Abstract
Polyphenolic compounds are known to possess many beneficial health effects, including the antioxidative activities of scavenging reactive oxygen species and chelating metals, such as iron and zinc. Tea and red wine are thought to be important sources of these compounds. However, some polyphenolic compounds can also reduce the absorption of iron, and possibly other trace metals, when included in a diet. There is very little information on the effect of dietary polyphenolic compounds on the status of trace elements other than iron. We examined the effects of epigallocatechin-3-gallate (EGCG), green tea extract (GT) and grape seed extract (GSE) on the absorption of 65Zn and compared them with their effects on 55Fe absorption in human intestinal Caco-2 cells grown on microporous membrane inserts. The levels of EGCG, GT and GSE used in this study were within the physiological ranges and did not affect the integrity of the Caco-2 cell monolayers. GSE significantly (P < 0.05) reduced zinc transport across the cell monolayer, and the decreased zinc transport was associated with a reduction in apical zinc uptake. However, EGCG and GT did not alter zinc absorption. In contrast, the polyphenolic compounds in EGCG, GT and GSE almost completely blocked transepithelial iron transport across the cell monolayer. The effect of GSE on zinc absorption was very different from that on iron absorption. While GSE decreased zinc absorption by reducing apical zinc uptake, the polyphenolic compounds inhibited iron absorption by enhancing apical iron uptake. GSE inhibited zinc absorption similarly to that observed for phytate. Phytate significantly (P < 0.05) decreased transepithelial zinc transport by reducing apical zinc uptake. The inhibition of zinc absorption may be due to the presence of procyanidins in GSE, which bind zinc with high affinity and block the transport of zinc across the apical membrane of enterocytes. Further research on the absorption of zinc as zinc-polyphenol complexes and free zinc should provide further insight into the process of dietary zinc absorption in the presence of GSE and other bioactive dietary polyphenols. The present study suggests that some individuals should consider their zinc status if they regularly consume procyanidin-containing foods in their diet. However, further studies, especially in vivo studies, are needed to confirm these results.
Keywords: zinc, iron, epigallocatechin-3-gallate (EGCG), grape seed extract, green tea extract, Caco-2 cells
INTRODUCTION
Bioactive dietary polyphenols have attracted increased attention due to their reported health benefits for a variety of disorders, including cardiovascular diseases, various cancers, diabetes, obesity, and neurological diseases (1–4). Bioactive polyphenols are naturally occurring chemicals found in foods, including fruits, some types of grains, wine, and tea. Tea, which is made from the leaves of the plant Camellia sinensis, is a popular beverage worldwide. The beneficial effects of green tea have been attributed to its polyphenolic compounds, particularly the catechins. (−)-Epigallocatechin-3-gallate (EGCG) is the most abundant green tea catechin and is regarded as the most bioactive disease-preventing polyphenol compound in green tea. Grape seed extract (GSE) contains various polyphenols, including gallic acid, catechin, EGCG, epigallocatechin (EGC), epicatechin-3-gallate (ECG), epicatechin (EC) and proanthocyanidins. Polyphenol-rich extracts derived from both green tea and grape seed have the potential to reduce the oxidation of foods and beverages and are widely used as supplements or food additives. Their antioxidant activities have been shown to be dependent on the ability of their constituent polyphenolic compounds to scavenge free radicals and chelate metals, not only the redox-active transition metals, such as iron and copper, but also redox-inactive metals, such as zinc (5–7).
Although it has been suggested that polyphenols chelate a number of trace metals, including iron, copper and zinc, very few studies have documented the effects of polyphenols on zinc absorption. While bioactive dietary polyphenols are known to have no effect on zinc absorption in animals and humans (8–11), some studies indicated that dietary polyphenols decrease zinc absorption in animals (12–13). In addition, a recent study suggested that some polyphenol-rich beverages, such as green tea, red wine and red grape juice, could enhance zinc absorption by increasing apical zinc uptake (14). Thus, while it is not clear whether dietary polyphenols affect zinc absorption, these previous studies have suggested that some dietary polyphenols may modulate intestinal zinc absorption and metabolism. In contrast, the inhibitory effect of the dietary bioactive polyphenolic compounds on iron absorption has been well described. The intake of the dietary polyphenolic compounds inhibited iron absorption (15–19).
Because of the increasing interest in EGCG, green tea extract (GT) and GSE as dietary supplements and food additives and a growing understanding of the potential health benefits of polyphenolic compounds, we determined the potential effects of the bioactive dietary polyphenols in EGCG, GT and GSE on zinc absorption and utilized the human intestinal Caco-2 cell line as a model system to study this process. On confluence, these cells spontaneously differentiate to exhibit many of the morphological and functional features of normal mature small intestinal enterocytes and have been widely used as a model of normal human intestinal epithelium (20–21). In particular, fully differentiated Caco-2 cells are a well-established in vitro model of human intestinal zinc absorption and metabolism (14, 22–24)
Dietary zinc is thought to be transported across the apical membrane via ZIP4, a zinc transporter, into enterocytes. In the intracellular step of zinc absorption, the newly transported zinc is intracellularly distributed to the basolateral surface or to zinc binding proteins (e.g., metallothionein (MT)). Finally, the newly acquired zinc is transferred across the basolateral membrane of the mucosa via ZnT1, a zinc exporter, and released into the circulation (25–26).
