Abstract
Selenium deficiency has been linked with increased cancer risk and, in some studies, selenium supplementation was protective against certain cancers. Previous studies suggest that selenium chemoprevention may involve reduced oxidative stress through enhanced glutathione (GSH). Our objectives were to examine the relationships between selenium and GSH in blood and modifying effects of race and sex in free living adults and individuals supplemented with selenium. Plasma selenium concentrations and free and bound GSH concentrations and γ-glutamyl cysteine ligase (GCL) activity in blood were measured in 336 healthy adults, (161 blacks, 175 whites). Plasma selenium and blood GSH were also measured in 36 healthy men from our previously conducted placebo-controlled trial of selenium-enriched yeast (247 μg/day for 9 months). In free-living adults, selenium concentrations were associated with increased blood GSH concentration and GCL activity (P<0.05). Further, selenium was significantly higher in whites than in blacks (P<0.01). After 9 months of supplementation, plasma selenium was increased 114% in whites and 50% in blacks (P<0.05) and blood GSH was increased 35% in whites (P<0.05) but was unchanged in blacks. These results indicate a direct association between selenium and GSH in blood of both free-living and selenium-supplemented individuals, with race being an important modifying factor.
Introduction
Many epidemiological and laboratory investigations show a protective effect of selenium against the development of cancer at numerous sites including prostate, colon and lung (1,2). Low soil selenium concentration is highly correlated with cancer mortality throughout the world (3-5). Both prospective and case control studies have shown associations between low blood or toenail selenium and increased risk for cancer, particularly of the prostate (6,7). In a randomized, placebo-controlled clinical trial of selenium-enriched yeast (Nutritional Prevention of Cancer [NPC]), reductions in prostate, colon and lung cancer incidence were observed with selenium supplementation in men (8-10). Based upon these finding, multiple large-scale clinical trials have been initiated worldwide to determine the role of selenium (in various forms and doses) in the prevention of prostate cancer (11-13). In the US, the SELECT trial was designed to assess the efficacy of selenomethionine and vitamin E, either alone or in combination, on prostate cancer (12), but was prematurely closed in 2008, at which time, no observable reduction in prostate cancer was apparent (14). However, the test agent in this study, selenomethionine, was different from that used in the NPC trial, selenium yeast; the later contains several forms of selenium that are more effective than selenomethionine in preclinical animal models (15).
Although the mechanisms of chemoprevention by selenium remain unclear, enhanced protection against oxidative stress may be involved (16-18). Selenium mediates the biological activity of selenium-containing antioxidant enzymes including glutathione peroxidase (8) and thioredoxin reductase (19). Preclinical studies have indicated that selenium supplementation, in various forms, can enhance concentrations the major intracellular antioxidant, glutathione (GSH) and the activity of its rate-limiting biosynthetic enzyme, γ-glutatmyl cysteine ligase (GCL), and decrease concentrations of its oxidized products in tissues and blood (20-24). GSH is a first line of defense against oxidative stress (25), and decreased GSH can result in increased risk for cancer (26). Glutathionylated proteins (GSSP) are major products of GSH oxidation, and their formation represents a potential mechanism by which oxidative stress can impact carcinogenesis (27,28). In healthy adult men, we observed that supplementation with selenium-enriched yeast (247 μg/d) resulted in a progressive increase in GSH and decrease in glutathionylated proteins in blood over 9 months (29).
In the current study, we examined the relationships between selenium and free and bound GSH concentrations and GCL activity in blood and dietary selenium intake in a large group of healthy un-supplemented adults and determined the modifying effects of factors such as race and sex. In addition, we examined these associations in participants of a selenium-enriched yeast intervention study (29).
Subjects and Methods
Materials
GSH, glutathione disulfide (GSSG), Cys, and cystine, were obtained from the Sigma Chemical Co. All other chemicals of high purity or HPLC grade were obtained from the Aldrich Chemical Co., Mallinckrodt, or EM Science.
Study of racial differences in smoking-related metabolism in Mt. Vernon, NY
Data and biological samples for the current analysis was obtained from a community-based study of >600 current smokers and non-smokers from Mount Vernon, Yonkers, Valhalla, and other areas in southeastern-mid Westchester County, NY (30,31). This recruitment strategy was aimed at minimizing differences in socioeconomic status which are thought to contribute to elevated cancer rates in blacks (32) since Mt. Vernon NY has a population that is approximately 50% black and 50% white and comparable sociodemographic characteristics among its residents (31,33). The methods and details of the study recruitment were described previously (30,31). In brief, subjects were healthy adults recruited using fliers, public service announcements, assistance from community and church leaders and other methods. Trained personnel interviewed the subjects using a structured questionnaire that contained items on demographics, smoking history, and other lifestyle habits. All subjects signed a consent form that was approved by the former Institute for Cancer Prevention (originally known as the American Health Foundation, Valhalla, NY) Institutional Review Board.
