Abstract
The microvasculature assumes an inflammatory and procoagulant state in a variety of different diseases, including sickle cell disease (SCD), which may contribute to the high incidence of ischemic stroke in these patients. This study provides evidence for accelerated thrombus formation in arterioles and venules in the cerebral vasculature of mice that express hemoglobin-S (βs mice). Enhanced microvascular thrombosis in βs mice was blunted by immunologic or genetic interventions that target tissue factor, endothelial protein C receptor, activated protein C, or thrombin. Platelets from βs mice also exhibited enhanced aggregation velocity after stimulation with thrombin but not ADP. Neutropenia also protected against the enhanced thrombosis response in βs mice. These results indicate that the cerebral microvasculature is rendered vulnerable to thrombus formation in βs mice via a neutrophil-dependent mechanism that is associated with an increased formation of and enhanced platelet sensitivity to thrombin.
Introduction
Sickle cell disease (SCD) is a chronic, genetic disease affecting the vasculature of various organs, including the lungs and brain, with affected tissues assuming an inflammatory phenotype.1 Sickle cell anemia is characterized by recurring acute vasoocclusive episodes and chronic damage to multiple organs.2 Many morbid consequences of SCD, such as stroke (the prevalence of stroke in SCD patients is 8%-10%), are believed to result from microvascular blood flow impairment.3,4 Histopathologic evaluation indicates that large vessel narrowing with superimposed thrombosis is the most common cause of ischemic stroke in children with SCD.5 Risk factors for ischemic stroke in SCD patients include: a hemoglobin-S (HbS) phenotype, low steady-state Hb concentrations, high leukocyte counts, and elevated systolic blood pressure.6
A fine balance normally exists between the procoagulant and anticoagulant functions of blood, allowing vessel walls to prevent unwanted hemorrhage and thrombosis.3 SCD is associated with abnormalities in coagulant/anticoagulant pathways that tend to favor thrombus formation; these include: increased tissue factor (TF), accelerated thrombin generation, increased D-dimer (a marker of increased fibrolysis) and prothrombin fragment 1.2 (a marker of thrombin generation), and increased circulating fibrinogen, VWF, and clotting factors VII and VIII.7–9 Patients with SCD also have reduced protein C and S levels.10 Despite the large body of evidence supporting a hypercoagulable and prothrombotic state in SCD, it remains unclear whether increased thrombin and fibrin generation and platelet activation are primary or secondary events in this disease.11 Furthermore, the few clinical studies on the use of antiplatelet agents (eg, aspirin and ticlopidine) and anticoagulant agents (eg, heparin and warfarin) in SCD patients have not provided convincing evidence to support this therapeutic strategy for the prevention or treatment of vasoocclusive complications.11 Whereas mechanisms promoting procoagulant and prothrombotic conditions in SCD remain poorly understood, the underlying causes of this condition may relate to coagulation and inflammation being closely linked, interdependent processes.12
Inflammation, coagulation, vascular stasis, reperfusion injury, reduced NO bioavailability, iron-based oxidative biochemistry, and red-cell sickling have all been implicated in the pathobiology of sickle cell anemia.13 A variety of inflammatory cytokines (eg, TNFα, IL-6, IL-1β, and IL-8) are elevated in SCD patients.7,14 Patients experiencing acute painful episodes exhibit elevated leukocyte counts and increased C-reactive protein and endothelin-1 levels, with evidence of monocyte, neutrophil, platelet, and endothelial-cell activation.4 Animal studies have revealed increased expression of various endothelial-cell adhesion molecules (eg, VCAM-1, ICAM-1, and P- and E-selectins) and enhanced leukocyte–endothelial-cell adhesion in the vasculature.15 Patients with SCD also exhibit increased expression of adhesion molecules on circulating endothelial cells.16 A multistep model of blood-cell recruitment in the microvasculature has been proposed, implicating sickle cells and/or secondary inflammatory stimuli as mediators of endothelial-cell activation and the consequent expression of adhesion molecules and leukocyte–endothelial-cell adhesion.14 Whereas strong evidence from animal models supports the view that the microvasculature assumes an inflammatory phenotype during SCD, it remains unclear whether and how this condition predisposes arterial and venous microvessels to thrombus formation.
Following up on the results from our previous studies,17 we used βs mice and bone marrow chimeras produced from βs mice to make the novel observation that both arterioles and venules of the brain exhibit enhanced thrombus formation after light/dye–induced vessel injury. Our findings implicate TF, the protein C pathway, and thrombin generation in the accelerated microvascular thrombosis elicited in βs mice. A role for thrombin is further supported by the observation that the aggregation velocity of platelets isolated from βs mice is greatly enhanced after exposure to thrombin but not to ADP. A link between inflammation and thrombosis is supported by experiments showing a reversal of the enhanced microvascular thrombosis in βs chimeras rendered neutropenic with antineutrophil serum.
Methods
Mice
Male wild-type (WT) C57BL/6 mice, 25-35 g, were obtained from The Jackson Laboratory. The βs mice (also called heterozygous BERK, trait BERK, or sickle cell trait model) used in these studies were developed on a mixed genetic background (FVB/N, 129, DBA/2, C57BL/6, and Black Swiss),7 are homozygous for knockout of murine α-globin and heterozygous for knockout of murine β-globin, and have one copy of the linked transgenes for human α- and βs-globins. Phenotypically, βs mice exhibit the inflammatory features of SCD rather than its vasoocclusive effects,15,19 although this feature of βs mice is not due to a strain difference, because the same strain control, an HbA-BERK mouse fully humanized to express only normal human HbA, does not exhibit such features.7,20 The percentage of HbS in βs mice (∼ 26%) predicts that intracellular concentration is not sufficient for polymerization. However, the apparent lower oxygen-affinity state suggests a deficiency of oxygen loading that could increase the degree of physiologic oxygen saturation and, in combination with perturbed pH and osmolality (eg, in the kidneys), might generate sufficient augmentation of polymerization tendency. The inflammatory phenotype of these mice may reflect a combination of effects of altered oxygen affinity, HbS, and enhanced oxidative stress. Hematocrit (Hct) is reduced and the reticulocyte count is elevated in these mice, but not as much as in BERK mice.2 It should be noted that we (the laboratory of D.N.G.) have used homozygotes in early experiments and noted that the mice could not tolerate the stress (eg, of anesthesia and surgery) associated with preparation for intravital microscopy. The high mortality of the more severely ill homozygotes excluded their use in the present study.
Mice were 6-8 months old, raised and housed under specific pathogen–free conditions, and maintained on standard laboratory chow fed ad libitum. Experimental procedures were reviewed and approved by Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee, and were performed according to the criteria outlined in the National Institutes of Health guidelines. Mice were age- and sex-matched.
