Abstract
VEGF165, the major angiogenic growth factor, is known to activate various steps in proangiogenic endothelial cell behavior, such as endothelial cell migration and invasion, or endothelial cell survival. Thereby, the urokinase-type plasminogen activator (uPA) system has been shown to play an essential role not only by its proteolytic capacities, but also by induction of intracellular signal transduction. Therefore, expression of its cell surface receptor uPAR is thought to be an essential regulatory mechanism in angiogenesis. We found that uPAR expression on the surface of confluent endothelial cells was down-regulated compared with subconfluent proliferating endothelial cells. Regulation of uPAR expression was most probably affected by extracellular signal-regulated kinase 1/2 (ERK1/2) activation, a downstream signaling event of the VEGF/VEGF-receptor system. Consistently, the receptor-like protein tyrosine phosphatase DEP-1 (density enhanced phosphatase-1/CD148), which is abundantly expressed in confluent endothelial cells, inhibited the VEGF-dependent activation of ERK1/2, leading to down-regulation of uPAR expression. Overexpression of active ERK1 rescued the DEP-1 effect on uPAR. That DEP-1 plays a biologic role in angiogenic endothelial cell behavior was demonstrated in endothelial cell migration, proliferation, and capillary-like tube formation assays in vitro.
Introduction
Angiogenesis is currently in the focus of basic and translational research: a detailed analysis of the complex mechanisms involved in its regulation led to various therapeutic concepts1 in oncology for tumor angiogenesis, ophthalmology as in macular degeneration or choroidal neovascular disease, rheumatology in pannus formation, or dermatology for psoriasis.
We have demonstrated previously that initial steps of angiogenesis are dependent on vascular endothelial growth factor receptor-2 (VEGFR-2) induced pro-urokinase (pro-uPA) activation.2 Thereby, pro-uPA bound to its glycosylphosphatidylinositol-anchored cell surface receptor uPAR becomes activated, a process that involves integrin-dependent membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 activation in a phosphatidylinositol-3 kinase-dependent manner.2,3 Active uPA in turn is blocked by its specific inhibitor plasminogen activator inhibitor-1 (PAI-1). The so-formed ternary complex uPAR-uPA-PAI-1 becomes internalized via a low-density lipoprotein-receptor like molecule.4,5 Apart from an eminent role of uPAR in cancer cell biology,6–9 this aforementioned process seems to mediate VEGF-induced endothelial cell (EC) migration in vitro as well as angiogenesis in vivo as shown by a Matrigel plaque angiogenesis assay.2 Furthermore, uPA was shown to mediate EC survival via induction of the X-linked inhibitor of apoptosis protein, a process that is also dependent on uPAR.10 These data suggest that the amount of cell surface uPAR is affecting (1) the initial migratory response of ECs toward angiogenic stimulation11 and (2) EC survival during angiogenesis. Thereby, it is known that activation of somatic cells,12 and specifically of ECs13,14 by growth factors, is greatly limited when cells are grown to confluence.
In this study, we show that uPAR surface expression is dependent on EC density. Thereby, density enhanced phosphatase-1 (DEP-1) negatively regulates extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation, which is involved in uPAR protein expression. These data indicate that DEP-1 is a regulator of uPAR, a central molecule for proangiogenic EC behavior.
Methods
Cell culture
Human umbilical vein ECs (HUVECs) were cultured at 37°C and 5% CO2 in M199 (Sigma-Aldrich) supplemented with 20% fetal calf serum (FCS) and bovine EC growth supplement ECGS (Technoclone), 2mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin (referred to as “complete growth medium”) as described previously.3 Experiments were performed using EC cultures up to passage 5 seeded in polystyrene 6- to 96-well plates (Iwaki microplate) coated with 1% gelatin. Human embryonic kidney cells (293 cells) were obtained and cultured as recommended (ATCC).
Expression plasmids
Expression plasmids for full-length human DEP-1 (pSRα DEP-1/HA), the catalytically inactive mutant (pSRα DEP-1 C-S/HA, C1239S), the cytoplasmic deletion mutant (pSRα DEP-1 ΔCyto/HA, terminated at amino acid 1051), the extracellular deletion mutant (pSRα DEP-1 Myr), and the empty vector (pSRα) were prepared by Ute Priglinger in Thomas O. Daniel's laboratory (Vanderbilt University School of Medicine, Nashville, TN).15 pEGFP-N1 plasmid was purchased from Clontech. pEGFP-ERK1 plasmid was kindly provided by Michael Freissmuth, Medical University Vienna.
Transfection
LipofectAMINE transfection.
HUVECs were seeded in 6-well plates and transfected after 24 hours at 50% confluence. Transient transfections were performed using the LipofectAMINE Plus reagent (Invitrogen) according to the manufacturer's protocol. Cells were incubated with a transfection mixture containing 1.5 μg of total DNA, 6 μL of Plus reagent, and 4 μL of LipofectAMINE in a total volume of 1 mL M199 for 130 minutes. Cells were then incubated with full growth medium and harvested after 12 hours.
Amaxa nucleofection.
Amaxa nucleofection was used for transient transfection with the HUVEC Nucleofector Kit according to the manufacturer's instructions. Briefly, 5 × 105 cells were resuspended in Nucleofector solution previously mixed with plasmids outlined in the respective figure legends, and incubated in full growth medium. After 48 hours, cells were harvested for the respective experiments.
Calcium-phosphate transfection.
293 Cells were transiently transfected for luciferase reporter assays using calcium phosphate16 and 1.5 μg of DNA as described.17
Enzyme assays
Human embryonic kidney 293 cells or HUVECs were transfected with the constructs outlined in the figure legends as well as with luciferase reporter constructs (Elk-1) and SV40-Renilla vector as an internal control. Luciferase and Renilla assays were performed 24 hours after transfection of the cells with a Dual Luciferase Assay Kit (Promega) as described.18 Luciferase activity was normalized to the respective Renilla values.