Data and biological samples were retrieved for a total of 336 randomly selected subjects for the current study. Subjects were equally divided by gender and race (Table 1). Questionnaire data included sociodemographic information, lifestyle factors, smoking and alcohol usage and medical history. Since the original study was designed to examine the effects of tobacco usage on parameters of health, the recruitment strategy included emphasis on smokers. Thus, nearly two thirds of the subjects were smokers, significantly greater than the prevalence of smoking in the general population. No major differences were noted by race for age, BMI, sociodemographic variables and smoking status (Table 1). As observed previously (31), whites smoked significantly more cigarettes per day than blacks, contributing to higher pack year values for whites compared to blacks. Data were also collected on occupation and related occupational exposures and no gender or race differences were observed (data not shown). Usual dietary intake data were collected using the National Cancer Institute's semi-quantitative food frequency questionnaire (Diet History Questionnaire, DHQ, http://riskfactor.cancer.gov/DHQ/). Nutrient data were calculated using the DIETSYS + Plus version 5·9 dietary analysis program (Block Dietary Data Systems, Berkeley, California).
Table 1.
Subject characteristics, Mt. Vernon Study
| Men | Females | ||||
|---|---|---|---|---|---|
| Groups | Black | White | Black | White | |
| N | 79 | 89 | 82 | 86 | |
| Age (yr) | |||||
| Mean ± SD | 34.2 ± 7.66 | 33.3 ± 10.2 | 34.7 ± 7.45 | 32.2 ± 10.6 | |
| Range | 20-56 | 18-59 | 18-52 | 18-55 | |
| BMI | |||||
| Mean ± SD | 25.6 ± 3.33 | 25.5 ± 3.72 | 25.6 ± 3.78 | 23.5 ± 3.97 | |
| Range | 18.7-35.0 | 18.7-37.0 | 19.4-35.5 | 17.0-32.7 | |
| Marital status (%) | |||||
| Single | 51.9 | 66.7 | 61.5 | 65.8 | |
| Married | 28.8 | 11.1 | 16.9 | 11.0 | |
| Divorced | 7.7 | 18.5 | 6.2 | 17.8 | |
| Separated | 11.5 | 3.7 | 10.8 | 1.4 | |
| Widowed | 0 | 0 | 4.6 | 4.1 | |
| Education (%) | |||||
| <12 yrs | 17.3 | 9.1 | 6.1 | 2.7 | |
| High school | 32.7 | 23.6 | 31.8 | 21.9 | |
| College | 48.1 | 63.6 | 51.5 | 61.6 | |
| Postgraduate | 1.9 | 3.6 | 10.6 | 13.7 | |
| No. residents/bedroom (%) | |||||
| <1 | 8.3 | 24.1 | 17.2 | 13.5 | |
| 1 – 1.9 | 62.5 | 61.1 | 67.2 | 63.5 | |
| 2 – 2.9 | 27.1 | 14.8 | 7.8 | 21.6 | |
| ≥3 | 2.1 | 0 | 7.8 | 1.4 | |
| Smoking Status, N (%) | |||||
| Current | 61 (77.2) | 70 (78.6) | 68 (82.9) | 66 (76.7) | |
| Non | 18 (22.8) | 19 (21.3) | 14 (17.1) | 20 (23.2) | |
| Cigarettes per day | |||||
| Mean ± SD | 15.9 ± 8.55 | 23.8 ± 12.4 | 14.0 ± 6.82 | 21.7 ± 9.33 | |
| Range | 4-60 | 6-70 | 3-40 | 5-50 | |
| Cigarette Pack Years | |||||
| Mean ± SD | 14.2 ± 13.1 | 22.0 ± 23.7 | 11.8 ± 8.57 | 18.2 ± 16.9 | |
Study of selenium yeast supplementation
The subject recruitment and clinical protocols were described in detail previously (29). In brief, a randomized, double-blinded, placebo-controlled study of Se-enriched yeast (247 μg/d) was conducted in Valhalla, NY to determine the effects on biomarkers of oxidative stress, glutathione, hormone status and circulating PSA concentrations. Subjects were healthy adult men (19–43 years of age) randomized into either the selenium or placebo arm (Table 2). Baseline data were collected on demographics, lifestyle habits, and usual dietary practices. Supplementation occurred daily for 9 months followed by a 3 month washout period. Blood and urine were collected at baseline and at 3, 9, and 12 months. Compliance was confirmed by pill count and plasma selenium concentrations. The study design was approved by the institutional review board of the former Institute for Cancer Prevention.
Table 2.
