Abstract
Human secondary lymphoid tissues (SLTs) contain interleukin-22 (IL-22)–producing cells with an immature NK phenotype. Given their location, these cells are difficult to study. We have generated large numbers of NK22 cells from hematopoietic stem cells. HSC-derived NK22 cells show a CD56+CD117highCD94− phenotype, consistent with stage III NK progenitors. Like freshly isolated SLT stage III cells, HSC-derived NK22 cells express NKp44, CD161, CCR6, IL1 receptor, AHR, and ROR-γτ. IL-1β and IL-23 stimulation results in significant IL-22 but not interferon-γ production. Supernatant from these cells increases CD54 expression on mesenchymal stem cells. Thus, IL-22–producing NK cells can be generated in the absence of SLT. HSC-derived NK22 cells will be valuable in understanding this rare NK subset and create the opportunity for human translational clinical trials.
Introduction
Recent studies have identified human NK lineage cells, including lymphoid tissue inducer–like (LTi-like) cells,1,2 stage III NK progenitors,3 and/or NK22 cells4 that reside in secondary lymphoid tissue (SLT) and produce interleukin-22 (IL-22). Although these cells differ by tissue location (tonsil, lymph node, and Peyer patches) and age of donor (fetal and adult tissues), they have many unifying features, including the expression of Th17/Th22-associated transcription factors (ie, ROR-γτ and aryl hydrocarbon receptor [AHR])2,4 and surface phenotype characteristic of developing NK cells, including CD117, CD127, NKp44, and CCR6 and the lack of granzyme, perforin, and killer immunoglobulin receptors.3–5 IL-22–producing NK lineage cells are functionally characterized by low/absent cytotoxicity and the induction of adhesion molecules on mesenchymal stem cells (MSCs).3–5
The study of human IL-22–producing NK lineage cells is limited by their location in SLT. Accordingly, investigators use small quantities of material obtained from either fetal tissue or surgical specimens, potentially in the setting of pathology. To date, no reports have investigated the development of these cells from hematopoietic stem cells (HSCs). In prior studies, we cultured umbilical cord blood (UCB) CD34+ HSCs on murine fetal liver cells to characterize human NK development.6–10 This approach yields NK intermediates with phenotypic and functional features similar to those in SLT.5,11 Because this system closely recapitulates ontogeny, we hypothesized that NK22 cells could be obtained from HSCs.
Methods
NK differentiation cultures
Briefly, the murine embryonic liver cell line EL08.1D2 was cultured as previously described.6 Purified UCB CD34+ HSCs were cultured on irradiated EL08.1D2 (3000 cGy) in the presence of IL-3 (first week), Fms-like tyrosine kinase 3 ligand, stem cell factor, IL-7, and IL-15 as previously described.6
FACS
At specified times, cells were stained with antibodies and subjected to fluorescence-activated cell sorter (FACS). Antibodies included: CD54, CD56, CD117, CD94, CCR6, CD161, interferon-γ (IFN-γ, BD Biosciences), and IL-1βR (R&D Systems), NKp30 NKp44, and NKp46 (Beckman Coulter), AHR (Santa Cruz Biotechnology), and ROR-c, CD127, and IL-22 (eBioscience). Intracellular staining was performed using cytofix/cytoperm (BD Biosciences). Data were analyzed using FlowJo software Version 7.6.3.
PCR
Total RNA was isolated from specified populations and reverse-transcribed using standard techniques. Polymerase chain reaction (PCR) was performed using primers for IL-22: forward, CCCATCAGCTCCCACTGC; reverse, GGCACCACCTCCTGCATATA; and GAPDH: forward, GTCGGAGTCAACGGATT; reverse, AAGCTTCCCGTTCTCAG. AHR and ROR-γt expression by quantitative reverse-transcribed PCR was with primer/probe mix Hs00169233_m1, targeting NM_001621.4 and with specific primers (forward, 5′-AGG-CGC-TGC-TGA-GAG-G-3′; reverse, 5′-CCT-TGG-CTC-CCT-GTC-CTT-3′; and TaqMan probe, 5′-CCT-CGC-CCC-GCC-TCT-3′), respectively. The latter primers were designed based on NM_001001523.1. Analysis was with the ΔΔCt method, normalized to 18s RNA.