Bone marrow transplantation
We generated 3 combinations of bone marrow chimeras. WT→WT chimeras were WT (C57BL/6) animals (CD45.2-positive leukocytes) receiving bone marrow cells from CD45 congenic mice (CD45.1-positive leukocytes), resulting in increased leukocytes expressing CD45.1 (of donor origin) from < 5% in WT to > 90% in the WT→WT chimeras, as described previously.1 βs→WT chimeras were produced by transplanting bone marrow from βs mice (CD45.1-positive leukocytes) into WT mice (CD45.2-positive leukocytes).1 In some experiments, βs bone marrow was transferred into transgenic mice overexpressing the endothelial-cell protein C receptor (EPCR-TgN; Oklahoma Medical Research Foundation), and backcrossed into C57BL/6.21 Animals were tested 8 weeks after transplantation for reconstitution, at which time flow cytometry confirmed that > 90% of circulating leukocytes in transplanted recipients were from donor bone marrow.
Analysis of bleeding time
Mice were anesthetized with pentobarbital (50 mg/kg IP). A 3-mm tail segment was cut cleanly with a scalpel blade, and bleeding was monitored at 15-second intervals by absorbing the bead of blood with filter paper without contacting the wound site. When no blood was observed on the paper, bleeding was determined to have ceased.22 The experiment was terminated after 15 minutes.
Experimental preparation for intravital fluorescence microscopy
All animals were anesthetized IP with pentobarbital (50 mg/kg), and the jugular vein was cannulated for IV administration of FITC-dextran (Sigma-Aldrich). Mice were tracheostomized and artificially ventilated with room air. Core body temperature was maintained at 37 ± 0.5°C. A craniectomy was performed, the dura reflected, and artificial cerebrospinal fluid was placed on the cranial opening. The preparation was allowed to equilibrate for 30 minutes. Visualization of individual vessels and induction of thrombosis was performed using a 40× water-immersion objective attached to a Xanophot IVM microscope (HLX64610; Nikon).17 A silicon-intensified target camera (C-2400; Hamamatsu Photonics) projected the image onto a monitor (Trinitron PVM-2030; Sony) and recorded on a DVD player (SR-MV50; JVC).
Photoactivation thrombosis model
Ten milligrams per kilogram of 5% FITC dextran (150 000 mol wt; Sigma-Aldrich) was injected IV and allowed to circulate for 10 minutes before photoactivation. Photoactivation was initiated (excitation, 495 nm; emission, 519 nm) by exposing 100 μm of vessel length to epi-illumination with a 175-W xenon lamp (Lamda LS; Sutter) and a fluorescein filter cube (HQ-FITC; Chroma).23 The excitation power density was measured daily (ILT 1700 radiometer, SED033 detector; International Light) and maintained within 1% of 0.77 W/cm2, as described previously.23,24 Epi-illumination was applied continuously, and the time of blood flow cessation (≥ 60 seconds duration) was recorded in both venules and arterioles (30-70 μm). Epi-illumination was discontinued once blood flow ceased in the vessel under study. Typically, 2-4 thrombi were induced in each mouse, and the results of each vessel type (ie, venules and arterioles) were averaged. More information about the light/dye method and its advantages/disadvantages relative to the ferric chloride model and other models is available in an excellent review by Rumbaut et al.25
Administration of anticoagulant therapy
Hirudin (1 mg/kg; Sigma-Aldrich), anti–thrombin III (ATIII; 20 mg/kg; Calbiochem), murine activated protein C (mAPC; 40 μg/mouse)26 or a rat anti–mouse APC blocking mAb (mAb1609; 10 μg/mouse),27 were administered via the jugular vein (5 minutes before the initiation of thrombus formation). Rat anti–mouse TF-blocking mAb (1H1; 20 mg/kg)28 was administered via the jugular vein 20 minutes before the onset of thrombus formation.
Platelet aggregation
Freshly collected platelet-rich plasma from donor mice was used to monitor platelet aggregation velocity after agonist exposure using a laser-particle analyzer (Lumen). The concentration for half-maximum velocity (EC50) for aggregation velocity of WT platelets in response to either thrombin or ADP was determined as described previously.29 This method yielded an EC50 of 0.63 U/mL for thrombin and 733nM for ADP. The EC50 values were used to determine whether platelet aggregation velocity differed between control (WT→WT) and βs→WT mice.
Leukocyte counts
Leukocytes were stained with 3% citric acid and 10% crystal violet and quantified using a hemocytometer.
ANS
In some experiments, neutropenia was induced using mouse antineutrophil serum (ANS; RB6-8C5; eBiosciences). ANS was administered (75 μg/mouse) IP 24 hours before the experiment.29,30 A reduction in circulating neutrophils (1% ± 5% in WT→WT and 88% ± 6% in βs→WT) was verified by counting neutrophils in the peripheral blood before ANS injection and immediately before the experiment. ANS did not alter the number of circulating lymphocytes, monocytes, or platelets. Whereas coagulation parameters were not measured in ANS-treated mice, previous reports on other models (sepsis) have shown no dependence of the thrombosis response on neutrophils.24
Hct.
Red blood cells were collected in microhematocrit tubes (StatSpin Technologies) and spun in a microhematocrit centrifuge (StatSpin Technologies) for 30 seconds. The height of the blood cell column was measured as a percentage of the total blood column.31
Prothrombin time
Prothrombin time was measured using the INRatio/INRatio2 monitoring system (HemoSense).
Immunostaining
Brains from WT→WT and βs→WT chimeras were fixed in 4% paraformaldehyde and 0.5% glutaraldehyde, 0.1M sodium phosphate buffer (pH 7.4) for 2 hours at 4°C, and embedded in Paraplast (Sigma).32 Sections (3-μm) were incubated at 4°C with primary antibodies: goat –murine protein C, goat anti–mouse EPCR (from C.T.E.), and rat anti–mouse TF-blocking mAb (from R.P.H.). Some sections were incubated with nonimmune rabbit or goat serum (1:200; Sigma) as a negative control. Goat anti–rabbit IgG antibody conjugated to 5nM colloidal gold was used for murine TF antibody, and rabbit anti–goat IgG antibody conjugated to 5nM colloidal gold (1:100; British BioCell International) for the murine EPCR and monoclonal protein C antibodies. Analysis was conducted with an Axio-skop microscope (Zeiss) and densitometry (arbitrary 0-255 U scale) was determined with AxioVision Rel. 4.8.2 software (Zeiss).