RNA isolation and relative quantitative reverse transcriptase-polymerase chain reaction
RNA from HUVECs was extracted using Trizol reagent. Total RNA (900 ng) was reverse transcribed with MuLV-reverse transcriptase using the Gene Amp RNA PCR kit (Applied Biosystems) and oligo d(T)16 primers. The mRNA sequences for the genes to be analyzed were obtained from GenBank. The primers were designed using the PRIMER3 1.1.1 software (Whitehead Institute for Biomedical Research). The following forward and reverse primers were used for uPAR: forward, 5′-GAG AAG AGC TGG AGC TGG TG-3′; reverse, 5′-CTT CGG GAA TAG GTG ACA GC-3′; DEP-1: forward, 5′-AGC AGG CTC AGG ACT ATG GA-3′; reverse, 5′-AAC GAG GTA CCG GAA GTT GA-3′; dual-specificity phosphatase-1 (DUSP-1): forward, 5′-TCA AGA ATG CTG GAG GAA GG-3′; reverse, 5′-CAG CCT CTG CCG AAC AGT-3′; DUSP-4: forward, 5′-TAC TCG GCG GTC ATC GTC TA-3′; reverse, 5′-TCT GGG TAC TCG GAG GAA AA-3′; quantitative reverse transcriptase-polymerase chain reaction was performed by LightCycler technology using the Fast Start SYBR Green I kit for amplification and detection (Roche Diagnostics). In all assays, cDNA was amplified using a standardized program (10-second denaturing step and 55 cycles of 5 seconds at 95°C; 15 seconds at 65°C, and 15 seconds at 72°C; melting point analysis in 0.1°C steps; final cooling step). Each LightCycler capillary was loaded with 1.5 μL DNA Master Mix, 1.8 μL MgCl2 (25mM), 10.1 μL H2O, and 0.4 μL of each primer (10μM). The final amount of cDNA per reaction corresponded to 2.5 ng total RNA used for reverse transcription. Relative quantification of target gene expression was performed using a mathematical model described by Pfaffl.19 The expression of the target molecule was normalized to the expression of porphobilinogen deaminase as previously described.10
Immunocytofluorimetric analysis
Monolayers of HUVECs were treated as indicated and thereafter harvested with 0.05% ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS (15 minutes), and aliquots were permeabilized using 0.2% Tween 20 (GE Healthcare) for 30 minutes at room temperature. Primary antibodies as well as fluorochrome-conjugated secondary antibodies were incubated for at least 30 minutes at 4°C in concentrations ranging from 2 to 10 μg/mL. Samples were analyzed with LSR II flow cytometer (BD Biosciences). Antigen amount was calculated from geometric mean fluorescence values. Primary antibodies include the following: monoclonal mouse anti–human uPAR (domains 2 + 3) IgG1 3937 and polyclonal rabbit antihuman uPAR IgG1 399R (both from American Diagnostica) and monoclonal mouse antihuman DEP-1 (CD148) IgG1 AHT4802 (BioSource International, Invitrogen). Secondary antibodies included phycoerythrin-conjugated goat anti–mouse IgG P9670 (Sigma-Aldrich) and Alexa Fluor 488–conjugated goat anti–mouse IgG1 (Invitrogen). Total uPAR was measured in permeabilized cells, whereas surface uPAR was measured on nonpermeabilized cells.
Western blotting
HUVECs were washed with PBS and lysed in Laemmli buffer or in RIPA buffer at 4°C. Samples were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore). Membranes were blocked in PBS containing 0.1% Tween 20 and 5% nonfat dry milk and incubated with polyclonal rabbit anti–pERK1/2 antibody, 1:1000, polyclonal rabbit anti–pan-ERK1/2 antibody, 1:1000 (both Cell Signaling), monoclonal mouse anti–human DEP-1 (CD148) IgG1 AHT4802 (from Biosource, Invitrogen), and monoclonal R2 mouse anti–human uPAR (a kind gift of Gunilla Hoyer-Hansen, Finsen Laboratory, Copenhagen, Denmark) for 1 hour at room temperature. Incubation with the secondary horseradish peroxidase–labeled antibody (GE Healthcare) was done 1:2500 at room temperature for 45 minutes. Detection of bands was performed with ECL Plus (GE Healthcare) according to the manufacturer's protocol. Bands were visualized and quantified with FluorChem HD2 (Alpha Innotech).
DEP-1 knockdown
Transfection of siRNA into HUVECs was done using polyethylenimine (Sigma-Aldrich). Sparsely seeded HUVECs (5000 cells/cm2) were transfected after 24 hours of culture in complete growth medium. siRNA against DEP-1 was obtained from Ambion: 5′-GCAGUACAGCAGAAUCCUUdTdT-3′; 5′-AAGGAUUCUGCUGUACUGCdTdT-3′. For one 6-well, 8 μL of vortexed polyethylenimine (35μM, pH 7.0) was mixed with 93 μL of 2 times N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered saline buffer (280mM NaCl, 1.5mM Na2HPO4, 50mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 10 μL of siRNA (20μM) was mixed with 93μL of 2 times N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered saline buffer. The 2 solutions were unified and incubated in the dark at room temperature for 20 minutes. Afterward, 200 μL of transfection mix was added to 800 μL of serum-free medium (M199) per well, and HUVECs were incubated for 4 hours at 37°C and 5% CO2. The transfection solution was then replaced by complete growth medium, and cells were harvested for analysis after grown to confluence.
Cell proliferation assay
Amaxa-nucleofected HUVECs were seeded subconfluently in 96-well plates and incubated for 24 hours with M199 containing 10% FCS. Afterward, 20 μL M199 containing 1 mCi (37 × 103 Bq) of 3H-thymidine (PerkinElmer Life and Analytical Sciences) was added per well. Cultures were harvested 20 hours after labeling using the Harvester 96 (Tomtec), and 3H-thymidine incorporation into DNA was measured using the 1205 Betaplate Liquid Scintillation Counter (PerkinElmer Life and Analytical Sciences). Results of triplicate cultures are shown as mean counts per minute plus or minus SEM.