IL-22 enzyme-linked immunosorbent assay
IL-22 was detected by enzyme-linked immunosorbent assay using DuoSet ELISA kits (R&D Systems). In some experiments, recombinant human IL-1β and IL-23 or IL-12 and IL-18 (R&D Systems) was added.
Tonsil cell suspensions
After University of Minnesota Institutional Review Board–approved informed consent for research use, in accordance with the Declaration of Helsinki, tonsils were obtained from the tissue procurement facility (University of Minnesota). Single-cell suspensions were prepared from fresh tonsils as described.12
MSC cocultures
Supernatant from specified NK populations was cocultured with MSCs for 48 hours, and MSCs were subjected to FACS expression.
Results and discussion
Recently, LTi cells have been isolated from human fetal tissues.1,2 These cells lack CD56, produce IL-22, and acquire CD56 on culture with IL-15.2 Alternatively, CD56+NKp44+ cells that produce IL-22 have been isolated from human gastrointestinal lymphoid tissue.4 Still others have identified IL-22–producing stage III NK progenitors that are CD56+/−CD117+CD94−.3 We investigated whether IL-22–producing NK cells could be derived from CD34+ UCB HSCs. As shown in Figure 1A, after 21 days of culture, both CD56− and CD56+ cells are present. Based on CD56, 2 populations were purified and assayed for IL-22 transcripts. Neither constitutively produced IL-22. After IL-1β/IL-23 stimulation, only CD56+ cells produced IL-22 mRNA (Figure 1B) and protein (Figure 1C). Prior studies showed that stage III NK cells (lin−CD56+/−CD117highCD94−) isolated from tonsils produce IL-22, whereas stage IV cells (CD56+/−CD117lowCD94+) do not.3 Using this in vitro NK-cell developmental system, we previously characterized similar HSC-derived NK populations (Figure 1D).6 To test for IL-22 production, these populations were FACS purified. After stimulation, IL-22 mRNA and protein were only detected in the stage III, CD56+CD117highCD94− cells (Figure 1E-F).
Figure 1.
HSC-derived CD56+CD117highCD94− NK cells produce IL-22 on stimulation. (A) HSC-derived NK cultures at day 21, showing heterogeneous CD56 expression. (B-C) Purified CD56+ and CD56− cells were assessed for expression of IL-22 mRNA and protein at rest and after activation with IL-1β (10 ng/mL) and IL-23 (40 ng/mL). (D) FACS at day 28 of culture, showing that the CD56+ cells can be divided into CD117highCD94− and CD117low/−CD94+ fractions. (E-F) CD117highCD94− and CD117low/−CD94+ fractions were FACS-purified and assessed for IL-22 mRNA expression and protein expression at rest and after activation with IL-1β (10 ng/mL) and IL-23 (40 ng/mL). Results are representative of more than 3 donors.
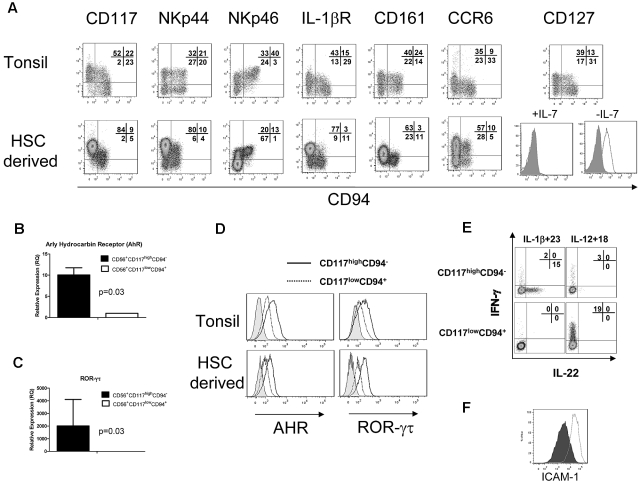
HSC-derived NK cells were compared with the CD3−CD19−CD14−CD56+/−CD117+ lymphoid fraction from tonsillar mononuclear cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Like tonsillar cells, the HSC-derived cells showed a high proportion of CD56+/−CD117highCD94− cells (Figure 2A). This fraction of HSC-derived cells expressed high levels of NKp44, heterogeneous NKp46, and lacked granzyme and perforin (Figure 2A; and data not shown).6 Similar to freshly isolated cells, HSC-derived NK cells expressed the IL-1 receptor.1,13 Interestingly, a minor fraction of HSC-derived stage IV cells (CD56+CD117low/−CD94+) also expressed IL-1 receptor but did not produce IL-22 after IL-1β/IL-23 stimulation (Figure 1E-F). Like stage III NK cells in tonsils, HSC-derived NK cells expressed CD161 and the lymph node homing receptor CCR6. The IL-7 receptor (CD127) was detected in tonsils, but not in HSC-differentiated cells. Removal of IL-7 from the culture media for 1 week led to CD127 reexpression on the CD56+CD117highCD94− fraction of HSC-derived cells (Figure 2A), suggesting that IL-7 down-regulates CD127 or occupies the CD127 epitope binding region.