Immunohistochemistry for EPCR
Brains from WT and EPCR-TgN was fixed in 4% paraformaldehyde for 5 hours, cryoprotected in 5% sucrose, and embedded in optimal cutting temperature medium. Cryosections were stained with rat anti–mouse EPCR (from C. Esmon), followed by donkey anti–rat FITC (1:200; Sigma). Nuclei were stained with TO-PRO3 (Molecular Probes). The samples were examined with a Nikon C1 confocal microscope.
Statistical analysis
Unless otherwise indicated, data are presented as means ± SEM. ANOVA was used with the Bonferroni post hoc test. Platelet aggregation was analyzed using the 2-tailed Student t test.
Results
Enhanced thrombus formation in cerebral microvessels of βs and βs→WT mice
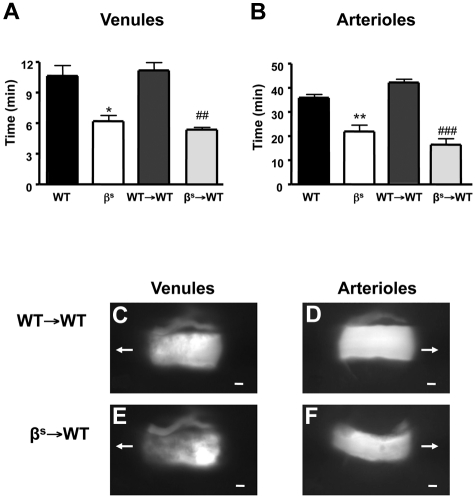
Thrombus formation, assessed as time for complete cessation of microvascular blood flow after light/dye injury, was quantified in WT, βs, WT→WT, and βs→WT mice using intravital fluorescence microscopy. In all mice studied, the time required for complete flow cessation was longer in arterioles than venules, as reported previously for the brain and other vascular beds.23,33,34 Significant reductions in flow-cessation time were observed in arterioles and venules of βs and βs→WT mice compared with their controls (WT and WT→WT) (Figure 1A-F). Because no differences were noted between βs and βs→WT chimeras or between WT and WT→WT chimeras, all subsequent studies were performed using bone marrow chimeras (WT→WT and βs→WT mice).
Figure 1.
Thrombus formation in sickle cell–transgenic mice. Sickle cell–transgenic mice (βs) and bone marrow chimeras produced from these mice (βs→WT) exhibit accelerated thrombus formation in both cerebral venules (A) and arterioles (B) compared with their WT (WT and WT→WT) counterparts. Images of thrombus formation visualized at 10 minutes demonstrate the significant differences between WT→WT (C-D) and βs→WT (E-F) in both venules (C-E) and arterioles (D-F) of the brain. The arrow indicates the direction of blood flow before cessation. Scale bar indicates 10 μm. Data are shown as the means ± SEM. *P < .05 compared with WT; **P < .01 compared with WT; ##P < .01 compared with WT→WT; ###P < .001 compared with WT→WT. n = 5-6 mice/group.
Blood parameters
SCD patients have elevated total leukocyte counts that are correlated with the severity of acute painful episodes and the risk of premature death.35 Table 1 compares total leukocyte and neutrophil counts between WT→WT and βs→WT mice. βs→WT mice exhibited elevated total leukocyte and neutrophil counts in the blood.15 (βs mice also exhibited elevated total leukocytes (15 004 ± 679 counts/μL in βs mice vs 7971 ± 297counts/μL in WT mice) and neutrophils (4105 ± 293 counts/μL in βs mice vs 1440 ± 484 counts/μL in WT mice) in the blood. Hct and platelet levels were reduced in βs→WT mice (Hct, 32.7% ± 1.8%; platelet counts, 466 ± 31counts/μL) compared with WT→WT mice (Hct, 39.4% ± 1.4%; platelet counts, 1261 ± 132counts/μL).12 No significant differences were seen in prothrombin or bleeding times (Table 1).
Table 1.
Blood parameters of WT→WT and βs→WT mice and immunohistochemistry densitometric analysis for murine TF (mTF), murine EPCR (mEPCR), and mAPC in WT→WT and βs→WT mouse brain vessels
| Parameter | WT→WT | βs→WT |
|---|---|---|
| Prothrombin time, s | 10.6 ± 0.5 | 9.9 ± 0.5 |
| Tail bleeding time, s | 316.7 ± 24.4 | 443.3 ± 84.2 |
| Hematocrit level, % | 39.4 ± 1.4 | 32.7 ± 1.8* |
| Total leukocyte count, /μL | 8500.0 ± 365.1 | 14 833.3 ± 823.3* |
| Neutrophil count, /μL | 1583.3 ± 200.7 | 3750.0 ± 359.4* |
| Platelet count, /μL | 1261 ± 132 | 466 ± 31.12* |
| mTF | ||
| Arteriole | 184.3 ± 8.9 | 123.6 ± 3.7* |
| Venule | 69.6 ± 4.2 | 65.3 ± 5.3 |
| mEPCR | ||
| Arteriole | 113.3 ± 1.5 | 92.8 ± 1.6* |
| Venule | 78.2 ± 2.2 | 63.9 ± 2.7 |
| mAPC | ||
| Arteriole | 111.3 ± 2.1 | 62.8 ± 1.6* |
| Venule | 66.1 ± 1.8 | 76.0 ± 1.9 |
Data for hemodynamic parameters of WT→WT and βs→WT mice are expressed as means ± SEM of 5-6 mice/group.
P < .05 compared with WT→WT. Immunohistochemical densitometric analysis for mTF, mPCR, and mAPC was done with AxioVision software. Values (densitometric units) are means ± SEM of 5 tissue sections analyzed from 3 mice per group.
TF contributes to enhanced microvascular thrombosis in βs→WT mice
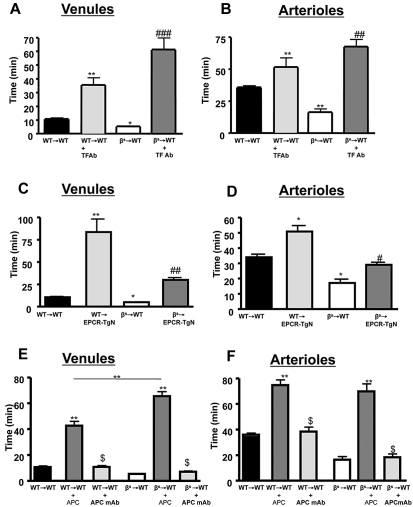
TF is the trigger of the coagulation system.7 Although it is not expressed on endothelial cells under normal conditions, it is elevated in patients with SCD.35 Endothelial TF expression was not increased in venules and was significantly decreased in arterioles (Table 1) of βs→WT mice compared with WT→WT mice, and TF expression was not observed on any blood cells. Administration of anti-TF antibody completely prevented the accelerated thrombus formation in WT→WT and βs→WT mice, prolonging time to flow cessation in both arterioles and venules after light/dye injury (see Figure 3A-B).