EC transmigration assay
Transmigration was performed in a modified Boyden chamber system using transwell membranes (8 μm) coated with 1% gelatin. Amaxa-nucleofected HUVECs were seeded to the top of the membrane, whereas VEGF165 (50 mg/mL) was added to the lower chamber. After 5 hours of incubation, filters were washed with PBS once, fixed with 10% buffered formalin, and stained with 4′-6-diamidino-2-phenylindole for nuclear staining. Migrated cells were counted using an AX70 Olympus microscope.
In vitro Matrigel tube-formation assay
Matrigel tube formation assays were performed as described before.20 Briefly, Amaxa-nucleofected HUVECs were seeded on Matrigel in 48-well plates and incubated in M199 supplemented with 1% FCS. Photographs were taken after 24 hours and the length of capillary-like structures were quantified using ImageJ 1.37 software (National Institutes of Health).
Statistics
Experimental values are expressed as mean plus or minus SEM if not otherwise indicated. Statistical significances were determined by unpaired Student t test and one-way analysis of variance. Significance was assigned at *P < .05 and **P < .01. All results were reproduced at least in 3 independent experiments.
Image acquisition
Cells were visualized using an Olympus IMT-2 microscope (20×/0.4 NA) and images were captured using an Olympus E620 camera (both Olympus Optical Co); pictures were imported to Adobe Photoshop CS4 software 11.0.1 (Adobe Systems); magnification was ×200 or as indicated.
Results
Confluence affects uPAR expression in ECs
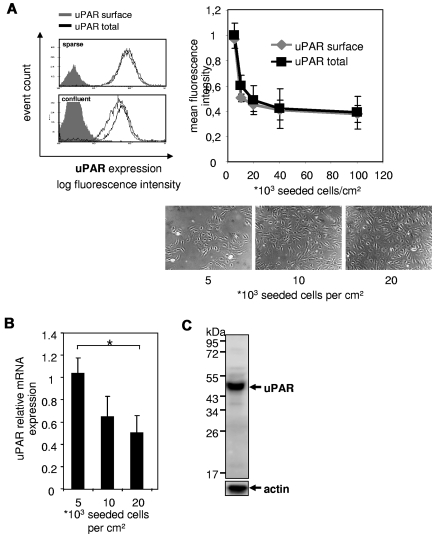
In an initial experiment, we analyzed possible effects of EC density on uPAR expression. By the use of fluorocytometric analyses, we found that confluent ECs express markedly reduced amounts of uPAR compared with sparsely seeded ECs, showing a consistent density dependence (Figure 1A). As surface and total uPAR expression was concomitantly decreased, we further analyzed uPAR mRNA expression and found a concordant decrease. Consistently, the degree of cell density was inversely correlated with uPAR mRNA expression (Figure 1B). Although it has been shown that both forms of uPAR, an intact approximately 44 kDa21 (composing 3 homologous domains D1, D2, and D3) as well as the cleaved form of uPAR (30 kDa, composing only D2 and D3), can be detected on cell surface of particular cultured cells,22-25 in ECs mainly intact uPAR was detected as shown by Western blot analysis (Figure 1C). This expression pattern was unaffected by cell density (data not shown), suggesting that regulation of uncleaved uPAR might play a functional role in cell surface bound proteolysis and extracellular matrix interaction.
Figure 1.
Expression of intact uPAR inversely correlates with cell density. HUVECs were seeded in different densities (indicated as cells per centimeter squared) and grown in complete growth medium for 4 days. Photographs were taken before harvest. (A) Immunocytofluorimetric detections of cell surface uPAR (nonpermeabilized cells; gray line) and total uPAR (permeabilized cells; black line). The total amount of uPAR decreases with cell density. Geometric means for fluorescence intensities were calculated. Filled gray bar represents IgG. Data are mean ± SEM. (B) Relative quantitative reverse transcriptase-polymerase chain reaction of uPAR mRNA normalized to porphobilinogen deaminase. Data are mean ± SEM. *P < .05. (C) Representative Western blot for uPAR of lysates of HUVECs. Mainly intact (ie, full-length) uPAR (∼ 44 kDa) was detected in contrast to cleaved uPAR (30 kDa).
ERK/MAPK mediates cell density–dependent uPAR expression
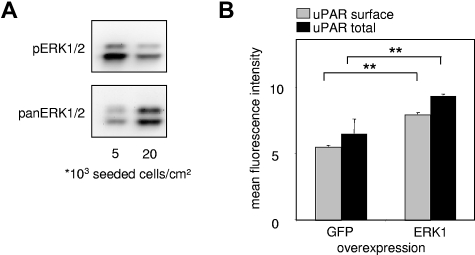
The mitogen-activated protein kinase (MAPK) pathway represents one of the most characterized signaling cascades in mitogenic stimulation26,27 and differentiation.28 It was previously shown that confluence of vascular ECs induces cell cycle exit by inhibition of the ERK1/2 signaling pathway, as shown by a decreased response to stimulation by different growth factors, such as fibroblast growth factor-2.14 When we analyzed EC lysates of experiments shown in Figure 1A, we found that sparsely seeded ECs had markedly higher amounts of basal pERK1/2 compared with confluent and thus more quiescent ECs (Figure 2A).
Figure 2.
Density-dependent uPAR expression is mediated by ERK/MAPK activity. (A) Western blots for phospho-(p)-ERK1/2 and pan-ERK1/2 from EC lysates. All samples were lysed in the same volume and prepared as described in “Western blotting” irrespective of cell density, and the same volume of samples was loaded. Consequently, the amount of loaded protein is higher in the 20 000 cells/cm2 sample than the 5000 cells/cm2 sample, reflected by the stronger signal in pan-ERK. Proteins were separated on a sodium dodecyl sulfate–10% polyacrylamide gel, and chemiluminescence of phosphorylated and pan-ERK1/2 was quantified with FluorTech HD2 from Alpha Innotech. (B) Immunocytofluorimetric detections of cell surface uPAR (nonpermeabilized cells; gray) and total (permeabilized cells; black) uPAR in HUVECs overexpressing either enhanced green fluorescence protein (EGFP) tagged ERK1 or EGFP alone. Transfected HUVECs were gated via EGFP fluorescence. Data are mean ± SEM. **P < .01.