Zinc is an essential trace element for humans. Although zinc is quite abundant in the environment, zinc deficiency is still a common nutritional deficiency, particularly in developing countries, which is due not only to low intake of this essential trace metal but also to poor bioavailability. Zinc deficiency is associated with growth retardation, hypogonadism, immune dysfunction and cognitive impairment and affects nearly 2 billion subjects in the developing world (27). Because the antioxidant properties of the polyphenols include chelation of metals, such as zinc, it is prudent to examine the effects of bioactive polyphenolic compounds on intestinal zinc absorption. The purpose of this study was to assess the effects of different dietary bioactive polyphenol sources with different polyphenol profiles on the absorption of zinc and compare the effects of polyphenols on zinc absorption with their effects on iron absorption.
MATERIALS AND METHODS
Reagents
Tissue culture media, Hanks’ balanced salt solution (HBSS), glutamine, non-essential amino acids and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum was obtained from Hyclone (Logan, UT). EGCG (TEAVIGO, >95% pure), GT and GSE were obtained from DSM Nutritional Products (Parsippany, NJ), Pharmanex Inc. (Provo, UT) and Partoeno (Bordeaux, France), respectively. The chemical characteristics and degree of polymerization for the GSE used in these studies have been documented (28). GT is a mixture of catechins, including EGCG (43.0% by weight), epicatechin-3-gallate (13.7%), epicatechin (6.0%), gallocatechin gallate (5.6%), epigallocatechin (4.0%), gallocatechin (2.3%), catechin (2.0%) and catechin gallate (1.4%) (29). 65Zn (as ZnCl2) was purchased from Oak Ridge National Laboratory (Oak Ridge, TN), and 55Fe (as FeCl3) was purchased from PerkinElmer (Boston, MA). Unless otherwise noted, all other reagents were purchased from Sigma Chemical (St. Louis, MO), Fisher Scientific (Springfield, NJ), or VWR (West Chester, PA).
Cell culture
The human Caco-2 cell line was purchased from the American Type Culture Collection (Rockville, MD). Stock cultures were maintained in complete medium at 37°C and in a humidified atmosphere of 95% air and 5% CO2 and employed for experiments within 20 serial passages. The complete culture medium contained Dulbecco’s Modified Essential Medium (DMEM) supplemented with 25 mmol/L glucose, 2 mmol/L glutamine, 100 μmol/L non-essential amino acids, 100 U/L penicillin G, 100 mg/L streptomycin, and 10% FBS. Stock cultures were seeded at 10,000 cells/cm2, and at ~85% confluence, they were split by treatment with 0.5 g/L trypsin-0.5 mmol/L EDTA in HBSS. For experiments, 50,000 cells/cm2 in a volume of 1.5 mL of complete DMEM were seeded on 3 μm microporous membrane inserts (4.9 cm2; BD Biosciences, Bedford, MA) coated with collagen (5 μg/cm2; BD Biosciences, Bedford, MA). The basolateral (bottom) chamber contained 2.5 mL of complete DMEM. The culture medium was changed every 2 days, and cells were used after d 17 post-confluence for experiments. The Caco-2 cell monolayer formed tight junctions at d 17 post-confluence as defined by the transepithelial electrical resistance (TEER) values of >250 Ω/cm2. Cells are fully differentiated at d 17 post-confluence in normal cell culture conditions (30–31).
65Zn transport and uptake
The transepithelial zinc transfer from the apical compartment to the basolateral compartment and the apical zinc uptake were determined (22). After washing the cell monolayer three times with Ca2+- and Mg2+-free HBSS at 37°C, the cells were incubated at 37°C with 1.5 mL of 10 μmol/L 65ZnCl2 in uptake buffer containing either 100 μmol/L phytate or 46 mg/L of the indicated bioactive compounds in the apical compartment and 2.5 mL of DMEM in the basolateral compartment. The effect of polyphenols and phytate on the solubility of Zn in the uptake buffer was determined by measuring the level of radioactivity in vortexed aliquots before and after centrifugation at 10,600 × g for 10 min at 20°C. All test solutions were freshly prepared before each experiment. Stock solutions of 10 mmol/L ZnCl2 were prepared fresh in 1 mmol/L HCl and diluted 10-fold in sterile, deionized water. The diluted stock solutions were mixed with 65ZnCl2 (specific radioactivity, 185 GBq/mmol; Oak Ridge National Laboratory) to provide 18.5 kBq per well for uptake and transport studies. The uptake buffer contained 130 mmol/L NaCl, 10 mmol/L KCl, 1 mmol/L MgSO4, 5 mmol/L glucose, and 50 mmol/L HEPES, pH 7.0. Two hundred microliters was removed from the basolateral chamber every hour and replaced with an equivalent volume of pre-warmed DMEM; the time course data were corrected to account for this sample replacement. The rate of radiolabeled zinc transfer across the cell monolayer increased during the 3-h incubation, and transport rates [pmol/(h · mg cellular protein)] were calculated by linear regression analysis (r2 > 0.995). The integrity of the tight junctions between cells was monitored by measuring TEER and phenol red transport; any leaking cell monolayers were discarded. To measure the cellular levels of 65Zn, cell monolayers were washed three times with ice-cold wash buffer containing 150 mmol/L NaCl, 10 mmol/L HEPES, pH 7.0, and 1 mmol/L EDTA to remove any nonspecifically bound radioisotope. Cells were homogenized in PBS containing 1 mmol/L EDTA and 0.2% Triton X-100, and 65Zn was quantified by gamma counting. Cellular protein levels were assessed using the Bio-Rad protein assay kit (Bio-Rad Laboratory Inc., Hercules, CA). A green tea bag contains up to 20–200 mg EGCG. The contents of the extracted polyphenols in tea depend on the origin of the tea, the manufacturer, and brewing conditions, such as water temperature, brewing time and tea-to-water ratio (32). Thus, a cup of green tea can provide from ~ 1 mg EGCG up to 200 mg EGCG. Most GSE supplements contain 100–500 mg GSE per capsule, while red wine contains about 132–366 mg/L catechin equivalents (33). It was reported that the contents of phytic acid in many crops exceed 1.0 % dry weight (34). Because the total gastric volume during meals can be 1–2 L and depends on the amount of food consumed (35), the concentrations of polyphenols (46 mg/L) and phytate (66 mg/L, 100 μmol/L) used in the present study are within practical ranges.