Figure 2.
HSC-derived CD56+CD117highCD94− NK cells are phenotypically and functionally similar to SLT-derived stage III cells or NK22 cells. (A) HSC-derived CD56+CD117highCD94− NK cells were compared with CD3−CD19−CD14−CD56+/−CD117+ lymphoid fraction of tonsillar mononuclear cells (supplemental Figure 1, gating strategy). Expression of the key receptors was compared, including CD117, NKp44, NKp46, IL-1 receptor, CD161, and CCR6. CD127 (IL-7R) expression is shown with cultures containing IL-7 (left) and when IL-7 is removed from the media for 7 days (right; open histogram). FACS plots for CD127 are gated on the CD56+CD117highCD94− cell fraction. Expression of HSC-derived NK22 cells for AHR (B) and ROR-γτ (C) by quantitative reverse-transcribed PCR. (D) The expression of AHR and ROR-γτ by intracellular FACS staining. Histograms represent isotype controls (gray) or electronic gating on CD56+/−CD117highCD94− (solid) or CD56+/−CD117lowCD94− (dotted). Tonsillar cells were first gated on the CD3−CD14−CD19− fraction. HSC-derived cells, which lack T and B cells, were first gated on the CD56+ fraction. (E) Intracellular expression of IL-22 and IFN-γ after stimulation of HSC-derived NK cells with either IL-1β + IL-23 or IL-12 + IL-18. Cells were obtained at day 28 of culture and stimulated, and intracellular staining for IL-22 or IFN-γ was performed. Results are representative of 3 donors. (F) Supernatant from HSC-derived NK cells can increase expression of ICAM-1 on MSCs. Shown are the FACS plots of ICAM-1 expression after 48 hours of coculture with media supplemented with IL-1β and IL-23 (closed histogram) or with supernatant from in vitro derived CD56+CD117highCD94− NK cells activated with IL-1β and IL-23 (open histogram). Results are representative of more than 3 donors.
Similar to fresh tonsillar cells, HSC-derived CD56+CD117highCD94− cells showed expression of the transcription factors AHR and ROR-γτ by both quantitative reverse-transcribed PCR and FACS (Figure 2B-D). Intracellular IL-22 was absent from resting HSC-derived CD56+CD117highCD94− cells but was present in freshly isolated tonsils (Figure 1F; and data not shown). On stimulation with IL-1β/IL-23, a fraction of HSC-derived CD117highCD94− NK cells showed detectable intracellular IL-22. In contrast, IL-12/IL-18 stimulation did not induce IL-22 but did lead to IFN-γ production mainly in the CD117lowCD94+ fraction (Figure 2E). There were essentially no cells that coexpressed IL-22 and INF-γ. To further characterize HSC-derived NK22 cells for LTi-like activity, supernatant from IL-1β/IL-23 activated cells was cocultured with MSCs. An increase in ICAM expression on MSCs was observed (Figure 2F). To establish the potential “yield” of HSC-derived NK22 cells from these cultures, cells with a CD56+CD117highCD94−IL-1 receptor+ phenotype were defined as NK22 cells. Using this definition, we observed 1036 plus or minus 259-fold expansion from UCB CD34+ progenitors over 21 to 28 days of culture (n = 4 donors).