Previous findings29 suggest that uPAR expression is regulated via the MAPK pathway, and uPAR expression can be down-regulated by the MAPKK-MEK1/2 inhibitors PD 098059 or U0126.30 Consistently, overexpression of active ERK1 led to an increase in uPAR expression in resting ECs (Figure 2B).
Increasing EC confluence up-regulates phosphatases and blunts activation of ERK/MAPK induced by VEGF165
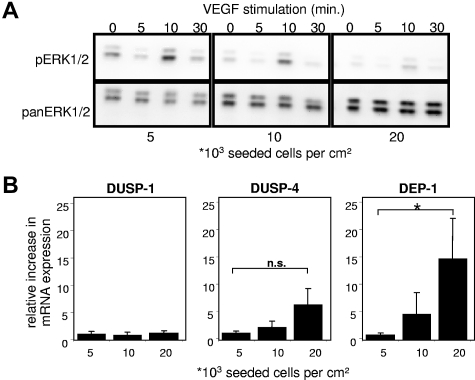
To determine whether confluence affects EC responses toward VEGF, HUVECs seeded at different densities were stimulated with VEGF165 and ERK1/2 phosphorylation was analyzed. Confluent ECs were much less responsive to VEGF165 stimulation compared with subconfluent ECs (Figure 3A). Identical activation patterns of ERK1/2 were observed in sparse and confluent cells; however, the amplitude of activation was attenuated with increasing cell density. By evaluating possible mechanisms responsible for the decrease of pERK1/2 levels, we examined HUVECs for major phosphatases regulating the MAPK signaling pathway,31,32 including DUSP-1 (or MAPK phosphatase-1), dual-specificity phosphatase-4 (DUSP-4 or MAPK phosphatase-2), and DEP-1.
Figure 3.
Influence of cell density on modulators of the MAPK signaling pathway in ECs. (A) Western blots for phospho-(p)-ERK1/2 and pan-ERK1/2 from EC lysates. Confluent (20 000), subconfluent (10 000), and sparse (5000) HUVECs were grown for 4 days in complete growth medium and afterward rendered quiescent for 4 hours by serum deprivation (4% bovine serum albumin in M199). Cells were then stimulated with 20 ng/mL VEGF165 for the time indicated. All samples were lysed in same volumes and obtained as described in “Western blotting.” Quantification of pERK1/2 chemiluminescence normalized to pan-ERK1/2 at 10 minutes of VEGF165 stimulation (RLU): 0.36/1 (5000), 0.16/0.38 (10 000), 0.05/0.1 (20 000); ERK1 = p44mapk, ERK2 = p42mapk. (B) Relative quantitative reverse transcriptase-PCR of HUVECs seeded in different densities (indicated as cells per centimeter squared) and grown in complete growth medium for 4 days. Among major phosphatases impacting the MAPK signaling pathway, only DEP-1 mRNA levels increased significantly; values were normalized to porphobilinogen deaminase. Data are mean ± SEM. *P < .05. n.s. indicates not significant.
Expression of the nuclear ERK/MAPK regulator phosphatases DUSP-1 and DUSP-4 was not significantly affected by cell density. With increase of cell density, the mRNA levels of the transmembrane tyrosine phosphatase DEP-1 were significantly up-regulated (Figure 3B), which pointed us to further investigate effects of DEP-1 on uPAR expression.
DEP-1 decreases uPAR surface expression via regulation of the ERK/MAPK signaling pathway
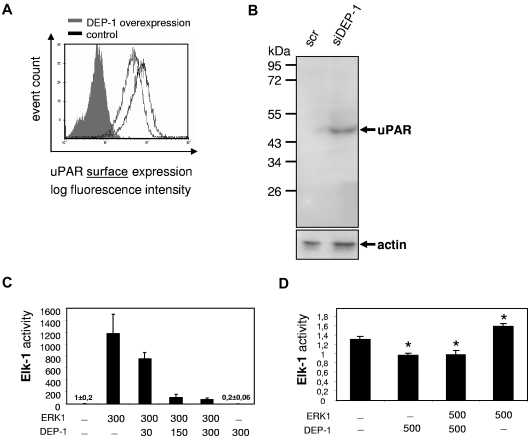
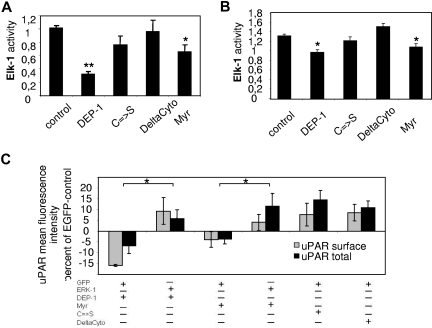
Expression of DEP-1 is regulated by cell confluence; therefore, the role of DEP-1 in confluence-related changes of uPAR expression was investigated. Subconfluent HUVECs were transfected with plasmids either encoding wild-type DEP-1 or mock. We observed a significant decrease in uPAR surface expression down to 64.8% plus or minus 2.2% (Figure 4A) when DEP-1 was overexpressed. The expression of CD59, another glycosylphosphatidylinositol-anchored protein, was unaltered in the presence or absence of DEP-1 (data not shown). Consistently, inhibition of DEP-1 expression by siRNA revealed an increase in total uPAR expression by 3.03- plus or minus 0.46-fold (Figure 4B). As a next step, we investigated the influence of DEP-1 on the MAPK signaling pathway. Elk-1 is a transcription factor downstream of ERK1/233 and thus a potential surrogate for uPAR transcription. Because of high transfection efficacy, we have first overexpressed ERK-1 in the human embryonic kidney cell line 293 (Figure 4C), which resulted in a marked increase of Elk-1 activity. Cotransfection of DEP-1 strongly and dose-dependently decreased ERK1-induced Elk-1 activation (Figure 4C). These results could be confirmed in primary ECs (HUVECs) as shown in Figure 4D. These data are consistent with the observation that DEP-1 has an inhibitory effect on the MAPK signaling cascade13 via direct dephosphorylation of ERK1/2.32
Figure 4.