55Fe transport and uptake
Similarly, the basolateral iron transfer from cells to the basolateral compartment and the apical uptake of iron were determined (30, 36–37). After washing the cell monolayer three times with Ca2+- and Mg2+-free HBSS at 37°C, cells were incubated at 37°C with 1.5 mL of 10 μmol/L 55Fe-nitrilotriacetic acid (NTA)2 in uptake buffer containing the 46 mg/L of the indicated bioactive compounds in the apical compartment and 2.5 mL of DMEM in the basolateral compartment. The effect of polyphenols on the solubility of Fe in the uptake buffer was also determined as described above for Zn. All test solutions were prepared fresh before each experiment. Stock solutions of 10 mmol/L FeCl3·6H2O and 20 mmol/L NTA were prepared in 1 mmol/L HCl and diluted 10-fold in sterile, deionized water. The diluted stock solutions were mixed with 55FeCl3 (specific radioactivity, 192 GBq/mmol; PerkinElmer) to provide 185 kBq per well for the uptake and transport studies. To measure the cellular levels of 55Fe, cell monolayers were washed three times with ice-cold wash buffer containing 150 mmol/L NaCl, 10 mmol/L HEPES, pH 7.0, and 1 mmol/L EDTA to remove any nonspecifically bound radioisotope. This washing step was effective in removing all surface bound iron because additional wash steps using solution containing 100 μmol/L bathophenanthroline disulfonate (BPS; an Fe2+ chelator) or desferrioxamine (DFO; an Fe3+ chelator) did not further change the cellular 55Fe content after washing. Cells were homogenized in PBS containing 1 mmol/L EDTA and 0.2% Triton X-100, and 55Fe was quantified by liquid scintillation counting in glass vials. Cellular protein levels were assessed using the Bio-Rad protein assay kit (Bio-Rad Laboratory Inc., Hercules, CA).
Western blot analysis
Protein samples were extracted with cell lysis buffer (0.3% Triton X-100, 150 mmol/L NaCl, and 10 mmol/L Tris-HCl, pH 7.4) and a protease inhibitor cocktail (Sigma, St. Louis, MO). Total protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratory Inc., Hercules, CA). Protein samples (80 μg) were solubilized in Laemmli buffer, boiled for 7 min, and separated by 4-20% SDS-PAGE. Similar loading and transfer of proteins were verified by staining the blots with Ponceau S. Proteins were transferred by electroblotting to nitrocellulose membranes, which were then blocked with 5% non-fat powdered milk in 10 mmol/L Tris-HCl, pH 7.4, and 150 mmol/L NaCl (TBS) at room temperature for 1 h. The membranes were then incubated for 2 h at room temperature with an anti-MT antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:500 dilution) in TBS containing 0.05% Tween 20 (TBST). The membranes were washed several times with TBST and then incubated for 1 h at room temperature with peroxidase-linked goat anti-rabbit IgG (PerkinElmer, Boston, MA; 1:3,000 dilution). The antigens were visualized by enhanced chemiluminescence (PerkinElmer Life Sciences, Boston, MA), detected using the ChemiDoc XRS system (Bio-Rad, Hercules, CA), and quantified using the Quantity-One application software (Bio-Rad, Hercules, CA). The membranes were stripped and reprobed with an anti-calnexin antibody to confirm equal loading and transfer.
Statistical analysis
All variables were tested in at least triplicate wells for each experiment, and experiments were repeated more than three times. Data were analyzed using 1-way or 2-way (treatment x time) ANOVA with the Bonferroni’s multiple comparison tests post hoc for multiple comparisons using the Prism 5.0 software (GraphPad). Data were log-transformed as necessary to attain homogeneity of variance, and data are reported as the nontransformed means. The REG (regression) procedure was used to perform the simple linear regression analysis for Table 1. Differences were considered significant at P < 0.05.
Table 1.
Effects of dietary polyphenols and phytate on the rate of apical 65Zn transfer across the cell monolayer in fully differentiated Caco-2 cells 1
| Treatments | Rate of 65Zn transfer (AP to BL chamber) [pmol/(h · mg cellular protein)] |
|---|---|
| Control | 534.4 ± 9.5a |
| + Phytate (66 mg/L, 100 μmol/L) | 129.4 ± 1.5d |
| + EGCG (46 mg/L, 100 μmol/L) | 500.9 ± 29.9ab |
| + GT (46 mg/L) | 472.8 ± 18.6b |
| + GSE (46 mg/L) | 337.2 ± 23.3c |
The rate of transepithelial 65Zn transport across the differentiated Caco-2 cell monolayers was calculated during 3 h of incubation by linear regression analysis (control: r2 > 0.999, +phytate: r2 > 0.999, +EGCG: r2 > 0.996, +GT: r2 > 0.998, +GSE: r2 > 0.995). Data are the means ± SEM for six replicate wells from a representative experiment (n = 3). Means without a common letter differ at P < 0.05.