Subject characteristics, Selenium Yeast Intervention Trial
| Black | White | |||
|---|---|---|---|---|
| Placebo | Se-yeast | Placebo | Se-yeast | |
| No. of Subjects | 6 | 5 | 13 | 12 |
| Age (yr) | ||||
| Mean ± SD | 28.5 ± 4.59 | 30.0 ± 6.41 | 30.4 ± 6.81 | 31.8 ± 5.19 |
| Range | 23-36 | 25-43 | 23-41 | 23-41 |
| BMI | 25.8 ± 4.61 | 24.9 ± 5.10 | 24.8 ± 4.01 | 24.0 ± 4.32 |
Blood analyses
Plasma selenium determinations were performed at Huffman Laboratories, Inc. (Golden, CO) by ICP-MS using a Perkin-Elmer Sciex 6100 DRC Plus instrument. Free and bound GSH was measured in whole blood as described previously (30). Protein concentration in the RBC lysates was measured by the bicinchonic acid procedure (Pierce, Rockford, IL). GCL activity was determined by measuring the amount of the GCL product, γ-glutamylcysteine, formed after incubating the red blood cell protein lysates with cysteine and glutamic acid as described previously (34). In brief, protein lysates from packed red blood cells were purified by centrifugal ultrafiltration (molecular weight cutoff 3000; 12,000 g/25 min) and were incubated for 30 min with 20-fold of 100 mM Tris buffer containing substrates glutamic acid (20 mM), cysteine (5 mM) and ATP (10 mM). The reaction was stopped by precipitating proteins with 1 volume of 5% MPA. Precipitated proteins were separated by centrifugation and the concentrations of γ-glutamylcysteine in supernatants were determined by HPLC with coulometric detection using a Bio-Sil ODS-5S, 5-μm, 4.0 × 250 mm, C18 column (Bio-Rad, Life Science Research Group, Hercules, CA) eluted with a mobile phase consisting of 50 mM NaH2PO4, 0.05 mM octane sulfonic acid, 1% (v/v) acetonitrile and 0.5% N,N dimethylformamide (v/v) (pH 2.52) at a flow rate of 1 ml/min.
Statistical analyses
The overall differences in biochemical parameters were tested using one-way analysis of variance (ANOVA). Pairwise differences between treated groups and their respective controls were determined using Scheffe's procedure. All P values are two-tailed and considered statistically significant below the 0·05 level. Chi-squared analyses were performed to compare differences in distributions of plasma selenium concentrations between blacks and whites.
Results
Mount Vernon Study
Plasma selenium
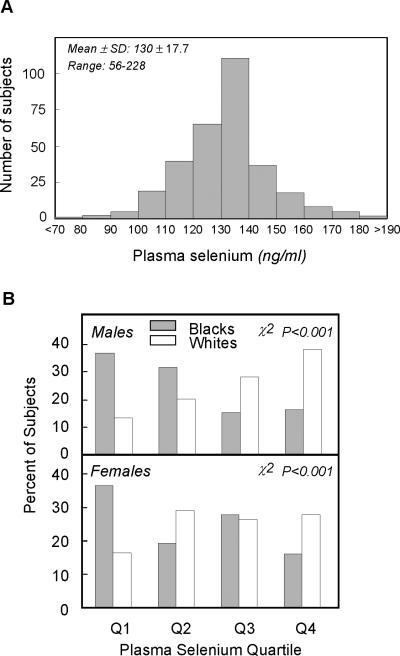
Plasma selenium concentrations were normally distributed ranging from 56 to 228 ng/ml with a mean ± SD of 130 ± 17.7 ng/ml (Figure 1A). Selenium concentrations fell within the expected range for US residents (100 ± 30 ng/ml) (1). When plasma selenium was analyzed by race and gender, significantly different distributions were observed between blacks and whites for both males and females (Figure 1B). When examined by quartile, a greater representation of blacks was observed in the lowest selenium group compared to whites while a greater representation of whites was observed in the highest selenium group compared to blacks (P<0.001). These racial differences were apparent for both males and females. Significant differences were also observed in mean plasma selenium concentrations between blacks and whites (Table 3). Plasma selenium was 9% higher in whites vs. blacks (P<0.01).
Figure 1.
Plasma selenium concentrations in participants of the Mt. Vernon Study. A. Frequency distribution of plasma selenium. B. Racial differences in plasma selenium by quartile in men and women.
Table 3.
Plasma selenium concentrations by race and sex, Mt. Vernon Study
| Blacks |
Whites |
||||||
|---|---|---|---|---|---|---|---|
| Plasma Selenium (ng/ml) |
Plasma Selenium (ng/ml) |
||||||
| Gender | N | Mean ± SD | Range | N | Mean ± SD | Range | P valuea |
| Males | 82 | 124 ± 15.3 | 92·3 - 165 | 86 | 133 ± 21.6 | 56.4 - 228 | 0.00004 |
| Females | 79 | 122 ± 18.0 | 81·5 - 175 | 89 | 135 ± 19.5 | 62.6 - 188 | 0.0027 |
| All | 161 | 1293 ± 16.7 | 81·5 - 175 | 175 | 134 ± 20.5 | 56.4 - 228 | <0.000001 |
comparison between racial groups
Dietary selenium intake
To determine if differences in selenium intake could explain the observed racial differences in plasma selenium concentrations, dietary selenium intake was measured by analysis of food frequency questionnaire data (Table 4). Selenium intake values were not different by race in either males or females. However, significantly lower intake values were observed for females compared to males, regardless of race (P<0.005).