Figure 3.
Effects of TF immunoneutralization and role of EPCR and APC on light/dye–induced thrombus formation in cerebral vessels. Administration of TF-blocking antibody to WT→WT and βs→WT chimeras extended blood flow-cessation time in cerebral venules (A) and arterioles (B). The accelerated thrombus formation observed in βs→WT chimeras was significantly reversed in βs→EPCR-TgN mice in both cerebral venules (C) and arterioles (D). WT (WT→WT) or sickle cell–transgenic (βs→WT) bone marrow chimeras were treated with either APC (40 μg/mouse) or an APC-neutralizing mAb (10 μg/mouse) 5 minutes before inducing thrombosis in both venules (E) and arterioles (F). Data are shown as the means ± SEM. *P < .05 compared with the corresponding control group (WT→WT or βs→WT); **P < .01 compared with the corresponding control group (WT→WT or βs→WT); #P < .05 compared with βs→WT or WT-EPCR-TgN; ##P < .01 compared with βs→WT or WT-EPCR-TgN; ###P < .001 compared with βs→WT or WT-EPCR-TgN; $P < .001 compared with WT→WT + APC or βs→WT + APC. n = 5-6 mice/group.
Role of the protein C pathway
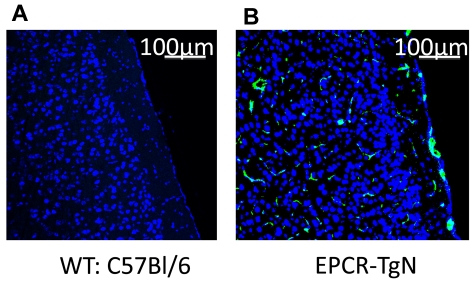
Alterations in the protein C pathway have been described in patients with SCD.12,36 The expression of EPCR was determined in EPCR-TgN mice (Figure 2A-B). Whereas EPCR was nearly absent in the brains of WT mice, EPCR-TgN mice expressed EPCR both in large vessels and in the capillary network (Figure 2B). A comparison of the thrombosis responses to light/dye injury between βs→WT mice and βs→EPCR-TgN mice (βs bone marrow transplantation into EPCR-transgenic mice) revealed a significantly prolonged time to flow cessation in the venules and arterioles of βs→EPCR-TgN mice (Figure 3C-D). We next tested whether exogenous mAPC administration exerts beneficial effects against cerebral microvascular thrombosis in βs→WT mice. As shown in Figure 3E-F, mAPC significantly extended the flow-cessation time of WT→WT and βs→WT mice. The effect of mAPC administration in venules of βs→WT was significantly greater than in WT→WT mice (Figure 3E). In contrast, immunoneutralization of endogenous APC had no effect on flow-cessation time in either the arterioles or venules of WT→WT and βs→WT mice, suggesting that APC is normally low in the cerebral microvasculature and that a further reduction does not accelerate thrombus formation. Densitometric analysis of EPCR and protein C expression in the cerebral microvasculature of WT→WT and βs→WT mice indicated that protein C and its receptor on endothelial cells (EPCR) exhibit reduced arteriolar expression in βs→WT mice (Table 1).
Figure 2.
Localization of EPCR in brains from WT and EPCR-transgenic mice. These images show the absence of EPCR in WT mice (A) and the presence of EPCR in both the large vessels and the capillary network of EPCR-TgN mice (B). TO-PRO3 nuclear (N) staining is shown as blue. Scale bars indicate 100 μm.
Thrombin mediates accelerated cerebral microvascular thrombosis in βs→WT mice
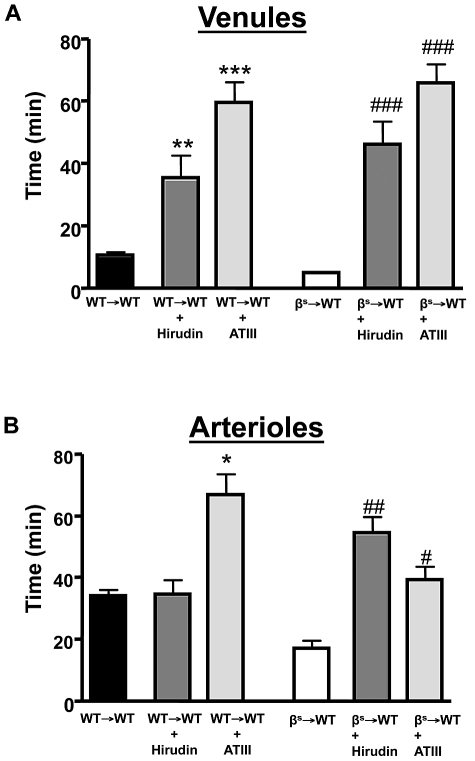
A role for accelerated thrombin production in SCD is supported by studies describing reduced ATIII and increased thrombin-antithrombin complexes in SCD patients.37 We examined the effects of 2 direct thrombin inhibitors (ATIII and hirudin) on thrombus formation in cerebral arterioles and venules of βs→WT mice (Figure 4). Both inhibitors effectively reversed the accelerated thrombus formation in βs→WT mice and prolonged flow-cessation time in the venules of WT→WT mice subjected to light/dye injury.
Figure 4.
The effect of thrombin inhibition on light/dye–induced thrombus formation in cerebral vessels. Effect of treatment with a direct thrombin inhibitor on light/dye–induced thrombus formation in cerebral venules (A) and arterioles (B) of WT and βs mice. WT→WT and βs→WT received either hirudin (1 mg/kg) or ATIII (20 mg/kg) 5-20 minutes before the onset of light/dye–induced thrombosis, and the time taken for flow to cease was recorded in cerebral venules (A) and arterioles (B). Data are shown as the means ± SEM. *P < .05 compared with the corresponding WT→WT group; **P < .05 compared with the corresponding WT→WT group; ***P < .05 compared with the corresponding WT→WT group; #P < .05 compared with the corresponding βs→WT group; ##P < .05 compared with the corresponding βs→WT group; ###P < .05 compared with the corresponding βs→WT group. n = 5-6 mice/group.