RESULTS
GSE inhibits transepithelial zinc transport
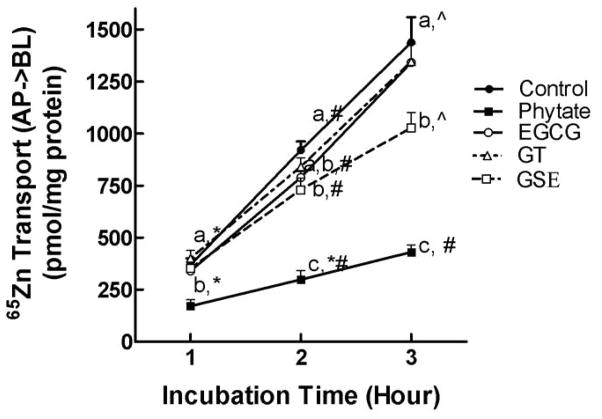
The solubility of zinc was not altered when 46 mg/L of polyphenols was also added to the uptake buffer. The amounts of 65Zn in the supernatants of samples containing EGCG, GSE, GT and phytate were same as that in the control solution (≥ 97%). The quantity of 65Zn transferred from the apical to the basolateral compartment of the Caco-2 cell monolayer increased between 1 and 3 h of incubation. Addition of GSE to the uptake buffer significantly (P < 0.05) decreased the rate of 65Zn transfer across the cell monolayer (Table 1). GSE decreased the transepithelial 65Zn transfer across the cell monolayer to 79.3 ± 6.3% and 71.3 ± 5.2 % of control after 2 and 3 h of incubation, respectively (Figure 1). However, neither EGCG nor GT modulated 65Zn transport across the cell monolayers after 3 h of incubation. Interestingly, the rate of 65Zn transfer was significantly (P < 0.05) decreased by GT during a 3 h transport study (Table 1). As shown in Figure 1, because the amounts of 65Zn transported into the basolateral chamber were higher (not significant) after 1 h of incubation but lower (not significant) after 2 and 3 h of incubation in the presence of GT, the overall transport rate was lower in the presence of GT compared to the control. As predicted, phytate significantly (P < 0.05) decreased 65Zn transport across the cell monolayer after 3 h of incubation. Phytate was used as a negative control for zinc absorption. The addition of bioactive dietary polyphenols did not alter the TEER values, which confirmed the integrity of the monolayer of EGCG, GT or GSE-treated cells.
Figure 1.
The effects of phytate, EGCG, GT and GSE on 65Zn transport across fully differentiated Caco-2 cell monolayers. The reactions included 10 μmol/L 65ZnCl2 and 100 μmol/L phytate or 46 mg/L EGCG, GT or GSE. Values are the means ± SEM for four (control and phytate) or five (EGCG, GSE and GT) replicate wells from a representative experiment (n = 3). Means at an incubation time point without a common letter differ at P < 0.05. Within a treatment, means without a common symbol differ at P < 0.05. AP, apical; BL, basolateral.
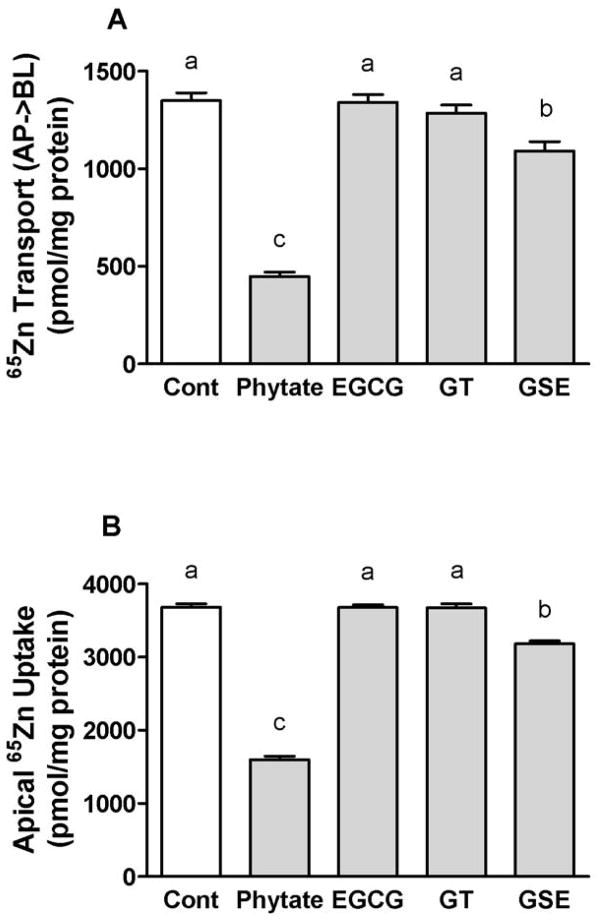
Apical zinc uptake is reduced by GSE but not by EGCG and GT
The addition of GSE significantly (P < 0.05) reduced the uptake of 65Zn across the brush-border membrane and its assimilation by Caco-2 cells to 86% of the control during a 3-h assay (Figure 2B). Basolateral 65Zn release was also significantly (P < 0.05) decreased by GSE to 80% of the control during the 3 h transport assay (Figure 2A). GSE inhibited zinc absorption in a manner similar to phytate. Phytate significantly (P < 0.05) decreased the basolateral transport of 65Zn by reducing the apical uptake of 65Zn. However, EGCG and GT did not affect the absorption of zinc. To examine whether the effect of polyphenols on zinc absorption differs from the effects on other metals, we next tested the effects of these bioactive polyphenolic compounds on iron absorption.
Figure 2.
The effects of bioactive dietary polyphenols on the basolateral 65 Zn transport (A) and the apical 65Zn uptake (B) after 3 h of incubation. The reactions included 10 μmol/L 65ZnCl2 and 100 μmol/L phytate or 46 mg/L EGCG, GT or GSE. Data are the means ± SEM for nine (control and phytate) or thirteen (EGCG, GSE and GT) replicate wells from three independent experiments. Means without a common letter differ at P < 0.05. AP; apical, BL; basolateral.