DEP-1 decreases uPAR expression. (A) Immunocytofluorimetric detection of cell surface uPAR in nonpermeabilized HUVECs overexpressing DEP-1 (gray line) compared with mock-transfected HUVECs (black line). DEP-1-positive cells were detected by monoclonal mouse anti-DEP-1 antibody. Filled gray bar represents IgG. Data are mean ± SEM. (B) Western blot for uPAR from EC lysates. HUVECs were seeded sparsely (5000 cells/cm2) and were allowed to grow for 24 hours in complete growth medium. Consequently, cells were transfected with DEP-1 siRNA, which resulted in an efficient and sustained reduction of DEP-1 expression compared with transfection with scrambled (scr)RNA (data not shown). Cells were then incubated in complete growth medium until all samples had reached confluence. All samples were lysed and obtained as described in “Western blotting.” Knockdown of DEP-1 yielded a 3.03- ± 0.46-fold increase in uPAR protein expression in confluent ECs. (C) HEK 293 or (D) HUVECs were transfected for reporter gene analysis (ie, Elk-1 activity, a downstream target of ERK1/2) with DEP-1 and/or ERK1 overexpressing plasmids (indicated as amounts of DNA in nanograms per milliliter). Cells were harvested 24 hours after transfection and analyzed for luciferase activity. pSRα was taken as empty plasmid (control). Data are mean ± SEM. *P < .05.
Phosphatase activity of DEP-1 is required for confluence-dependent regulation of uPAR expression
DEP-1 is a receptor protein tyrosine phosphatase that consists of an extracellular domain, including 8 fibronectin III domains, a transmembrane segment, and an intracellular tail with a single protein tyrosine phosphatase domain.34–36 To reveal the structural and functional involvement of DEP-1 in the regulation of uPAR expression, mutated variants of the protein were generated. We introduced the mutated constructs of DEP-1 either lacking phosphatase activity (C → S) or the entire intracellular (DeltaCyto) or the extracellular (Myr) domain into HEK293 cells (Figure 5A). These cells were transiently transfected with elements of the Elk-1 reporter system. We found that the cytoplasmic domain of DEP-1 is required and sufficient to regulate Elk-1 activity. Activation of Elk-1 is affected by the DEP-1 phosphatase domain because mutants of DEP-1 lacking phosphatase activity were not capable of reducing basal Elk-1 activity. These results could be confirmed in primary human ECs (Figure 5B). Finally, we were interested in which domain of DEP-1 is involved in the regulation of uPAR expression. Therefore, the mutated DEP-1 variants were introduced by transient transfection in HUVECs and uPAR protein levels were investigated. The cytoplasmic domain of DEP-1 was required and sufficient to reduce uPAR protein levels. The down-modulating effect of DEP-1 on uPAR could be prevented by coexpression of ERK1 and was again dependent on phosphatase activity, as mutants of DEP-1 lacking phosphatase activity were not able to decrease uPAR expression (Figure 5C). From these data, we conclude that DEP-1 regulates uPAR expression via the MAPK signaling pathway.
Figure 5.
MAPK-mediated uPAR expression is regulated by DEP-1 phosphatase activity. (A) HEK 293 cells and (B) HUVECs were transfected for reporter gene analysis (Elk-1 activity) with plasmids overexpressing either wild-type DEP-1 or its mutated forms. Cells were harvested after 24 hours and analyzed for luciferase activity. Data are mean ± SEM. *P < .05. **P < .01. Only catalytically active DEP-1 was able to reduce Elk-1 activity. (C) Immunocytofluorimetric detections of cell surface (nonpermeabilized cells; gray) and total (permeabilized cells; black) uPAR in HUVECs overexpressing different DEP-1 mutants and either EGFP or EGFP-ERK1 gated for EGFP fluorescence. *P < .05. Data are mean ± SEM. Values calculated as percentage over EGFP control. Only catalytically active mutants of DEP-1 were able to decrease uPAR expression. This effect could be rescued by ERK1 coexpression. DEP-1 indicates wild-type; C → S, inactivated tyrosine phosphatase resulting from cysteine to serine mutation in the catalytic domain; DeltaCyto, deletion of the intracellular domain (including phosphatase domain); and Myr, deletion of the extracellular domain).
DEP-1 inhibits key steps of angiogenesis in vitro
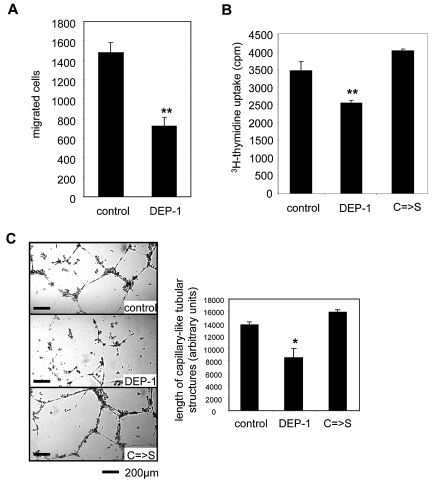
As shown before,2 uPAR plays a central role in VEGF-induced EC migration. Therefore, we analyzed the effect of DEP-1 overexpression on VEGF165-induced EC migration. As expected, DEP-1 significantly inhibited transmigration of HUVECs compared with mock-transfected cells (Figure 6A). Another prerequisite for efficient angiogenesis is EC proliferation,1 which was also significantly reduced whenever DEP-1 was overexpressed. Notably, this effect was dependent on intact phosphatase activity of DEP-1 (Figure 6B). Finally, we assessed capillary-like tube formation on Matrigel, another surrogate of angiogenic cell behavior.20 Capillary-like structures were significantly reduced in HUVECs transfected with wild-type DEP-1 compared with ECs overexpressing either a phosphatase inactive mutant (C → S) or mock (Figure 6C).