Thrombin- but not ADP-induced platelet aggregation is enhanced in βs→WT mice
Because our in vivo studies implicated thrombin in the accelerated thrombosis of βs→WT mice, we examined whether platelet sensitivity to thrombin and ADP differs between WT→WT and βs→WT mice (Figure 5). Using EC50 concentrations for ADP, we found no difference in platelet aggregation responses between WT→WT and βs→WT mice (Figure 5A-B). However, a marked difference was noted when murine thrombin was used as the agonist, yielding a more intense platelet aggregation response in βs→WT mice compared with WT→WT mice (Figure 5C-D).
Figure 5.
Comparison of ADP- or thrombin-induced aggregation responses of platelets isolated from WT→WT or βs→WT mice. (A,C) Mean aggregation responses to EC50 doses of ADP (733nM) and thrombin (0.63 U/mL). (B,D) Representative aggregation responses to the EC50 doses as detected by a laser-particle analyzer. Data are shown as the means ± SEM. *P < .05 compared with WT→WT. n = 5 mice/group.
Neutrophils are involved in cerebral microvascular thrombosis in βs→WT mice
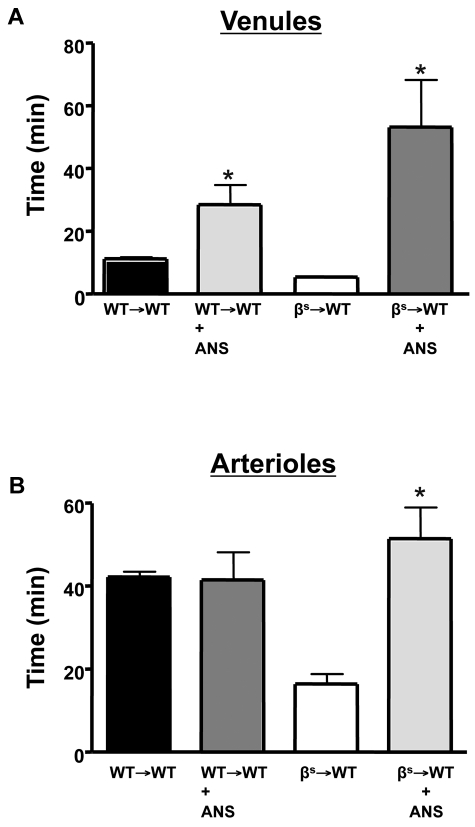
The cerebral microvasculature assumes an inflammatory phenotype in βs and βs→WT chimeras, with increased leukocyte adhesion in cerebral venules.1,17 Evidence implicates neutrophils in the vasoocclusive events associated with experimental SCD.38,39 To determine whether neutrophils contribute to the enhanced thrombosis response observed in our model, we rendered WT→WT and βs→WT mice neutropenic with ANS (Figure 6). With neutropenia, both cerebral venules and arterioles of βs→WT mice exhibited a significant prolongation in flow-cessation time after light/dye injury (Figure 6A-B). Although neutropenia did not alter thrombus formation rate in the cerebral arterioles of WT→WT mice, it significantly attenuated thrombosis in venules after injury.
Figure 6.
Role of neutrophils in light/dye–induced thrombus formation in cerebral vessels. Neutropenia was produced by administering ANS 24 hours before induction of thrombus formation in cerebral venules (A) and arterioles (B) of WT and βs mice. Data are shown as the means ± SEM. *P < .05 compared with the corresponding controls (WT→WT or βs→WT). n = 5-6 mice/group.
Discussion
SCD patients experience acute painful episodes that are a manifestation of microvascular occlusion. Thrombosis is an important aspect of the clinical spectrum of SCD, increasing the risk of thrombosis-associated events such as stroke.5 There are 3 stroke syndromes that affect patients with SCD: (1) clinically silent strokes that result from multifocal small vessel disease and affect ∼ 20% of children, (2) hemorrhagic strokes that tend to affect adults in their 20s, and (3) regional ischemic strokes (probably microvascular) seen in older adults. One of these syndromes affects approximately 40% of patients with sickle cell anemia, although prevalence diminishes in less-severe disease variants.30 Approximately 10% of children (peak age ∼ 5 years) with sickle cell anemia develop classic ischemic stroke, with risk factors such as increased white blood cell counts, lower Hb, and elevated systolic blood pressure.3 However, occlusive disease at the circle of Willis (affecting vessels that are moderate to large in size) is most common and is believed to be pathogenic.6,40 Whereas the etiology of circle of Willis disease in children with sickle cell anemia is not firmly established, a recent report suggests an association with altered expression of endothelial-cell genes that encode inflammatory signaling pathways.41 Factors other than inflammation that may also contribute to the site-specific occlusive disease include red blood cell/endothelial interactions and vessel wall responses to thrombin.
Biochemical evidence supports the existence of a hypercoagulable, prothrombotic state in SCD patients, as evidenced by elevated levels of activated coagulation factors, increased factor VII turnover and thrombin-antithrombin complexes, and impaired anticoagulation mechanisms such as those in the protein C pathway.7–11 Murine models of SCD offer the potential to better understand the pathophysiology of SCD and to identify novel therapeutic targets.42 Studies using a variety of SCD models have revealed increased adhesive interactions between circulating erythrocytes and leukocytes with the endothelium, which can impede microvascular perfusion and promote vasoocclusive events.1 However, it is important to recognize the limitations of the currently available SCD models, because none of them truly mimics human disease (mainly because of the complexities of SCD and the apparent contribution of multiple biologic processes to the vasculopathy associated with this disease). In this study, we used βs mice (heterozygous for knockout of murine β-globin) to extend our previous efforts to understand how the murine microvasculature responds to the expression of sickle hemoglobin.1,17,43 Multiple laboratories have used this model because it has proven to be valuable for simulating different aspects of the pathophysiology of SCD. We attempted to address potential mechanisms that promote the procoagulant and prothrombotic state that accompanies sickle hemoglobin expression. In the present study, we have produced the first evidence that thrombus formation is significantly accelerated in both the arterioles and venules of the cerebral microvasculature in mice that express sickle hemoglobin. Our study also implicates TF and thrombin activation and an impaired protein C pathway in the prothrombotic phenotype of βs mice.
The thrombotic responses generated in this study are secondary to phototoxicity-induced endothelial injury elicited by the photoactivation of FITC, which leads to endothelial-cell activation without denudation of the endothelial-cell layer. Other models, such as ferric chloride, generally initiates severe vessel damage that begins outside of the vessel wall and leads to endothelial-cell denudation with occlusive thrombi involving platelet adhesion to subendothelial matrix components.25,43 The photochemical activation model used to induce microvascular thrombosis in our study has been applied to a variety of different vascular beds and in different animal models of disease.44 The light/dye method allows for repeated observations in different vessels (arterioles or venules) of the same tissue. A detailed discussion of the light/dye thrombosis method, its advantages/disadvantages relative to other models, and its application in different mouse strains is provided in a recent review by Rumbaut et al.25 Whereas some concerns have been raised about the use of C57BL/6 mice (which express a form of ADAMTS13, a metalloprotease for which the only known substrate is VWF) to study thrombosis,45 most of the available data on microvascular thrombosis and many of the current concepts regarding this process have been generated using the C57Bl/6 strain or in mutant mice developed on this background.