Table 4.
Dietary Selenium intake concentrations by race and sex, Mt. Vernon Study
| Blacks |
Whites |
|||||
|---|---|---|---|---|---|---|
| Selenium intake (μg/day) |
Selenium intake (μg/day) |
|||||
| Gender | N | Mean ± SD | Range | N | Mean ± SD | Range |
| Males | 44 | 177 ± 127 | 29.7 – 554 | 53 | 179 ± 104 | 28.9 - 486 |
| Females | 51 | 111 ± 78.8a | 17.2 – 454 | 59 | 109 ± 64.6a | 19.3 - 309 |
significantly different from males (P<0.005)
Blood glutathione in blacks and whites
Whole blood free and protein-bound GSH were determined for all subjects and gender and race differences were examined (Table 5). Values for both free and bound GSH were similar to those observed previously in healthy adults (30,35). In women, GSH concentrations were 16% higher among whites than blacks (P<0.005), however, no differences were observed among men. Protein-bound GSH concentrations in women and men combined were 35% greater in whites than in blacks (P<0.05), whereas, differences by race within each gender group were not significant. Gender differences for GSH were observed in blacks with men having 16% higher concentrations than women (P<0.01).
Table 5.
Blood glutathione concentrations by race and sex, Mt. Vernon Study
| Blacks |
Whites |
|||||
|---|---|---|---|---|---|---|
| Glutathione (μmol/ml) |
Glutathione (μmol/ml) |
|||||
| Gender | N | Mean ± SD | Range | N | Mean ± SD | Range |
| (free glutathione) | ||||||
| Males | 80 | 0.998 ± 0.340 | 0.280 – 2.23 | 87 | 1.09 ± 0.484 | 0.202 – 2.32 |
| Females | 77 | 0.858 ± 0.311b | 0.212 – 2.50 | 84 | 1.00 ± 0.358a | 0.366 – 2.50 |
| All | 157 | 0.929 ± 0.333 | 0.212 – 2.50 | 171 | 1.05 ± 0.428a | 0.202 – 2.50 |
| (protein-bound glutathione) | ||||||
| Males | 44 | 0.116 ± 0.04 | 0.061 – 0.176 | 26 | 0.162 ± 0.04 | 0.107 – 0.234 |
| Females | 26 | 0.113 ± 0.03 | 0.062 – 0.166 | 22 | 0.144 ± 0.07 | 0.064 – 0.234 |
| All | 70 | 0.114 ± 0.04 | 0.061 – 0.176 | 48 | 0.154 ± 0.06c | 0.064 – 0.234 |
significantly different from blacks, P<0.005
significantly different from males, P<0.01
Significantly different from blacks, P<0.05
Associations between plasma selenium and blood GSH and GCL
To examine if plasma selenium concentrations were associated with blood GSH concentration and/or GCL activity, mean free and bound GSH values and GCL activity were compared between selenium concentration quartile groups (Table 6). A trend of increasing GSH was observed from the lowest to the highest selenium quartile (P<0.01) with mean GSH concentrations in the highest quartile being 21% higher than those in the lowest quartile (P<0.05). A similar trend was observed for GCL activity, which increased from lowest to highest selenium quartile by 38% (P<0.01). These relationships between GSH and GCL and selenium levels were observed in both smokers and non-smokers (data not shown). For protein-bound GSH, the highest concentrations were observed in the lowest selenium quartile which were 45%, 54% and 37% higher than those observed for quartiles 2, 3 and 4, respectively (P<0.01). However, in contrast to GSH and GCL, no consistent dose response relationship was observed for protein-bound GSH.
Table 6.
Mean blood free and bound glutathione concentrations and GCL activity, by quartiles of blood selenium concentrations, Mt. Vernon Study
| Quartile | N | Selenium (ng/ml)a | GSH (μmol/ml)a | Bound GSH (μmol/ml)a | GCL Activity (nmol/min/g)a |
|---|---|---|---|---|---|
| Q1 | 85 | 106 ± 11.1 | 0.892 ± 0.348 | 0.179 ± 0·047 | 0.755 ± 0.228 |
| Q2 | 84 | 123 ± 3.03 | 0.973 ± 0.322 | 0.123 ± 0.040c | 0.804 ± 0.393 |
| Q3 | 83 | 133 ± 3.41 | 1.01 ± 0.400 | 0.116 ± 0.040c | 0.869 ± 0.442 |
| Q4 | 84 | 153 ± 14.6 | 1.08 ± 0.457b | 0.131 ± 0.064c | 1.04 ± 0.617b |
Values are mean ± SD
Significantly different from Q1, P<0·05.