Figure 6.
Dep-1 inhibits proliferation, transmigration, and capillary-like tube formation of ECs. (A) EC migration of transfected HUVECs was assessed in a modified Boyden chamber assay in the presence of 50 ng/mL VEGF165. Migrated cells were fixed, stained, and quantified by microscopic counting. Overexpression of DEP-1 significantly decreased EC migration toward VEGF165 compared with mock-transfected cells. Data are mean ± SEM. **P < .01. (B) 3H-thymidine incorporation in HUVECs transfected with plasmids overexpressing either wild-type DEP-1, a mutant lacking phosphatase activity (C → S), or mock. After 24 hours of culture in full growth medium, 3H-thymidine (1 μCi/well) uptake of proliferating cells was measured (20 hours). Overexpression of DEP-1 significantly inhibited cell growth. Data are mean (cpm) ± SEM. **P < .01. (C) Capillary-like tube formation of HUVECs transfected with plasmids either overexpressing wild-type DEP-1, the C → S mutated form lacking phosphatase activity, or mock. Transfected cells were seeded on Matrigel in the presence of 1% FCS and analyzed 24 hours after seeding. Tubular-like structures were quantified as described in “In vitro Matrigel tube-formation assay.” DEP-1 inhibited capillary-like tube formation dependent on phosphatase activity. Data are mean ± SEM. *P < .05. **P < .01. DEP-1 indicates wild-type; and C → S, inactivated tyrosine phosphatase resulting from cysteine to serine mutation in the catalytic domain.
From these in vitro angiogenesis assays, we conclude that DEP-1 affects EC behavior.
Discussion
A variety of proangiogenic growth factors and chemokines activate ECs (ECs) to migrate and invade surrounding tissue, an essential step in angiogenesis. For invasion, the coordinated formation of a localized proteolytic machinery is essential. Focusing uPAR toward the leading edge of migrating cells provides such armor,2,37 whereas inhibition of uPA binding to its receptor prevents invasion of ECs.38 The functionality of uPAR critically depends on the presence of its intact form, as cleavage of uPAR disrupts its ability to bind uPA or the extracellular matrix protein vitronectin.39 VEGF induces EC migration and invasion by activating pro-uPA and mediating uptake of uPA-uPAR-PAI-1 complexes via LRP-like proteins.5 These initial steps of VEGF-induced EC migration are independent of transcriptional activity.2 Therefore, the amount of surface uPAR and pro-uPA bound to its receptor are expected to mediate EC responses toward VEGF. Here we show that uPAR expression is strongly regulated by cell density and that, in confluent ECs, the amount of uPAR is only approximately 30% compared with sparsely seeded ECs (Figure 1). Consistently, uPAR was found to be down-regulated in myoblasts when they reached confluence, cease migration, and start to differentiate.40 Therefore, it can be expected that confluent ECs respond much less to VEGF-induced EC migration or survival compared with sparsely seeded cells. Indeed, different responses toward fibroblast growth factor-2 depending on cell density were described before.14 Here we show that indeed the response of confluent ECs toward VEGF stimulation is dramatically reduced with respect to the activation of the MAPK signaling pathway (Figure 3A). Therefore, confluent ECs, as expected to be found in mature vessels, not only showed decreased responses toward VEGF stimulation but also express less uPAR. Notably, intact uPAR is the most prominent form expressed on the surface of ECs (Figure 1C). As potential factors responsible for down-regulating ERK1/2 activation as well as decreased uPAR expression, we suggest DEP-1 as a hitherto undescribed regulator for uPAR. As indicated by its name, expression of DEP-1 is regulated by cell density.34 Thereby, DEP-1 is up-regulated with increasing density in ECs35 and inhibits the MAPK pathway13,15,32 in confluent compared with sparsely seeded ECs (Figure 2A). Indeed, overexpression of DEP-1 not only decreased ERK1/2 activity but also uPAR expression (Figure 4). This effect of DEP-1 was dependent on the intact phosphatase-domain because mutants deficient in DEP-1 phosphatase activity were not active, whereas a mutant deficient in the extracellular domain showed activity, but not to the same extent as full-length DEP-1 (Figure 5). Indeed, it was shown before that certain extracellular matrix proteins increased protein tyrosine phosphatase activity of DEP-1; however, only when its extracellular domain was present.41 The remaining activity of the extracellular deficient mutant indicates that the extracellular domain is not responsible for linking DEP-1 to growth factors or other extracellular surface molecules guiding DEP-1 phosphatase activity to intracellular targets. Our results further support the data from Takahashi et al,15 who reported that an increase in DEP-1 phosphatase activity by an activating antibody strongly inhibits angiogenesis in vivo using a murine corneal pocket model. Consistently, DEP-1 is shown to be important for contact inhibition of VEGF-induced proliferation13 and during neo-intima formation.42 That DEP-1 affects cell behavior has been shown for many cell types.13,43–46 For primary ECs, we here report that DEP-1 inhibited transmigration (Figure 6A), proliferation (Figure 6B), and formation of capillary-like structures (Figure 6C). This is consistent with previous findings that the disrupted DEP-1 gene led to early embryonic lethality at midgestation because of impaired vascularization.44
In this study, we suggest that DEP-1, which is up-regulated during confluence, not only decreases VEGF-induced MAPK activity but also impairs MAPK-dependent uPAR expression. As it has been shown that uPAR represents a central player for VEGF-induced proangiogenic EC behavior,2,3,10,47 we suggest DEP-1 as a novel target for agents modulating angiogenesis.
Acknowledgments
The authors thank Melanie Gschaider, Christine Wagner, and Marina Poettler for technical advice.
This work is dedicated to Bernd R. Binder, who was an important contributor to this project. He died on August 28, 2010.