As demonstrated in other vascular beds (eg, in the intestines and lungs),23,46 thrombus formation in cerebral venules occurs more rapidly after vessel injury than in arterioles. Whereas the difference between arterioles and venules was evident for all groups studied (WT, WT→WT, βs, and βs→WT mice), the influence of the βs genotype on thrombus formation was equally impressive in both vessel populations. Thrombi formed in the venous system are characteristically rich in fibrin and trapped red blood cells and poor in platelets, whereas arterial thrombi are rich in aggregated platelets. Venules also differ from arterioles in a variety of other ways that could explain the differences in thrombi, including a nonuniform distribution/production of pro- and anticoagulation factors (eg, TF) between vessel types or less dilution of locally generated procoagulants (eg, thrombin) by more slowly moving blood in venules.7 Regardless of the mechanism(s) underlying the differential responses of arterioles and venules to thrombus development, our observation that both vessel populations are similarly affected by the βs genotype suggests that similar prothrombotic processes are involved in both types of vessels.
Coagulation is initiated by the binding of factor VII to TF. TF is expressed by various blood cells, activated endothelial cells, and vascular smooth muscle. Microparticles shed from monocytes and platelets are also a rich source of TF that can initiate coagulation. Patients with SCD exhibit an increased number of (TF-positive) microparticles,47 although it remains unknown whether mouse models of SCD produce such microparticles. TF has been detected in the pulmonary veins of mild sickle-cell–phenotypic mice; however, hypoxia/reoxygenation was required to induce TF, whereas mice with the more severe sickle-cell phenotype express moderately high TF levels even in ambient air.7 Our assessment of TF in cerebral microvessels did not reveal increased expression in βs mice. Nonetheless, TF immunoneutralization effectively prevented the accelerated thrombus formation observed in these mutants. In the absence of increased TF expression by the endothelium in βs mice, the responses noted with the anti-TF antibody suggests that blood cell- and/or microparticle-associated TF may contribute to the βs-enhanced thrombosis response.
The protein C anticoagulant pathway is activated by thrombin binding to thrombomodulin, with subsequent activation of protein C by the thrombin-thrombomodulin complex and EPCR. Evidence from patients with SCD suggests an impaired protein C pathway, accompanied by decreased blood levels of protein C and protein S.9,10 Immunohistochemical detection of murine protein C revealed a significant reduction in cerebral microvessels of βs→WT mice. Similarly, a small but significant reduction in arteriolar EPCR expression was noted in the cerebral vasculature of βs→WT mice (EPCR staining was absent in WT mice, whereas EPCR-TgN mice expressed abundant EPCR on endothelial cells in large vessels and in capillaries, where EPCR is generally low, as reported for other tissues21). The mechanisms underlying the altered protein C pathway in SCD remain unknown. However, cytokines (eg, TNF-α and IL-6), which are elevated in SCD patients, have been shown to down-regulate EPCR on microvascular endothelial cells and to reduce the capacity of these cells to activate protein C.48 A role for impaired protein C activation is supported by our observation that genetic overexpression of EPCR and administration of murine protein C were both effective in preventing the accelerated thrombus formation observed in arterioles and venules of βs chimeras. Because a protein C–blocking antibody had no effect on light/dye–induced thrombus formation in either vessel type, it appears that the production/activation of protein C in the cerebral vasculature of βs→WT mice is too low to afford protection against thrombosis.
Increased thrombin activity has been shown to correlate with pain episodes in SCD,3 and elevated levels of thrombin-antithrombin complexes and D-dimers are observed in SCD.11 Activation of the thrombin receptor on endothelial cells is associated with increased adhesiveness to leukocytes and sickled red blood cells.49 We used 2 direct thrombin inhibitors (hirudin and ATIII) with distinct mechanisms of action to assess the role of thrombin in the accelerated thrombosis in βs→WT mice. Hirudin inhibits all biologic functions of thrombin but does not inhibit other enzymes in the coagulation or fibrinolytic pathways.50 Hirudin had no effect on arteriolar blood flow-cessation time of WT→WT mice, suggesting that thrombin is less important in inhibiting blood flow cessation in arterioles of WT→WT mice compared with venules. The efficacy of hirudin and ATIII were previously compared using the light/dye model of microvascular thrombosis in the ears of hairless mice.51 This study revealed that ATIII was far more effective in preventing arteriolar thrombotic vessel occlusion, suggesting that ATIII is the most effective thrombin inhibitor. Whether the beneficial effects of the 2 thrombin inhibitors on βs-associated thrombosis relate entirely to their influence on coagulation factor generation or to thrombin-mediated actions on endothelial and/or blood cells remains unclear. However, our finding that platelets from βs→WT mice exhibit an enhanced aggregation velocity in response to thrombin but not ADP suggests that thrombin-mediated platelet aggregation may be an important feature of the βs genotype that promotes microvascular thrombosis. Increased sensitivity of platelets to thrombin may also contribute to the increased P-selectin expression detected on platelets in SCD patients52 and the enhanced recruitment of platelets in cerebral microvessels of βs and βs→WT mice.1
Inflammation has been implicated in the pathogenesis of SCD. Studies on different tissues in sickle cell–transgenic mice have suggested that the microvasculature assumes an inflammatory phenotype, as evidenced by enhanced leukocyte–endothelial-cell adhesion in venules2 and increased reactive oxygen species production in arterioles and venules.19,53 Systemically, inflammation is reflected in increased leukocyte counts in both βs mice and patients with SCD. In view of evidence in other disease models linking inflammation to thrombosis,12 with inflammation promoting thrombosis and vice versa, we addressed the possibility that neutrophils may contribute to the enhanced microvascular thrombosis in βs mice by rendering them neutropenic with ANS. Our findings reveal a major role for neutrophils in accelerated thrombus formation. Whereas it remains unclear how neutrophils may promote thrombus formation, the ability of these blood cells to secrete cytokines and produce superoxide may provide a link between neutrophils and thrombosis. There is evidence that neutrophils can produce and secrete TNF-α and IL-1β, which can down-regulate EPCR and promote thrombin generation.48 The superoxide anion generation that accompanies neutrophil activation can also result in inactivation of NO produced by endothelial cells. Reduced NO availability has been implicated in the enhanced arteriolar thrombosis associated with hypercholesterolemia.53
Neutrophils may also promote thrombosis via the release of neutrophil extracellular traps (NETs).54 Bacterial endotoxin and activated platelets can induce neutrophils to make NETs in the microvasculature.55 NETs are abundant in thrombi associated with deep-vein thrombosis, and it has been shown that platelets under flow in vitro bind avidly to NETs and are able to promote thrombosis.56 It has been suggested that the backbone of NETs, which is made of chromatin, provides a structure on which platelets can adhere, become activated, and aggregate, thereby contributing to thrombus initiation and/or stability. Finally, the protective effects of neutropenia against microvascular thrombosis in βs→WT mice may also relate to the findings of a recent study showing a major role for adherent neutrophils in capturing circulating platelets and in the subsequent exacerbation of superoxide production and microvascular thrombosis mediated by platelet-neutrophil aggregates.56
Acknowledgments
We thank Drs N. Esmon and F. Lupu (Oklahoma Medical Research Foundation) for their work on EPCR.