Significantly different from Q1, P<0·01.
In all subjects, free GSH was significantly correlated with plasma selenium (r=0.19, P<0.05). However, this association was limited to whites only (r=0.20, P<0.05) as there was no significant correlation between GSH and selenium in black subjects (r=0.08). GCL activity measured in a random subset of subjects (n=76) was also significantly correlated with plasma selenium concentrations (r=0.25, P<0.05). This association was stronger in blacks (r=0.37, n=37, P<0.02) than in whites (r=0.19, n=39, P=0.2). Protein-bound GSH was inversely correlated with selenium in all subjects (r=–0.25, P<0.05). As with GSH, this association was observed in whites only (r=–0.36, P<0.05) as there was no significant correlation between GSSP and selenium in blacks (r=–0.04).
Selenium Intervention Trial
Racial differences in effects of selenium supplementation on plasma selenium
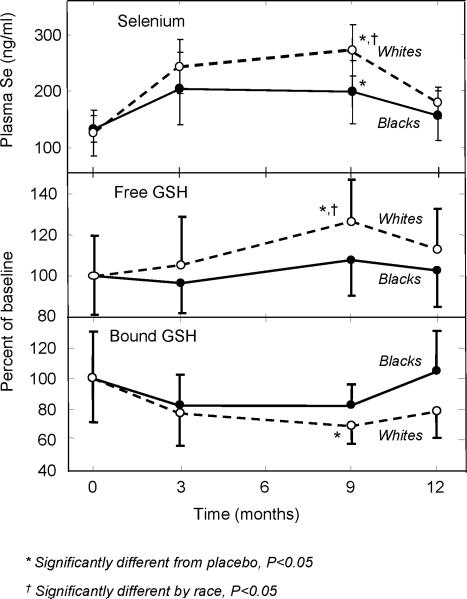
To determine if the racial differences in plasma selenium concentrations were also apparent in selenium-supplemented individuals, we re-analyzed the data from a previously conducted randomized double-blinded placebo-controlled trial of selenium-enriched yeast (29). Initially, plasma selenium was compared at baseline, 3 and 9 months of selenium supplementation and after a 3 month washout period between blacks (n=5) and whites (n=12) in the selenium arm (Figure 2, top panel). At baseline, the concentrations of selenium were similar in both blacks and whites. After both 3 and 9 months, a progressive increase was observed for both racial groups reaching a maximum at 9 months, after which concentrations returned toward baseline values. Racial differences in the response to selenium supplementation was apparent as the mean selenium concentration reached at 9 months in whites was 35% higher than that observed in blacks (P<0.05). The overall increase in selenium from baseline was 116% for whites compared to 50% for blacks. No differences in selenium was observed in either blacks or whites in the placebo group (data not shown).
Figure 2.
Racial differences in plasma selenium and blood free and protein-bound glutathione concentrations during selenium yeast supplementation in healthy adult men. Subjects were randomized into placebo (black n=6; white n=13) or selenium yeast, 247 □g/day, (black n=5; white n-12) groups for 9 months. Blood samples were obtained at baseline and 3, 9 and 12 months and analyzed for selenium and glutathione as described in text. Values are mean ± SD.
Racial differences in effects of selenium supplementation on free and bound GSH in healthy men
Previously we observed in healthy men that supplementation with selenium enriched yeast was associated with an increase in blood GSH and a decrease in blood protein-bound GSH (29). To examine for racial differences in these responses, we re-analyzed the GSH data generated from our previous trial. For GSH, baseline concentrations were similar between blacks and whites and thereafter, a progressive increase in GSH was observed after 3 and 9 months for whites only, which returned toward baseline during the 3 month washout period (Figure 2, middle panel). While there was a trend toward increased GSH among blacks after 9 months, this increase was not significant. After 9 months, blood GSH was 18% greater in whites than in blacks (P<0.05).
For protein-bound GSH, baseline concentrations were similar between blacks and whites and thereafter, a progressive decrease was observed after 3 and 9 months for whites only, which returned toward baseline during the 3 month washout period (Figure 2, bottom panel). While there was a trend toward decreased bound GSH among blacks after 3 and 9 months, these changes were not significant. After 9 months, bound GSH was 9% lower in whites than in blacks, however, this difference was not significant. For both free and protein bound GSH, no differences in selenium concentrations were observed in either blacks or whites in the placebo group (data not shown).