The study was supported by the Hans and Blanc Moser Foundation, the Integrated Project of the 6th European Union framework program Cancerdegradome (contract no. LSHC-CT-2003-503297), and Initiactive Krebsforschung (UE71104018) as well as the Austrian Science Foundation project (FWF P21301; G.W.P.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M.B., P.C.H., and G.W.P. performed experiments; U.P. prepared plasmids; J.M.-B. analyzed results; and P.M.B., G.W.P. and B.R.B. designed the research project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of P.M.B. is Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University Vienna, Vienna, Austria.
Correspondence: Gerald W. Prager, Clinical Division of Oncology, Department of Medicine I and Comprehensive Cancer Center, Medical University Vienna, Vienna, Austria; e-mail: gerald.prager@meduniwien.ac.at.
References
- 1.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 2.Prager GW, Breuss JM, Steurer S, et al. Vascular endothelial growth factor receptor-2-induced initial endothelial cell migration depends on the presence of the urokinase receptor. Circ Res. 2004;94(12):1562–1570. doi: 10.1161/01.RES.0000131498.36194.6b. [DOI] [PubMed] [Google Scholar]
- 3.Prager GW, Breuss JM, Steurer S, Mihaly J, Binder BR. Vascular endothelial growth factor (VEGF) induces rapid prourokinase (pro-uPA) activation on the surface of endothelial cells. Blood. 2004;103(3):955–962. doi: 10.1182/blood-2003-07-2214. [DOI] [PubMed] [Google Scholar]
- 4.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71(3):411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 5.Nykjaer A, Conese M, Christensen EI, et al. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J. 1997;16(10):2610–2620. doi: 10.1093/emboj/16.10.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrati S, Margheri F, Fibbi G, et al. Endothelial cells and normal breast epithelial cells enhance invasion of breast carcinoma cells by CXCR-4-dependent up-regulation of urokinase-type plasminogen activator receptor (uPAR, CD87) expression. J Pathol. 2008;214(5):545–554. doi: 10.1002/path.2309. [DOI] [PubMed] [Google Scholar]
- 7.Henic E, Borgfeldt C, Christensen IJ, Casslen B, Hoyer-Hansen G. Cleaved forms of the urokinase plasminogen activator receptor in plasma have diagnostic potential and predict postoperative survival in patients with ovarian cancer. Clin Cancer Res. 2008;14(18):5785–5793. doi: 10.1158/1078-0432.CCR-08-0096. [DOI] [PubMed] [Google Scholar]
- 8.Andronicos NM, Chen EI, Baik N, et al. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010;115(7):1319–1330. doi: 10.1182/blood-2008-11-188938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magdolen V, Kruger A, Sato S, et al. Inhibition of the tumor-associated urokinase-type plasminogen activation system: effects of high-level synthesis of soluble urokinase receptor in ovarian and breast cancer cells in vitro and in vivo. Recent Results Cancer Res. 2003;162:43–63. doi: 10.1007/978-3-642-59349-9_4. [DOI] [PubMed] [Google Scholar]
- 10.Prager GW, Mihaly J, Brunner PM, Koshelnick Y, Hoyer-Hansen G, Binder BR. Urokinase mediates endothelial cell survival via induction of the X-linked inhibitor of apoptosis protein. Blood. 2009;113(6):1383–1390. doi: 10.1182/blood-2008-06-164210. [DOI] [PubMed] [Google Scholar]
- 11.Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemost. 2007;97(3):336–342. [PubMed] [Google Scholar]
- 12.Fagotto F, Gumbiner BM. Cell contact-dependent signaling. Dev Biol. 1996;180(2):445–454. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- 13.Grazia Lampugnani M, Zanetti A, Corada M, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161(4):793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinals F, Pouyssegur J. Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Mol Cell Biol. 1999;19(4):2763–2772. doi: 10.1128/mcb.19.4.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Takahashi K, Mernaugh RL, Tsuboi N, Liu H, Daniel TO. A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood. 2006;108(4):1234–1242. doi: 10.1182/blood-2005-10-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins ND, Edwards NL, Duckett CS, Agranoff AB, Schmid RM, Nabel GJ. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12(9):3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucerna M, Mechtcheriakova D, Kadl A, et al. NAB2, a corepressor of EGR-1, inhibits vascular endothelial growth factor-mediated gene induction and angiogenic responses of endothelial cells. J Biol Chem. 2003;278(13):11433–11440. doi: 10.1074/jbc.M204937200. [DOI] [PubMed] [Google Scholar]
- 18.Gruber F, Hufnagl P, Hofer-Warbinek R, et al. Direct binding of Nur77/NAK-1 to the plasminogen activator inhibitor 1 (PAI-1) promoter regulates TNF alpha-induced PAI-1 expression. Blood. 2003;101(8):3042–3048. doi: 10.1182/blood-2002-07-2331. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponce ML. Tube formation: an in vitro matrigel angiogenesis assay. Methods Mol Biol. 2009;467:183–188. doi: 10.1007/978-1-59745-241-0_10. [DOI] [PubMed] [Google Scholar]
- 21.Serrati S, Cinelli M, Margheri F, et al. Systemic sclerosis fibroblasts inhibit in vitro angiogenesis by MMP-12-dependent cleavage of the endothelial cell urokinase receptor. J Pathol. 2006;210(2):240–248. doi: 10.1002/path.2048. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer-Hansen G, Ronne E, Solberg H, et al. Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J Biol Chem. 1992;267(25):18224–18229. [PubMed] [Google Scholar]
- 23.Ronne E, Behrendt N, Ellis V, Ploug M, Dano K, Hoyer-Hansen G. Cell-induced potentiation of the plasminogen activation system is abolished by a monoclonal antibody that recognizes the NH2-terminal domain of the urokinase receptor. FEBS Lett. 1991;288(1):233–236. doi: 10.1016/0014-5793(91)81042-7. [DOI] [PubMed] [Google Scholar]