One complexity in understanding human IL-22–producing NK cells is their location within SLT. This leads to difficulty in obtaining sufficient quantities of cells for study. It has been speculated that, through IL-22 elaboration, these cells play an important role in lymph node homeostasis and maintenance of mucosal barriers.4,14 After allogeneic hematopoietic cell transplantation, damage to SLT architecture occurs15 and is associated with delayed immune reconstitution.16 As well, gastrointestinal tract injury has been implicated in graft-versus-host disease induction through a cascade involving lipopolysaccharide translocation from the gastrointestinal tract, resulting in accessory cell activation followed by IL-1 and tumor necrosis factor production.17 In the SLT, stage III NK cells are in close proximity to dendritic cells that produce IL-1β.13 Here we describe a simple method leading to more than 1000 times expansion of IL-22–producing NK lineage cells from HSCs. This is the first time that cells with functional properties previously described for NK22 and IL-22–producing stage III NK cells have been differentiated from HSCs. This suggests that the NK22 cells and NK cells are tightly linked in ontogeny and that their final fate can be determined by cytokines encountered locally. Moreover, SLT is not required for their development. HSC-derived IL-22–producing NK cells will facilitate understanding of the lineage development of these cells and has the potential for translational studies, including posttransplantation adoptive transfer.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health National Cancer Institute (P01-CA65493; B.R.B., J.S.M., and M.R.V.), Children's Cancer Research Fund (B.R.B. and M.R.V.), American Cancer Society (RSG-08-181-LIB) (M.R.V.), and Leukemia Research Fund (M.R.V.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Q.T. and Y.-O.A. planned and performed experiments; P.S. provided critical reagents and critical review of the manuscript; B.R.B. assisted with experimental design and critical review of the manuscript; J.S.M. assisted with experimental design and critical review of the manuscript; and M.R.V. wrote the manuscript and oversaw all aspects of this project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. Verneris, University of Minnesota, Department of Pediatrics, Division of Hematology/Oncology and Blood and Marrow Transplantation, 660 CCRB, 425 E River Rd, Minneapolis, MN 55455; e-mail: verneris@umn.edu.
References
- 1.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207(2):281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cupedo T, Crellin NK, Papazian N, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10(1):66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 3.Hughes T, Becknell B, McClory S, et al. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113(17):4008–4010. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 6.Grzywacz B, Kataria N, Sikora M, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108(12):3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachanova V, McCullar V, Lenvik T, et al. Activated notch supports development of cytokine producing NK cells which are hyporesponsive and fail to acquire NK cell effector functions. Biol Blood Marrow Transplant. 2009;15(2):183–194. doi: 10.1016/j.bbmt.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83(9):2594–2601. [PubMed] [Google Scholar]
- 9.Miller JS, McCullar V, Punzel M, Lemischka IR, Moore KA. Single adult human CD34(+)/Lin−/CD38(−) progenitors give rise to natural killer cells, B-lineage cells, dendritic cells, and myeloid cells. Blood. 1999;93(1):96–106. [PubMed] [Google Scholar]
- 10.McCullar V, Oostendorp R, Panoskaltsis-Mortari A, et al. Mouse fetal and embryonic liver cells differentiate human umbilical cord blood progenitors into CD56-negative natural killer cell precursors in the absence of interleukin-15. Exp Hematol. 2008;36(5):598–608. doi: 10.1016/j.exphem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22(3):295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Maher DM, Zhang ZQ, Schacker TW, Southern PJ. Ex vivo modeling of oral HIV transmission in human palatine tonsil. J Histochem Cytochem. 2005;53(5):631–642. doi: 10.1369/jhc.4A6534.2005. [DOI] [PubMed] [Google Scholar]
- 13.Hughes T, Becknell B, Freud AG, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32(6):803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trifari S, Spits H. IL-22-producing CD4(+) T cells: middle-men between the immune system and its environment. Eur J Immunol. 2010;40(9):2369–2371. doi: 10.1002/eji.201040848. [DOI] [PubMed] [Google Scholar]
- 15.Horny HP, Horst HA, Ehninger G, Kaiserling E. Lymph node morphology after allogeneic bone marrow transplantation for chronic myeloid leukemia: a histological and immunohistological study focusing on the phenotype of the recovering lymphoid cells. Blut. 1988;57(1):31–40. doi: 10.1007/BF00320632. [DOI] [PubMed] [Google Scholar]
- 16.Kelly RM, Goren EM, Taylor PA, et al. Short-term inhibition of p53 combined with keratinocyte growth factor improves thymic epithelial cell recovery and enhances T-cell reconstitution after murine bone marrow transplantation. Blood. 2010;115(5):1088–1097. doi: 10.1182/blood-2009-05-223198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17(4):187–194. doi: 10.1016/s0268-960x(03)00009-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.