Discussion
These results support hypothesis that plasma selenium concentrations as well as selenium supplementation are associated with enhanced GSH and reduced glutathionylated proteins concentrations in blood. We had previously demonstrated this relationship in a clinical study of selenium supplementation, but here we have observed an association in free-living un-supplemented adults where plasma selenium concentrations and the overall daily intake of selenium were substantially lower (29). This link between selenium and GSH may represent an important mechanism responsible for the anti-cancer properties of selenium. It has long been known that selenium has antioxidant properties in biological systems based on its essential roles in selenoproteins including glutathione peroxidase and thioredoxin reductase (17). Selenium supplementation has been shown to reduce biomarkers of oxidative stress in humans (29,36,37). In the present study, decreases in oxidative stress associated with both increasing plasma selenium and selenium supplementation were indicated based on our findings of decreased protein glutathionylation, a redox sensitive post-translational regulatory mechanism for many diverse cellular and metabolic processes including cell proliferation and apoptosis (27,30,38). These results suggest that increased GSH may also be playing a role in the protection against oxidative stress by selenium. In addition to protecting against oxidation, GSH also plays numerous roles in defense against cancer development (39).
The mechanism by which selenium enhances GSH is likely to involve the upregulation of its rate limiting biosynthetic enzyme GCL. In previous studies, we observed that organoselenium compounds were highly effective at enhancing lung GSH in a preclinical mouse model (20) and others have shown that other selenium containing compounds, including sodium selenite and ebselen, were effective at increasing GSH and activity of GCL in liver or mammary tissues of rats (21-24). One potential pathway for GCL upregulation involves the activation of the nuclear factor-erythroid 2-related factor 2 (nrf2)/antioxidant response element (ARE) signaling pathway. Activation of this pathway has been implicated in the induction of GCL and GSH by ebselen (40). Recently we observed that the induction of GSH and GCL by the selenium-containing chemopreventive agent 1,4-phenylenebis(methylene)selenocyanate (p-XSC) also occurred through the activation of the nrf2/ARE pathway (20) (unpublished results).
The current results also indicate that race is a determinant of plasma selenium concentration. Plasma selenium as significantly lower in blacks vs. whites in both men and women. The reanalysis of our previous selenium clinical trial data (29) indicated that the increase in plasma selenium by selenium yeast supplementation was much lower in black men than in white men. These results are consistent with recent analyses of US population data from the National Health and Nutrition Examination Survey (NHANES III) (41), where selenium concentrations were~6% lower in blacks than in whites after adjustment for known predictors of serum selenium (P<0.0001). It is not likely that the race differences are a result of differences in smoking or occupational exposures as black smokers tended to smoke fewer cigarettes per day, similar to previous reports and national trends (31,42) and no differences were noted for occupation or related exposures between the races. Given the proposed protective roles of selenium in cancer and the inverse relationship observed between selenium and cancer risk in both black and white men (6,7,43), our findings suggest that lower selenium in blacks may, in part, contribute to their higher rates of cancer, particularly prostate cancer (44).
Our findings suggest that diet may not be responsible for differences in plasma selenium between blacks and whites. No differences in selenium intake were observed between the racial groups based upon food frequency questionnaire data, consistent with previous studies where no differences in selenium intake or supplement usage between different racial groups were observed (41,45,46). It is of interest to note that selenium intake values were significantly lower for females than for males, while no differences were observed for plasma selenium by gender. While the nature of this gender discrepancy is not known, an identical trend has also been observed in NHANES data (47). It should be noted that dietary selenium intake data derived from food frequency questionnaire are often fraught with inaccuracies due to the large variation in the selenium content of foods based upon geographic differences in selenium concentrations in soil (48). However, in our study, we would expect that variation in the selenium content of foods to be rather low as all subjects were recruited from the same relatively small geographic location in southeastern Westchester County, (Mt. Vernon) NY. Plasma selenium is not solely dependant on dietary selenium intake but can also be impacted by other factors such as absorption, metabolism and distribution in the body. Thus, variation in plasma selenium may not necessarily be driven by differences in dietary intake.
The finding that selenium supplementation was less effective at increasing plasma selenium in black men than in white men are consistent with our population based data of lower plasma selenium in blacks than in whites and may reflect potential racial differences in selenium absorption, metabolism or distribution. To our knowledge, there are no previous data comparing the chemopreventive efficacy of selenium supplementation in blacks and whites. It should be noted that the trial from which these data were derived was small, consisting of only 5 blacks, and was not specifically designed to test for racial differences in selenium efficacy. Thus, further investigations into this possibility are warranted. However, if confirmed, these findings might indicate that higher doses of selenium may be required in blacks to attain the same blood concentrations and, hence, level of protection against cancer than whites.
In summary, using data from a community-based biomarker study of blacks and whites as well as a small-scale selenium intervention trial, we observed decreased concentrations of plasma selenium in blacks compared to whites that appears to be due to reduced bioavailability of supplemental dietary selenium. In addition, we observed associations between both blood selenium and selenium supplementation with blood GSH and reduced levels of oxidative stress which were significantly stronger in whites than in blacks. Together these results suggest that decreases in selenium status and its subsequent effects on GSH and oxidative stress may be playing an important role in the mechanism by which blacks are at greater risk for certain cancers, including prostate cancer. These findings may translate into race-specific prevention strategies involving selenium-containing compounds, many of which are currently under development for use as chemopreventive agents.