This work was funded by the National Institutes of Health (NIH; Bethesda, MD) National Heart, Lung and Blood Institute (grant HL26441 to D.N.G. and grant P01-HL55552 to R.P.H.). F.G. is funded by the Biotechnology and Biological Sciences Research Council (Wiltshire, United Kingdom) Integrative Mammalian Biology Fund. C.T.E. is an investigator with Howard Hughes Medical Institute (Chevy Chase, MD), which is funded by NIH grant GM091739, and is also supported by a Transatlantic Network for Excellence in Cardiovascular Research grant from the Foundation Leducq (Paris, France). A.S.D. is funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brasilia, Brazil).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.N.E.G. and D.N.G. designed and supervised research, analyzed data, and wrote the paper; F.N.E.G., E.L.S., L.D.A.P., and A.S.D. performed experiments; J.R. supervised research and analyzed data; and all other authors assisted in data interpretation and manuscript revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Felicity N. E. Gavins, Wolfson Neuroscience Laboratories, Imperial College Faculty of Medicine, Hammersmith Hospital Campus, Burlington Danes Bldg, Du Cane Rd, London, W12 0NN, United Kingdom; e-mail: f.gavins@imperial.ac.uk.
References
- 1.Wood K, Hebbel RP, Granger DN. Endothelial cell P-selectin mediated a proinflammatory and prothrombogenic phenotype in cerebral venules of sickle cell transgenic mice. Am J Physiol Heart Circ Physiol. 2004;286(5):H1608–1614. doi: 10.1152/ajpheart.01056.2003. [DOI] [PubMed] [Google Scholar]
- 2.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106(3):411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 4.Hebbel RP, Embury SH, Mohandas N, Steinberg MH. Sickle Cell Disease: Basic principles and clinical practice. Philadelphia, PA: Lippincott Williams & Wilkins; 1994. [Google Scholar]
- 5.Merkel KH, Ginsberg PL, Parker JC, Jr, Post MJ. Cerebrovascular disease in sickle cell anemia: a clinical, pathological, and radiological correlation. Stroke. 1978;9(1):945–952. doi: 10.1161/01.str.9.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Prengler M, Pavlakis SG, Prohovnik I, Adams RJ. Sickle Cell Disease: The neurological complications. Ann Neurol. 2002;51(5):543–552. doi: 10.1002/ana.10192. [DOI] [PubMed] [Google Scholar]
- 7.Solovey A, Kollander R, Shet A, Hebbel RP, et al. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104(3):840–846. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- 8.Kurantsin-Mills J, Ofosu FA, Safa TK, Siegel RS, Lessin LS. Plasma factor VII and thrombin-antithrombin III levels indicate increased tissue factor activity in sickle cell patients. Br J Haematol. 1992;81(4):539–544. doi: 10.1111/j.1365-2141.1992.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 9.Westerman MP, Green D, Gilman-Sachs A, et al. Antiphospholipid antibodies, protein C and S, and coagulation changes in sickle cell disease. J Lab Clin Med. 1999;134(4):352–362. doi: 10.1016/s0022-2143(99)90149-x. [DOI] [PubMed] [Google Scholar]
- 10.Wright JG, Malia R, Cooper P, Thomas P, Preston FE, Serjeant GR. Protein C and S in homozygous sickle cell disease: does hepatic dysfunction contribute to low levels? Br J Haematol. 1997;98(3):627–631. doi: 10.1046/j.1365-2141.1997.2663083.x. [DOI] [PubMed] [Google Scholar]
- 11.Ataga KI, Orringer EP. Hypercoagulability in sickle cell disease: a curious paradox. Am J Med. 2003;115(9):721–728. doi: 10.1016/j.amjmed.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2008;61(1–2):122–131. doi: 10.1016/j.maturitas.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Hebbel RP, Vercellotti GM, Pace BS, et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood. 2010;115(12):2483–2490. doi: 10.1182/blood-2009-02-204990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavins F, Yilmaz G, Granger DN. The evolving paradigm for blood cell-endothelial cell interactions in the cerebral microcirculation. Microcirculation. 2007;14(7):667–681. doi: 10.1080/10739680701404903. [DOI] [PubMed] [Google Scholar]
- 15.Kaul DK, Liu XD, Choong S, Belcher JD, Vercellotti GM, Hebbel RP. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2004;287(1):H293–H301. doi: 10.1152/ajpheart.01150.2003. [DOI] [PubMed] [Google Scholar]
- 16.Solovey AA, Solovey AN, Harkness J, Hebbel RP. Modulation of endothelial cell activation in sickle cell disease: a pilot study. Blood. 2001;97(7):1937–1941. doi: 10.1182/blood.v97.7.1937. [DOI] [PubMed] [Google Scholar]
- 17.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005;19(8):989–891. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi CT, Gladwin M, Diwan B, et al. Pathophysiology of a sickle cell trait mouse model: human alpha(beta)(S) transgenes with one mouse beta-globin allele. Blood Cells Mol Dis. 2001;27(6):971–977. doi: 10.1006/bcmd.2001.0469. [DOI] [PubMed] [Google Scholar]
- 19.Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 20.Kollander R, Solovey A, Milbauer LC, Abdulla F, Kelm RJ, Jr, Hebbel RP. Nuclear factor-kappa B (NFkappaB) component p50 in blood mononuclear cells regulates endothelial tissue factor expression in sickle transgenic mice: implications for the coagulopathy of sickle cell disease. Transl Res. 2010;155(4):170–177. doi: 10.1016/j.trsl.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Zheng X, Gu J, et al. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3(7):1351–1359. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 22.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida H, Russell J, Stokes KY, Yilmaz CE, Esmon CT, Granger DN. Role of the protein C pathway in the extraintestinal thrombosis associated with murine colitis. Gastroenterology. 2008;135(3):882–888. doi: 10.1053/j.gastro.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rumbaut RE, Bellera RV, Randhawa JK, et al. Endotoxin enhances microvascular thrombosis in mouse cremaster venules via a TLR4-dependent, neutrophil-independent mechanism. Am J Physiol Heart Circ Physiol. 2006;290(4):H1671–H1679. doi: 10.1152/ajpheart.00305.2005. [DOI] [PubMed] [Google Scholar]