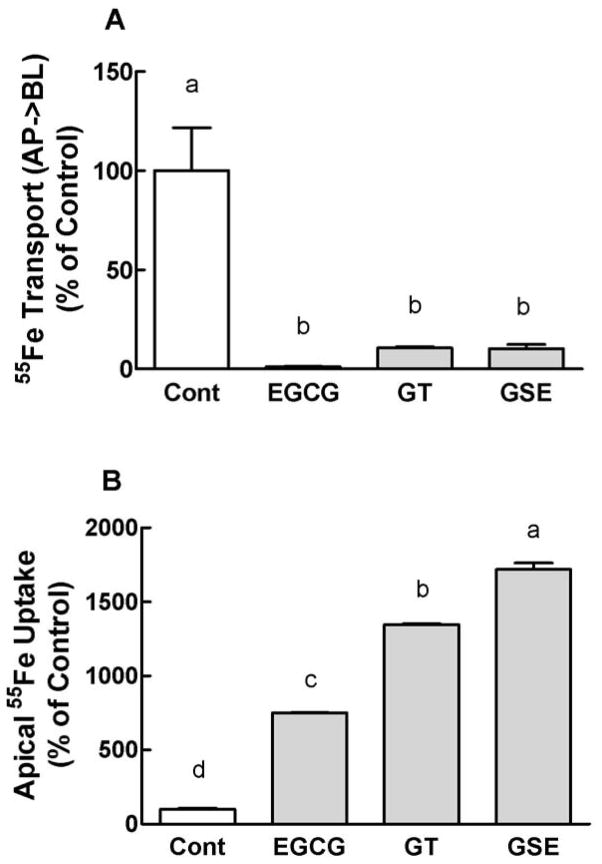
Dietary polyphenolic compounds inhibit basolateral 55Fe transport but increase the apical uptake of 55Fe
Transepithelial 55Fe transport across the cell monolayer was almost completely inhibited by EGCG, GT and GSE over 3 h of incubation (Figure 3A). Interestingly, the addition of these dietary polyphenols tremendously enhanced apical uptake of 55Fe over 3 h of incubation (Figure 3B). These dietary polyphenols significantly (P < 0.05) enhanced the apical iron uptake within 1 h of incubation (Kim and Han, unpublished data). The solubility of iron was ≥ 98% and was not changed when polyphenols were also added to the uptake buffer.
Figure 3.
The inhibitory effects of dietary bioactive polyphenols on basolateral 55 Fe transport (A) and the apical 55Fe uptake (B) after 3 h of incubation. The reactions included 10 μmol/L 55Fe(NTA)2 and 46 mg/L of the indicated polyphenolic compounds. Data are the means ± SEM for six (control, EGCG and GSE) and nine (GT) replicate wells from two independent experiments. The experiments were repeated more than six times with similar results. Means without a common letter differ at P < 0.05.
Effects of polyphenols and phytate on the expression of MT in Caco-2 cells
To explore the possible mechanisms by which GSE inhibits zinc absorption, the expression of the zinc binding protein MT was assessed. Our analysis of quantified western blots showed that the MT protein level was not changed by phytate and selected dietary polyphenols over 3 h of incubation (Figure 4).
Figure 4.
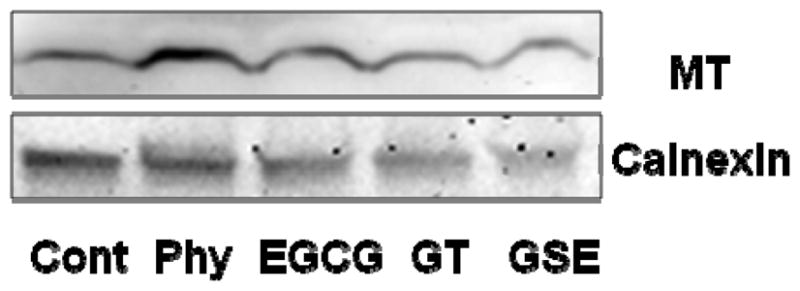
The effects of phytate, EGCG, GT and GSE on the expression of MT. A representative western blot of MT protein expression from Caco-2 cells treated with 100 μmol/L phytate (Phy) or 46 mg/L EGCG, GT or GSE for 3 h. Calnexin was used to confirm equal loading and transfer.
DISCUSSION
Polyphenolic compounds are known to possess many beneficial health effects, including the antioxidative activities of scavenging reactive oxygen species and chelating metals. Tea and red wine are thought to be important sources of these compounds. However, some polyphenolic compounds also reduce the absorption of iron, and possibly other trace metals, when included in a diet. Multiple galloyl groups are known to be responsible for the inhibition of iron absorption from foods; however, there is very little information on the effect of dietary polyphenolic compounds on the status of trace elements other than iron. It is known that polyphenolic compounds are also able to chelate zinc (7, 38). In the present study, the effects of EGCG, GT and GSE on the absorption of 65Zn were examined and compared their effects on 55Fe absorption in human intestinal Caco-2 cells grown on microporous membrane inserts. We also investigated the effects of the polyphenolic compounds on the expression of MT, which binds zinc and may determine the amounts of zinc that are transported across the basolateral membrane of enterocytes. The levels of EGCG, GT and GSE used in this study were within the physiological ranges and did not affect the integrity of the Caco-2 cell monolayers.