Acknowledgements
Supported in part by National Cancer Institute grants PO1 CA68384, R01 CA127729, P01 CA70972 and K07-CA104231.
References
- 1.El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutat Res. 2001;475:123–139. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 2.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res. 2005;591:224–236. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Shamberger RJ, Frost DV. Possible protective effect of selenium against human cancer. Canadian Medical Association journal. 1969;100:682. [PMC free article] [PubMed] [Google Scholar]
- 4.Shamberger RJ, Tytko SA, Willis CE. Antioxidants and cancer. Part VI. Selenium and age-adjusted human cancer mortality. Archives of environmental health. 1976;31:231–235. doi: 10.1080/00039896.1976.10667225. [DOI] [PubMed] [Google Scholar]
- 5.Schrauzer GN, White DA, Schneider CJ. Cancer mortality correlation studies--III: statistical associations with dietary selenium intakes. Bioinorganic chemistry. 1977;7:23–31. doi: 10.1016/s0006-3061(00)80126-x. [DOI] [PubMed] [Google Scholar]
- 6.Platz EA, Helzlsouer KJ. Selenium, zinc, and prostate cancer. Epidemiologic reviews. 2001;23:93–101. doi: 10.1093/oxfordjournals.epirev.a000801. [DOI] [PubMed] [Google Scholar]
- 7.Vogt TM, Ziegler RG, Graubard BI, Swanson CA, Greenberg RS, et al. Serum selenium and risk of prostate cancer in U.S. blacks and whites. International journal of cancer. 2003;103:664–670. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- 8.Clark LC, Combs GF, Jr., Turnbull BW, Slate EH, Chalker DK, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 9.Clark LC, Dalkin B, Krongrad A, Combs GF, Jr., Turnbull BW, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. British journal of urology. 1998;81:730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 10.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr., Slate EH, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 11.Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, et al. SELECT: the Selenium and Vitamin E Cancer Prevention Trial: rationale and design. Prostate cancer and prostatic diseases. 2000;3:145–151. doi: 10.1038/sj.pcan.4500412. [DOI] [PubMed] [Google Scholar]
- 12.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 13.Costello AJ. A randomized, controlled chemoprevention trial of selenium in familial prostate cancer: Rationale, recruitment, and design issues. Urology. 2001;57:182–184. doi: 10.1016/s0090-4295(00)00969-9. [DOI] [PubMed] [Google Scholar]
- 14.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Bayoumy K. The negative results of the SELECT study do not necessarily discredit the selenium-cancer prevention hypothesis. Nutrition and cancer. 2009;61:285–286. doi: 10.1080/01635580902892829. [DOI] [PubMed] [Google Scholar]
- 16.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annual review of pharmacology and toxicology. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 17.Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Molecular aspects of medicine. 2005;26:256–267. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B. Oxidative stress and cancer: have we moved forward? The Biochemical journal. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 19.Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- 20.Richie JP, Jr., Kleinman W, Desai DH, Das A, Amin SG, et al. The organoselenium compound 1,4-phenylenebis(methylene)selenocyanate inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorgenesis and enhances glutathione-related antioxidant levels in A/J mouse lung. Chemico-biological interactions. 2006;161:93–103. doi: 10.1016/j.cbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Thompson HJ, Clement IP. Temporal changes in tissue glutathione in response to chemical form, dose, and duration of selenium treatment. Relevance to cancer chemoprevention by selenium. Biological trace element research. 1991;30:163–173. doi: 10.1007/BF02990351. [DOI] [PubMed] [Google Scholar]
- 22.Chidambaram N, Baradarajan A. Influence of selenium on glutathione and some associated enzymes in rats with mammary tumor induced by 7,12-dimethylbenz(a)anthracene. Molecular and cellular biochemistry. 1996;156:101–107. doi: 10.1007/BF00426331. [DOI] [PubMed] [Google Scholar]
- 23.Liu JZ, Zhang BZ, Milner JA. Dietary selenite modifies glutathione metabolism and 7,12-dimethylbenz(a)anthracene conjugation in rats. The Journal of nutrition. 1994;124:172–180. doi: 10.1093/jn/124.2.172. [DOI] [PubMed] [Google Scholar]
- 24.Chung AS, Maines MD. Effect of selenium on glutathione metabolism. Induction of gamma-glutamylcysteine synthetase and glutathione reductase in the rat liver. Biochemical pharmacology. 1981;30:3217–3223. doi: 10.1016/0006-2952(81)90521-9. [DOI] [PubMed] [Google Scholar]