- 25.Rumbaut RE, Slaff DW, Burns AR. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation. 2005;12(3):259–274. doi: 10.1080/10739680590925664. [DOI] [PubMed] [Google Scholar]
- 26.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32(1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 27.Liaw PC, Ferrell G, Esmon CT. A monoclonal antibody against activated protein C allows rapid detection of activated protein C in plasma and reveals a calcium ion dependent epitope involved in factor Va inactivation. J Thromb Haemost. 2003;1(4):662–670. doi: 10.1046/j.1538-7836.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost. 2005;3(5):1098–1099. doi: 10.1111/j.1538-7836.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 29.Senchenkova EY, Russell J, Almeida-Paula LD, Harding JW, Granger DN. Angiotensin II-mediated microvascular thrombosis. Hypertension. 2010;56(6):1089–1095. doi: 10.1161/HYPERTENSIONAHA.110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavins FN, Kamal AM, D'Amico M, Oliani SM, Perretti M. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J. 2005;19(1):100–102. doi: 10.1096/fj.04-2178fje. [DOI] [PubMed] [Google Scholar]
- 31.Han V, Serrano K, Devine DV. A comparative study of common techniques used to measure haemolysis in stored red cell concentrates. Vox Sang. 2010;98(2):116–123. doi: 10.1111/j.1423-0410.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 32.Damazo AS, Yona S, Flower RJ, Perretti M, Oliani SM. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176(7):4410–4418. doi: 10.4049/jimmunol.176.7.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Povlishock JT, Rosenblum WI, Sholley MM, Wei EP. An ultrastructural analysis of endothelial change paralleling platelet aggregation in a light/dye model of microvascular insult. Am J Pathol. 1983;110(2):148–160. [PMC free article] [PubMed] [Google Scholar]
- 34.Anyaegbu CC, Okpala IE, Akren'Ova YA, Salimonu LS. Peripheral blood neutrophil count and candidacidal activity correlate with the clinical severity of sickle cell anaemia (SCA). Eur J Haematol. 1998;60(4):267–268. doi: 10.1111/j.1600-0609.1998.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 35.Luther T, Mackman N. Tissue factor in the heart. Multiple roles in hemostasis, thrombosis, and inflammation. Trends Cardiovasc Med. 2001;11(8):307–312. doi: 10.1016/s1050-1738(01)00129-3. [DOI] [PubMed] [Google Scholar]
- 36.Hebbel RP, Vercellotti GM. The endothelial biology of sickle cell disease. J Lab Clin Med. 1997;129(3):288–293. doi: 10.1016/s0022-2143(97)90176-1. [DOI] [PubMed] [Google Scholar]
- 37.Onyemelukwe GC, Jibril HB. Anti-thrombin III deficiency in Nigerian children with sickle cell disease: possible role in the cerebral syndrome. Trop Geogr Med. 1992;44(1–2):37–41. [PubMed] [Google Scholar]
- 38.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A. 2002;99(5):3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117(4):850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillery CA, Panepinto JA. Pathophysiology of stroke in sickle cell disease. Microcirculation. 2004;11(2):195–208. doi: 10.1080/10739680490278600. [DOI] [PubMed] [Google Scholar]
- 41.Chang Milbauer L, Wei P, Enenstein J, et al. Genetic endothelial systems biology of sickle stroke risk. Blood. 2008;111(7):3872–3879. doi: 10.1182/blood-2007-06-097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shet AS, Hoffmann TJ, Jirouskova M, et al. Morphological and functional platelet abnormalities in Berkeley sickle cell mice. Blood Cells Mol Dis. 2008;41(1):109–118. doi: 10.1016/j.bcmd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115(12):3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proske S, Vollmar B, Menger MD. Microvascular consequences of thrombosis in small venules: an in vivo microscopic study using a novel model in the ear of the hairless mouse. Thromb Res. 2000;98(6):491–498. doi: 10.1016/s0049-3848(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 45.Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115(10):2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holtzclaw JD, Jack D, Aguayo SM, Eckman JR, Roman J, Hsu LL. Enhanced pulmonary and systemic response to endotoxin in transgenic sickle mice. Am J Respir Crit Care Med. 2004;169(4):687–695. doi: 10.1164/rccm.200302-224OC. [DOI] [PubMed] [Google Scholar]
- 47.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 48.Scaldaferri F, Sans M, Vetrano S, et al. Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J Clin Invest. 2007;117(7):1951–1960. doi: 10.1172/JCI31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsui NM, Varki A, Embury SH. Heparin inhibits the flow adhesion of sickle red blood cells to P-selectin. Blood. 2002;100(10):3790–3796. doi: 10.1182/blood-2002-02-0626. [DOI] [PubMed] [Google Scholar]
- 50.Markwardt F. Hirudin as alternative anticoagulant–a historical review. Semin Thromb Hemost. 2002;28(5):405–414. doi: 10.1055/s-2002-35292. [DOI] [PubMed] [Google Scholar]
- 51.Sorg H, Hoffmann JN, Menger MD, Lindenblatt N, Goehring P, Vollmar B. Antithrombin is as effective as heparin and hirudin to prevent formation of microvascular thrombosis in a murine model. Thromb Haemost. 2006;96(3):371–377. [PubMed] [Google Scholar]
- 52.Wun T, Paglieroni T, Field CL, et al. Platelet-erythrocyte adhesion in sickle cell disease. J Investig Med. 1999;47(3):121–127. [PubMed] [Google Scholar]
- 53.Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, oude Egbrink MG. Hypercholesterolemia enhances thromboembolism in arterioles but not venules: complete reversal by L-arginine. Arterioscler Thromb Vasc Biol. 2002;22(4):680–685. doi: 10.1161/01.atv.0000013287.08141.74. [DOI] [PubMed] [Google Scholar]
- 54.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thrombo-inflammatory injury. Nat Med. 2009;15(4):384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 56.Fuchs T. Neutrophil Extracellular Traps Induce Platelet Adhesion and Thrombus Formation.. 51st ASH annual meeting and exposition; Abstract No. 1345. [Google Scholar]