GSE reduced zinc transport across the cell monolayer, and the decreased zinc transport was associated with a reduction in apical zinc uptake. Similarly, phytate inhibited zinc absorption by decreasing apical zinc uptake. However, EGCG and GT did not alter zinc absorption. In contrast, the polyphenolic compounds in EGCG, GT and GSE almost completely blocked transepithelial iron transport across the cell monolayer. The effect of GSE on zinc absorption was very different from its effect on iron absorption. While GSE decreased zinc absorption by reducing apical zinc uptake, the polyphenolic compounds inhibited iron absorption by enhancing apical iron uptake. GSE may increase apical iron uptake by reducing ferric iron to ferrous iron as previously reported (36). Another possible explanation is that while GSE-iron complexes are transported into the cell, possibly via a polyphenol transport pathway(s), GSE-zinc complexes do not enter the cell.
Evidence has shown that green tea both suppresses and has no effect on zinc absorption. Greger and Lyle reported that GT at a level of 2.7% in a diet decreased the apparent zinc absorption in animals (13). Similarly, GT decreased zinc absorption in a dose-dependent manner in animals (12). GT at levels of ≥ 0.6% in a diet significantly inhibited zinc absorption (12). However, both 1 % green tea infusion and 0.2% GT feeding in a diet did not have significant effects on zinc status (8). Similarly, the results of our current study also showed that GT had no effect on the transepithelial transport and apical uptake of 65Zn in fully differentiated Caco-2 cells grown on microporous membrane inserts. Interestingly, a recent study proposed that green tea might enhance zinc absorption (14). The addition of green tea stimulated apical zinc uptake in intestinal Caco-2 cells grown in a 6-well plate and incubated for 3 h (14).
It was previously reported that long-term ingestion of red wine did not modulate zinc absorption (11), but there is no information on the effect of GSE on zinc absorption. The results of our present study showed that GSE decreased transepithelial 65Zn transport and was associated with a reduction in apical 65Zn uptake over a 3-h incubation in Caco-2 cells. Similar to our current study, a recent study reported that GSE inhibited the cellular uptake of zinc in hepatocytes (39). The GSE-mediated decrease in zinc uptake was not due to a reduction in the zinc importer because GSE increased the expression of the zinc importers (39). Interestingly, a recent study indicated that red wine could enhance zinc absorption, as the addition of red wine extract stimulated apical zinc uptake in intestinal Caco-2 cells (14). It has been suggested that polyphenols form complexes with zinc, and the variability in the structure and stoichiometry of polyphenols-zinc complexes may affect the different cell membrane permeability of polyphenols (40–41). However, the exact mechanism of the effects of dietary bioactive polyphenols on zinc uptake remains to be elucidated.
To test whether GSE decreased basolateral zinc release by decreasing intracellular labile zinc concentrations and increasing MT protein levels, we assessed MT protein expression in cells. Within the cytoplasm, zinc is bound by MT, and MT controls the concentrations of free and labile zinc (42). Our western blot data showed no changes in MT protein expression after treatment with GSE, GT and EGCG, suggesting that the GSE-mediated inhibition of zinc absorption is due to reduced apical zinc uptake. However, a polyphenol-mediated decrease in MT expression has been previously reported (39, 43). The expression of MT was reduced by GSE and EGCG in HepG2 and PC-3 cells (39, 43), and the decrease in MT expression was associated with elevated intracellular labile zinc concentrations in HepG2 cells (39).
In contrast to the effects of polyphenols on transepithelial transport and apical uptake of zinc, EGCG, GT and GSE almost completely blocked basolateral iron release but tremendously enhanced the apical iron uptake (Fig. 3A & B). These results indicate that polyphenols differentially modulate zinc and iron absorption in intestinal cells. Similarly, while EGCG increased zinc uptake, Cd uptake was decreased by EGCG in PC-3 cells (43). As discussed above, the effects of dietary polyphenolic compounds on the absorption and cellular uptake of metals are dependent on the metals applied or the cells used. Dietary polyphenolic compounds form complexes with metals (7, 38), and the structure and stoichiometry of polyphenol-metal complexes might determine the different membrane permeabilities of polyphenols and modulate the intestinal absorption and cellular uptake of metals.
Some dietary polyphenols, including EGCG, GT and GSE, have been shown to form complexes with metal ions, especially transitional metal ions, under various conditions. Our previous study showed that EGCG and GSE chelate iron (36). EGCG, GT and GSE have also been known to bind zinc in solution with higher affinity than the zinc-specific chelator Zinquin (39). It has been suggested that the content and type of polyphenols present in foods determine their effects on the absorption of metals, such as iron. However, the precise mechanism by which bioactive dietary polyphenolic compounds inhibit the absorption of metals has not been delineated. It is generally accepted that some dietary factors modulate the absorption of iron and zinc by altering their solubilities in the lumen of the small intestine (44–45). However, our results indicate that dietary polyphenols and phytate modulate the intestinal transport of iron and zinc without changing their solubilities as previously suggested by other investigators (46).
The results of the present study demonstrate that GSE, but not EGCG and GT, decreases zinc absorption at a physiological level. The GSE-mediated inhibition of zinc absorption was associated with a decrease in apical zinc uptake but not MT protein expression. The inhibition of zinc absorption may be due to the presence of procyanidins, which bind zinc with high affinity and block the transport of zinc across the apical membrane of enterocytes. In contrast, procyanidin-iron complexes stimulate iron transport across the apical membrane of enterocytes, as shown in Fig. 3B. Further research on the absorption of zinc as zinc-polyphenol and free zinc should provide further insight into the process of dietary zinc absorption in the presence of GSE and other various bioactive dietary polyphenols. The present study suggests that some individuals should consider their zinc status if they regularly consume procyanidin-containing foods. However, further studies, especially in vivo studies, are needed to confirm these results.
Acknowledgments
This work is supported by the College of Human and Health Development at the Pennsylvania State University and NIH grant AT005006 to OH.
