tolerant hemophilia B dogs. Blood. 2003;102(13):4393–4398. doi: 10.1182/blood-2003-05-1498. [DOI] [PubMed] [Google Scholar]
- 36.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 37.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 38.Konkle BA, Ebbesen LS, Erhardtsen E, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost. 2007;5(9):1904–1913. doi: 10.1111/j.1538-7836.2007.02663.x. [DOI] [PubMed] [Google Scholar]
- 39.Hedner U, Lee CA. First 20 years with recombinant FVIIa (NovoSeven). Haemophilia. 2011;17(1):e172–e182. doi: 10.1111/j.1365-2516.2010.02352.x. doi: 10.1111/j.1365-2516.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- 40.Gopalakrishnan R, Hedner U, Ghosh S, et al. Bio-distribution of pharmacologically administered recombinant factor VIIa (rFVIIa). J Thromb Haemost. 2010;8(2):301–310. doi: 10.1111/j.1538-7836.2009.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman M, Colina CM, McDonald AG, Arepally GM, Pedersen L, Monroe DM. Tissue factor around dermal vessels has bound factor VII in the absence of injury. J Thromb Haemost. 2007;5(7):1403–1408. doi: 10.1111/j.1538-7836.2007.02576.x. [DOI] [PubMed] [Google Scholar]
- 42.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 2009;361(17):1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 43.Schuettrumpf J, Herzog RW, Schlachterman A, Kaufhold A, Stafford DW, Arruda VR. Factor IX variants improve gene therapy efficacy for hemophilia B. Blood. 2005;105(6):2316–2323. doi: 10.1182/blood-2004-08-2990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.