- 25.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 26.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I. S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med. 2004;8:201–212. doi: 10.1111/j.1582-4934.2004.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z, Komninou D, Kleinman W, Pinto JT, Gilhooly EM, et al. Enhanced levels of glutathione and protein glutathiolation in rat tongue epithelium during 4-NQO-induced carcinogenesis. International journal of cancer. 2007;120:1396–1401. doi: 10.1002/ijc.22525. [DOI] [PubMed] [Google Scholar]
- 29.El-Bayoumy K, Richie JP, Jr., Boyiri T, Komninou D, Prokopczyk B, et al. Influence of selenium-enriched yeast supplementation on biomarkers of oxidative damage and hormone status in healthy adult males: a clinical pilot study. Cancer Epidemiol Biomarkers Prev. 2002;11:1459–1465. [PubMed] [Google Scholar]
- 30.Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, et al. Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic Biol Med. 2004;36:464–470. doi: 10.1016/j.freeradbiomed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Muscat JE, Djordjevic MV, Colosimo S, Stellman SD, Richie JP., Jr. Racial differences in exposure and glucuronidation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cancer. 2005;103:1420–1426. doi: 10.1002/cncr.20953. [DOI] [PubMed] [Google Scholar]
- 32.Baquet CR, Horm JW, Gibbs T, Greenwald P. Socioeconomic factors and cancer incidence among blacks and whites. Journal of the National Cancer Institute. 1991;83:551–557. doi: 10.1093/jnci/83.8.551. [DOI] [PubMed] [Google Scholar]
- 33.Richie JP, Jr., Carmella SG, Muscat JE, Scott DG, Akerkar SA, et al. Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol Biomarkers Prev. 1997;6:783–790. [PubMed] [Google Scholar]
- 34.Nichenametla SN, Ellison I, Calcagnotto A, Lazarus P, Muscat JE, et al. Functional significance of the GAG trinucleotide-repeat polymorphism in the gene for the catalytic subunit of gamma-glutamylcysteine ligase. Free radical biology & medicine. 2008;45:645–650. doi: 10.1016/j.freeradbiomed.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richie JP, Jr., Skowronski L, Abraham P, Leutzinger Y. Blood glutathione concentrations in a large-scale human study. Clin Chem. 1996;42:64–70. [PubMed] [Google Scholar]
- 36.Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Assadi Y, Ghaemmagami J, et al. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology (Carlton, Vic. 2008;13:294–298. doi: 10.1111/j.1440-1843.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- 37.Ardalan MR, Tubbs RS, Shoja MM. Vitamin E and selenium co-supplementation attenuates oxidative stress in haemodialysis patients receiving intra-dialysis iron infusion. Nephrol Dial Transplant. 2007;22:973–975. doi: 10.1093/ndt/gfl650. [DOI] [PubMed] [Google Scholar]
- 38.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 39.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell biochemistry and function. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai T, Kanayama M, Shibata T, Itoh K, Kobayashi A, et al. Ebselen, a seleno-organic antioxidant, as an electrophile. Chemical research in toxicology. 2006;19:1196–1204. doi: 10.1021/tx0601105. [DOI] [PubMed] [Google Scholar]
- 41.Vogt TM, Ziegler RG, Patterson BH, Graubard BI. Racial differences in serum selenium concentration: analysis of US population data from the Third National Health and Nutrition Examination Survey. American journal of epidemiology. 2007;166:280–288. doi: 10.1093/aje/kwm075. [DOI] [PubMed] [Google Scholar]
- 42.Novotny TE, Warner KE, Kendrick JS, Remington PL. Smoking by blacks and whites: socioeconomic and demographic differences. American journal of public health. 1988;78:1187–1189. doi: 10.2105/ajph.78.9.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Archives of internal medicine. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 44.IARC . Cancer incidence in five continents. IARC; Lyon: 1997. [Google Scholar]
- 45.Lewis SA, Hardison NW, Veillon C. Comparison of isotope dilution mass spectrometry and graphite furnace atomic absorption spectrometry with Zeeman background correction for determination of plasma selenium. Analytical chemistry. 1986;58:1272–1273. doi: 10.1021/ac00297a070. [DOI] [PubMed] [Google Scholar]
- 46.Niskar AS, Paschal DC, Kieszak SM, Flegal KM, Bowman B, et al. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988-1994. Biological trace element research. 2003;91:1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- 47.Kant AK, Graubard BI. Ethnicity is an independent correlate of biomarkers of micronutrient intake and status in American adults. The Journal of nutrition. 2007;137:2456–2463. doi: 10.1093/jn/137.11.2456. [DOI] [PubMed] [Google Scholar]
- 48.Holben DH, Smith AM. The diverse role of selenium within selenoproteins: a review. Journal of the American Dietetic Association. 1999;99:836–843. doi: 10.1016/S0002-8223(99)00198-4. [DOI] [PubMed] [Google Scholar]