Abbreviations used
- AP
apical
- BL
basolateral
- BPS
bathophenanthroline disulfonate
- DFO
desferrioxamine
- DMEM
Dulbecco’s Modified Essential Medium
- EC
epicatechin
- ECG
epicatechin-3-gallate
- EGC
epigallocatechin
- EGCG
(−)-epigallocatechin-3-gallate
- FBS
fetal bovine serum
- GSE
grape seed extract
- GT
green tea extract
- HBSS
Hanks’ balanced salt solution
- MT
metallothionein
- NTA
nitrilotriacetic acid
- TEER
transepithelial electrical resistance
Footnotes
Author disclosures: E-Y. Kim, T-K. Pai and O. Han, no conflicts of interest.
References
- 1.Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 3.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 4.Tijburg LB, Mattern T, Folts JD, Weisgerber UM, Katan MB. Tea flavonoids and cardiovascular disease: a review. Crit Rev Food Sci Nutr. 1997;37:771–785. doi: 10.1080/10408399709527802. [DOI] [PubMed] [Google Scholar]
- 5.Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996;1304:210–222. doi: 10.1016/s0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 6.Apak R, Guclu K, Ozyurek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 7.Hider RC, Liu ZD, Khodr HH. Metal chelation of polyphenols. Methods Enzymol. 2001;335:190–203. doi: 10.1016/s0076-6879(01)35243-6. [DOI] [PubMed] [Google Scholar]
- 8.Record IR, McInerney JK, Dreosti IE. Black tea, green tea, and tea polyphenols. Effects on trace element status in weanling rats. Biol Trace Elem Res. 1996;53:27–43. doi: 10.1007/BF02784542. [DOI] [PubMed] [Google Scholar]
- 9.Afsana K, Shiga K, Ishizuka S, Hara H. Reducing effect of ingesting tannic acid on the absorption of iron, but not of zinc, copper and manganese by rats. Biosci Biotechnol Biochem. 2004;68:584–592. doi: 10.1271/bbb.68.584. [DOI] [PubMed] [Google Scholar]
- 10.Ganji V, Kies CV. Zinc bioavailability and tea consumption. Studies in healthy humans consuming self-selected and laboratory-controlled diets. Plant Foods Hum Nutr. 1994;46:267–276. doi: 10.1007/BF01088999. [DOI] [PubMed] [Google Scholar]
- 11.Coudray C, Tressol JC, Feillet-Coudray C, Bellanger J, Pepin D, Mazur A. Long-term consumption of red wine does not modify intestinal absorption or status of zinc and copper in rats. J Nutr. 2000;130:1309–1313. doi: 10.1093/jn/130.5.1309. [DOI] [PubMed] [Google Scholar]
- 12.Zeyuan D, Bingying T, Xiaolin L, Jinming H, Yifeng C. Effect of green tea and black tea on the metabolisms of mineral elements in old rats. Biol Trace Elem Res. 1998;65:75–86. doi: 10.1007/BF02784115. [DOI] [PubMed] [Google Scholar]
- 13.Greger JL, Lyle BJ. Iron, copper and zinc metabolism of rats fed various levels and types of tea. J Nutr. 1988;118:52–60. doi: 10.1093/jn/118.1.52. [DOI] [PubMed] [Google Scholar]
- 14.Sreenivasulu K, Raghu P, Nair KM. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J Food Sci. 2010;75:H123–H128. doi: 10.1111/j.1750-3841.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- 15.Samman S, Sandstrom B, Toft MB, Bukhave K, Jensen M, Sorensen SS, et al. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001;73:607–612. doi: 10.1093/ajcn/73.3.607. [DOI] [PubMed] [Google Scholar]
- 16.Marouani N, Chahed A, Hedhili A, Hamdaoui MH. Both aluminum and polyphenols in green tea decoction (Camellia sinensis) affect iron status and hematological parameters in rats. Eur J Nutr. 2007;46:453–459. doi: 10.1007/s00394-007-0685-4. [DOI] [PubMed] [Google Scholar]
- 17.Layrisse M, Garcia-Casal MN, Solano L, Baron MA, Arguello F, Llovera D, et al. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J Nutr. 2000;130:2195–2199. doi: 10.1093/jn/130.9.2195. [DOI] [PubMed] [Google Scholar]
- 18.Gabrielli GB, De Sandre G. Excessive tea consumption can inhibit the efficacy of oral iron treatment in iron-deficiency anemia. Haematologica. 1995;80:518–520. [PubMed] [Google Scholar]
- 19.Cook JD, Reddy MB, Hurrell RF. The effect of red and white wines on nonheme-iron absorption in humans. Am J Clin Nutr. 1995;61:800–804. doi: 10.1093/ajcn/61.4.800. [DOI] [PubMed] [Google Scholar]
- 20.Jumarie C, Malo C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J Cell Physiol. 1991;149:24–33. doi: 10.1002/jcp.1041490105. [DOI] [PubMed] [Google Scholar]
- 21.Peterson MD, Bement WM, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993;105:461–472. doi: 10.1242/jcs.105.2.461. [DOI] [PubMed] [Google Scholar]
- 22.Han O, Failla ML, Hill AD, Morris ER, Smith JC., Jr Inositol phosphates inhibit uptake and transport of iron and zinc by a human intestinal cell line. J Nutr. 1994;124:580–587. doi: 10.1093/jn/124.4.580. [DOI] [PubMed] [Google Scholar]
- 23.Tupe RS, Agte VV. Effect of water soluble vitamins on Zn transport of Caco-2 cells and their implications under oxidative stress conditions. Eur J Nutr. 2010;49:53–61. doi: 10.1007/s00394-009-0048-4. [DOI] [PubMed] [Google Scholar]