- 24.Hoyer-Hansen G, Behrendt N, Ploug M, Dano K, Preissner KT. The intact urokinase receptor is required for efficient vitronectin binding: receptor cleavage prevents ligand interaction. FEBS Lett. 1997;420(1):79–85. doi: 10.1016/s0014-5793(97)01491-9. [DOI] [PubMed] [Google Scholar]
- 25.Sidenius N, Blasi F. Domain 1 of the urokinase receptor (uPAR) is required for uPAR-mediated cell binding to vitronectin. FEBS Lett. 2000;470(1):40–46. doi: 10.1016/s0014-5793(00)01282-5. [DOI] [PubMed] [Google Scholar]
- 26.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270(25):14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 27.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- 28.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 29.Lund LR, Ellis V, Ronne E, Pyke C, Dano K. Transcriptional and post-transcriptional regulation of the receptor for urokinase-type plasminogen activator by cytokines and tumour promoters in the human lung carcinoma cell line A549. Biochem J. 1995;310(1):345–352. doi: 10.1042/bj3100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi H, Suzuki M, Kanayama N, Nishida T, Takigawa M, Terao T. Suppression of urokinase receptor expression by bikunin is associated with inhibition of upstream targets of extracellular signal-regulated kinase-dependent cascade. Eur J Biochem. 2002;269(16):3945–3957. doi: 10.1046/j.1432-1033.2002.03068.x. [DOI] [PubMed] [Google Scholar]
- 31.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 32.Sacco F, Tinti M, Palma A, et al. Tumor suppressor density-enhanced phosphatase-1 (DEP-1) inhibits the RAS pathway by direct dephosphorylation of ERK1/2 kinases. J Biol Chem. 2009;284(33):22048–22058. doi: 10.1074/jbc.M109.002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang SH, Sharrocks AD. Convergence of the SUMO and MAPK pathways on the ETS-domain transcription factor Elk-1. Biochem Soc Symp. 2006;73:121–129. doi: 10.1042/bss0730121. [DOI] [PubMed] [Google Scholar]
- 34.Ostman A, Yang Q, Tonks NK. Expression of DEP-1, a receptor-like protein-tyrosine-phosphatase, is enhanced with increasing cell density. Proc Natl Acad Sci U S A. 1994;91(21):9680–9684. doi: 10.1073/pnas.91.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borges LG, Seifert RA, Grant FJ, et al. Cloning and characterization of rat density-enhanced phosphatase-1, a protein tyrosine phosphatase expressed by vascular cells. Circ Res. 1996;79(3):570–580. doi: 10.1161/01.res.79.3.570. [DOI] [PubMed] [Google Scholar]
- 36.Kuramochi S, Matsuda S, Matsuda Y, Saitoh T, Ohsugi M, Yamamoto T. Molecular cloning and characterization of Byp, a murine receptor-type tyrosine phosphatase similar to human DEP-1. FEBS Lett. 1996;378(1):7–14. doi: 10.1016/0014-5793(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 37.Kroon ME, Koolwijk P, van Goor H, et al. Role and localization of urokinase receptor in the formation of new microvascular structures in fibrin matrices. Am J Pathol. 1999;154(6):1731–1742. doi: 10.1016/S0002-9440(10)65429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min HY, Doyle LV, Vitt CR, et al. Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res. 1996;56(10):2428–2433. [PubMed] [Google Scholar]
- 39.Rasch MG, Lund IK, Almasi CE, Hoyer-Hansen G. Intact and cleaved uPAR forms: diagnostic and prognostic value in cancer. Front Biosci. 2008;13:6752–6762. doi: 10.2741/3186. [DOI] [PubMed] [Google Scholar]
- 40.Wells JM, Strickland S. Regulated localization confers multiple functions on the protease urokinase plasminogen activator. J Cell Physiol. 1997;171(2):217–225. doi: 10.1002/(SICI)1097-4652(199705)171:2<217::AID-JCP13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Sorby M, Sandstrom J, Ostman A. An extracellular ligand increases the specific activity of the receptor-like protein tyrosine phosphatase DEP-1. Oncogene. 2001;20(37):5219–5224. doi: 10.1038/sj.onc.1204581. [DOI] [PubMed] [Google Scholar]
- 42.Kappert K, Paulsson J, Sparwel J, et al. Dynamic changes in the expression of DEP-1 and other PDGF receptor-antagonizing PTPs during onset and termination of neointima formation. FASEB J. 2007;21(2):523–534. doi: 10.1096/fj.06-6219com. [DOI] [PubMed] [Google Scholar]
- 43.Jandt E, Denner K, Kovalenko M, Ostman A, Bohmer FD. The protein-tyrosine phosphatase DEP-1 modulates growth factor-stimulated cell migration and cell-matrix adhesion. Oncogene. 2003;22(27):4175–4185. doi: 10.1038/sj.onc.1206652. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi T, Takahashi K, St John PL, et al. A mutant receptor tyrosine phosphatase, CD148, causes defects in vascular development. Mol Cell Biol. 2003;23(5):1817–1831. doi: 10.1128/MCB.23.5.1817-1831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balavenkatraman KK, Jandt E, Friedrich K, et al. DEP-1 protein tyrosine phosphatase inhibits proliferation and migration of colon carcinoma cells and is upregulated by protective nutrients. Oncogene. 2006;25(47):6319–6324. doi: 10.1038/sj.onc.1209647. [DOI] [PubMed] [Google Scholar]
- 46.Kellie S, Craggs G, Bird IN, Jones GE. The tyrosine phosphatase DEP-1 induces cytoskeletal rearrangements, aberrant cell-substratum interactions and a reduction in cell proliferation. J Cell Sci. 2004;117(4):609–618. doi: 10.1242/jcs.00879. [DOI] [PubMed] [Google Scholar]
- 47.Kroon ME, Koolwijk P, van der Vecht B, van Hinsbergh VW. Urokinase receptor expression on human microvascular endothelial cells is increased by hypoxia: implications for capillary-like tube formation in a fibrin matrix. Blood. 2000;96(8):2775–2783. [PubMed] [Google Scholar]