- 24.Valentine RA, Jackson KA, Christie GR, Mathers JC, Taylor PM, Ford D. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J Biol Chem. 2007;282:14389–14393. doi: 10.1074/jbc.M701752200. [DOI] [PubMed] [Google Scholar]
- 25.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 26.Ford D. Intestinal and placental zinc transport pathways. Proc Nutr Soc. 2004;63:21–29. doi: 10.1079/PNS2003320. [DOI] [PubMed] [Google Scholar]
- 27.Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, et al. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr. 2005;94:170–181. doi: 10.1079/bjn20051480. [DOI] [PubMed] [Google Scholar]
- 29.Lu QY, Jin YS, Pantuck A, Zhang ZF, Heber D, Belldegrun A, et al. Green tea extract modulates actin remodeling via Rho activity in an in vitro multistep carcinogenic model. Clin Cancer Res. 2005;11:1675–1683. doi: 10.1158/1078-0432.CCR-04-1608. [DOI] [PubMed] [Google Scholar]
- 30.Han O, Wessling-Resnick M. Copper repletion enhances apical iron uptake and transepithelial iron transport by Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G527–G533. doi: 10.1152/ajpgi.00414.2001. [DOI] [PubMed] [Google Scholar]
- 31.Louvard D, Kedinger M, Hauri HP. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992;8:157–195. doi: 10.1146/annurev.cb.08.110192.001105. [DOI] [PubMed] [Google Scholar]
- 32.Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem. 2001;49:5340–5347. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Moreno C, Cao G, Ou B, Prior RL. Anthocyanin and proanthocyanidin content in selected white and red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J Agric Food Chem. 2003;51:4889–4896. doi: 10.1021/jf030081t. [DOI] [PubMed] [Google Scholar]
- 34.Lott JA, Ockenden I, Roboy V, Batten G. In: Global estimate of phytic acid and phosphorus in crop grains, seeds and fruits. Reddy KR, Sathe SK, editors. Boca Raton, Florida, USA: CRC Press; 2002. [Google Scholar]
- 35.Burton DD, Kim HJ, Camilleri M, Stephens DA, Mullan BP, O’Connor MK, et al. Relationship of gastric emptying and volume changes after a solid meal in humans. Am J Physiol Gastrointest Liver Physiol. 2005;289:G261–G266. doi: 10.1152/ajpgi.00052.2005. [DOI] [PubMed] [Google Scholar]
- 36.Kim EY, Ham SK, Shigenaga MK, Han O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J Nutr. 2008;138:1647–1651. doi: 10.1093/jn/138.9.1647. [DOI] [PubMed] [Google Scholar]
- 37.Han O, Failla ML, Hill AD, Morris ER, Smith JC., Jr Reduction of Fe(III) is required for uptake of nonheme iron by Caco-2 cells. J Nutr. 1995;125:1291–1299. doi: 10.1093/jn/125.5.1291. [DOI] [PubMed] [Google Scholar]
- 38.Scalbert A, Mila I, Expert D, Marmolle F, Albrecht AM, Hurrell R, et al. Polyphenols, metal ion complexation and biological consequences. Basic Life Sci. 1999;66:545–554. doi: 10.1007/978-1-4615-4139-4_30. [DOI] [PubMed] [Google Scholar]
- 39.Quesada IM, Bustos M, Blay M, Pujadas G, Ardevol A, Salvado MJ, et al. Dietary catechins and procyanidins modulate zinc homeostasis in human HepG2 cells. J Nutr Biochem. 2011;22:153–163. doi: 10.1016/j.jnutbio.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Yang JG, Yu HN, Sun SL, Zhang LC, He GQ, Das UN, et al. Epigallocatechin-3-gallate affects the growth of LNCaP cells via membrane fluidity and distribution of cellular zinc. J Zhejiang Univ Sci B. 2009;10:411–421. doi: 10.1631/jzus.B0820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun SL, He GQ, Yu HN, Yang JG, Borthakur D, Zhang LC, et al. Free Zn(2+) enhances inhibitory effects of EGCG on the growth of PC-3 cells. Mol Nutr Food Res. 2008;52:465–471. doi: 10.1002/mnfr.200700172. [DOI] [PubMed] [Google Scholar]
- 42.Krezel A, Maret W. Thionein/metallothionein control Zn(II) availability and the activity of enzymes. J Biol Inorg Chem. 2008;13:401–409. doi: 10.1007/s00775-007-0330-y. [DOI] [PubMed] [Google Scholar]
- 43.Yu HN, Shen SR, Yin JJ. Effects of interactions of EGCG and Cd(2+) on the growth of PC-3 cells and their mechanisms. Food Chem Toxicol. 2007;45:244–249. doi: 10.1016/j.fct.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Hallberg L, Rossander L, Skanberg AB. Phytates and the inhibitory effect of bran on iron absorption in man. Am J Clin Nutr. 1987;45:988–996. doi: 10.1093/ajcn/45.5.988. [DOI] [PubMed] [Google Scholar]
- 45.Lonnerdal B, Sandberg AS, Sandstrom B, Kunz C. Inhibitory effects of phytic acid and other inositol phosphates on zinc and calcium absorption in suckling rats. J Nutr. 1989;119:211–214. doi: 10.1093/jn/119.2.211. [DOI] [PubMed] [Google Scholar]
- 46.Liang J, Han BZ, Nout MJ, Hamer RJ. In vitro solubility of calcium, iron and zinc in relation to phytic acid levels in rice-based consumer products in China. Int J Food Sci Nutr. 2010;61:40–51. doi: 10.3109/09637480903229017. [DOI] [PubMed] [Google Scholar]